Ankfy1 is dispensable for neural stem/precursor cell development
Chao Weng, Man Ding, Lian-sheng Chang, Ming-xin Ren, Hong-feng Zhang, Zu-neng Lu,, Hui Fu, Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China Department of Anatomy and Embryology, School of Basic Medical Sciences, Wuhan University, Wuhan, Hubei Province, China Department of Pathology, Central Hospital of Wuhan, Wuhan, Hubei Province, China4 Hubei-MOST KLOS & KLOBME, Wuhan, Hubei Province, China
Ankfy1 is dispensable for neural stem/precursor cell development
Chao Weng1, Man Ding1, Lian-sheng Chang2, Ming-xin Ren2, Hong-feng Zhang3, Zu-neng Lu1,*, Hui Fu2,4,*
1 Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Anatomy and Embryology, School of Basic Medical Sciences, Wuhan University, Wuhan, Hubei Province, China
3 Department of Pathology, Central Hospital of Wuhan, Wuhan, Hubei Province, China
4 Hubei-MOST KLOS & KLOBME, Wuhan, Hubei Province, China
How to cite this article:Weng C, Ding M, Chang LS, Ren MX, Zhang HF, Lu ZN, Fu H (2016) Ankfy1 is dispensable for neural stem/precursor cell development. Neural Regen Res 11(11):1804-1809.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:Dr. Hui Fu was supported by the National Natural Science Foundation of China, No.81371338, and by Open Research Fund Program of Hubei-MOST KLOS & KLOBME. Dr. Zu-neng Lu was supported by grants from Health and Family Planning Commission of Hubei Province scientific research project, No. WJ2015MA007.
Graphical Abstract

There are few studies on the membrane protein Ankfy1. We have found Ankfy1 is specifically expressed in neural stem/precursor cells during early development in mice (murine). To further explore Ankfy1 function in neural development, we developed a gene knockout mouse with a mixed Balb/C and C57/BL6 genetic background. Using immunofluorescence andin situhybridization, neural defects were absent in mixed genetic Ankfy1 null mice during development and in adults up to 2 months old. However, Ankfy1 gene knockout mice with a pure genetic background were found to be lethal in the C57/BL6 inbred mice embryos, even after seven generations of backcrossing. Polymerase chain reaction confirmed homozygotes were unattainable as early as embryonic day 11.5. We conclude that Ankfy1 protein is dispensable in neural stem/precursor cells, but could be critical for early embryonic murine development, depending on the genetic background.
nerve regeneration; Ankfy1; neural development; genetic background; protein; function; gene knockout; neural stem/precursor cells; embryo; neural regeneration
Introduction
Ankfy1, a membrane-bound protein, was first reported in 1999 (Ito et al., 1999). Ankfy1 has a molecular weight of approximately 130 kDa in both humans and mice, as detected by western blotting (Ito et al., 1999; Kuriyama et al., 2000). Ankfy1 does not have a typical transmembrane hydrophobic domain (Ito et al., 1999), but consists of a coiled-coil structure and a BTB/POZ domain at the N-terminal, multiple ankyrin repeats in the middle, and a FYVE-finger motif at the C-terminal (Ito et al., 1999; Kuriyama et al., 2000).
Ankfy1 has several names including ANKHZN (Ito et al., 1999) and Rabakyrin-5 (Schnatwinkel et al., 2004) andis widely expressed in multiple tissues during development and in adults (Ito et al., 1999; Kuriyama et al., 2000). Functionally, Ankfy1 is involved in different types of endocytosis, especially in macropinocytosis (Schnatwinkel et al., 2004). Ankfy1 can be found in large vacuolar structures associated with macropinocytosis through direct binding with Rab5. Overexpression of Ankfy1 can increase macropinocytosis while knockdown suppresses the process (Schnatwinkel et al., 2004). Ankfy1 is also found in early endosomes, and as shown by the limited effect of knockdown experiments, has a minor role in early endosome fusion (Schnatwinkel et al., 2004). Another study found that Ankfy1 plays marginal roles in endosome-to-Golgi retrieval and biosynthetic transport, as it is localized in the retromerviaassociation with EH Domain Containing 1 (EHD1) and vacuolar protein sorting 26 (Vps26) (Zhang et al., 2012). In summary, there are few studies on Ankfy1 and all to date have been carried outin vitro. Based on these few studies, our current understanding indicates that Ankfy1 plays minor roles in endocytosis/vesicle shuffling.
From our previous genome-wide study in the developing mouse brain (Gray et al., 2004), we found Ankfy1 to be of significant interest. Ankfy1 displayed an interesting expression pattern during early development, with specific expression in the ventricular zone of the central nervous system (CNS), suggesting Ankfy1 functions within neural stem cells (NSCs)/precursor cells. Based on this observation, we developed Ankfy1 knockout (K/O) mice to carry out detailed studies on its function in neural development.
Materials and Methods
Animals
The chimera mouse was ordered from the Mutant Mouse Resource & Research Center (MMRRC, UC Davis, CA, USA), where a gene-trap embryonic stem cell (ESC) line against Ankfy1 had already been generated. C57/BL6 and Balb/C inbred mice were purchased from Charles River Laboratories (MA, USA). The mice were housed in the Animal Facility Center of the Dana-Farber Cancer Institute (Boston, MA, USA) and Wuhan University (Wuhan, China). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 8023, revised 1978) and were approved by the Institutional Animal Care and Use Committee at the Dana-Farber Cancer Institute and Wuhan University.
In situhybridization
The Ankfy1 probe was made by reverse transcription-polymerase chain reaction (PCR) from an embryonic day 13.5 (E13.5) mouse embryo. The primer sequences were 5′-CAC AAG TTT GTC TTG GCC GC and 3′-GGT CTA ATG GGT CTC CTG GG. The PCR product was 741 bp in size and cloned into a pSport1 plasmid.In situhybridization was performed as previously described (Fu et al., 2009).
Cell culture
Neural stem cell culture
Mouse NSCs were isolated from the cortex of embryonic (E14) CD-1 mice (SJA Lab Animal, China). The mouse cortices were dissected out and transferred into a 15-mL tube containing cold phosphate buffered saline (PBS). The tissues were triturated with a pipette to make single-cell suspension and passed through a 70 μm cell strainer (Falcon, USA). After centrifugation at 800 r/min (TDL-40B, Anke, Shanghai, China) for 5 minutes, the cells were resuspended in Dulbecco’s Modified Eagle Medium (DMEM)/F12 (Invitrogen, Carlsbad, CA, USA) containing L-glutamine (Sigma, St. Louis, MO, USA), B27 and N2 supplements (Invitrogen), 20 ng/mL epidermal growth factor, and 20 ng/mL fibroblast growth factor (Invitrogen). Cells were then seeded at a density of 2 × 105cells/mL on 6-well plates coated with poly 2-hydroxyethylmethacrylate (Sigma). Cells were passaged every 5–8 days, depending on their growth, by mechanical dissociation. For differentiation, cells were dissociated and resuspended in DMEM/F12 containing L-glutamine and B27 and N2 supplements, then seeded on 24-well plates containing cover slips coated with poly-L-ornithine (15 mg/L; Sigma) at 1 × 104cells/cm2. For immunostaining, NSCs were allowed to differentiate for 5 days.
Mixed glial cell culture
Cortices from neonatal mouse (P1–2) were digested with Accumax (Chemicon, Santa Cruz, CA, USA) for 10 minutes at 37°C. Cortices were triturated and passed through a 70-μm cell strainer (Falcon, USA) to form a single-cell suspension. After centrifugation at 1,000 r/min for 5 minutes, the cells were suspended in DMEM/F12 + 10 % fetal bovine serum (Hyclone, Logan, UT, USA). When the cultured cell mixture reached 65–75 % confluence, the culture medium was replaced with DMEM/F12 + 15 % B104 cell-conditioned medium + 1% N2supplement + 5 mg/ L insulin (Sigma, USA) (modified oligodendrocyte progenitor cell [OPC] growth-medium). The modified OPC growth-medium was replaced daily. On day 7 after incubation with modified OPC growth-medium, mixed glial cells were used for immunostaining.
Immunofluorescence staining
The immunofluorescence procedure was performed as previously described (Fu et al., 2009), and conducted in E13.5 and E18.5 spinal cords of Ankfy1 null and littermate control mice. Briefly, the slides were washed three times with PBS, 10 minutes each. Afterwards, slides were blocked by 5 % normal goat serum for 1 hour. After blocking, primary antibodies were added and incubated at 4°C overnight. Slides were then washed in PBS three times, 10 minutes each, followed by incubation with appropriate secondary antibodies (Jackson ImmunoResearch, PA, USA) in PBS for 1 hour. The slides were washed three more times in PBS. Pictures were taken using a Nikon Eclipse Ni fluorescent microscope (Nikon, Tokyo, Japan).
The antibodies used in this study included: anti-Olig2 (1:10,000, from Dr. Charles Stiles’ lab, DFCI, Boston, MA, USA), anti-glutamine synthetase (1:500; MAB302, Chemicon), anti-NeuN (1:200; MAB377, Chemicon), anti-glast
(1:4,000; AB1782, Chemicon), anti-Nkx2.2 (1:5; 74.5A5, Hybridoma Bank), anti-Sox9 (1:100; Ab5535, Chemicon), anti-glial fibrillary acidic protein (GFAP)-cy3 (1:200; C9205, Sigma), anti-Tuj1 (1:400; PRB-435P-100, Covance), anti-NG2 (1:50; Dr. Stallup’s Lab, Burnham Institute for Medical Research, Cancer Research Center, La Jolla, CA, USA), anti-Sox10 (1:10; Dr. Anderson’s Lab, Caltech, Pasadena, CA, USA), anti-Ankfy1 (B-6) (1:100; SC393353, Santa Cruz Biotechnology), and anti-Ankfy1 (1:100; 24890-I-AP, Proteintech, Rosemont, IL, USA).
Whole mouse necropsy
Whole mouse necropsy (overall histological examination) was performed at the DF/HCC Rodent Histopathology Core in Harvard Medical School (USA) with four Ankfy1 null mice and two heterozygotes.
Results
Ankfy1 expression matched neural precursor markers
In an earlier study, we performed genome-wide transcription factor screening during neural development (Gray et al., 2004). Since Ankfy1 protein contains a Zinc-finger motif, which could serve as a DNA-binding motif in many proteins, it was included in this study. Initial screening revealed that Ankfy1 was specifically expressed in the ventricular zone during early development; whilst a follow-up study highlighted its interesting expression patterns during neural development (Figure 1). In the spinal cord during neuronal development (E11.5), Ankfy1 was highly expressed in the ventral part of the ventricular zone, where NSCs/neuronal precursors are located. Two days later at E13.5, Ankfy1 had restricted expression in the ventral ventricular zone. At this time, the spinal cord starts gliogenesis, therefore the majority of cells in the ventricular zone are glial precursors. Simultaneously, Ankfy1 was also specifically expressed in the ventricular zone in the hindbrain and midbrain, but not in the forebrain. Later on, Ankfy1 was expressed in the migrating neural/glial progenitors which radiated out from the ventricular zone into the parenchyma (Figure 1B, from E15.5–P5). This expression pattern, especially in the developing spinal cord, matched that of glial progenitor markers (like Sox9), suggesting that Ankfy1 may play functional roles in NSCs and/or glial development.
Double staining experiments on cultured neural cells confirmed that Ankfy1 was expressed in glial progenitors (Figure 2). In mixed glia cell cultures, Ankfy1 was expressed in NG2-positive glial progenitors (Figure 2B). More than 95% NG2-immunoreactive cells were also positive for Ankfy1 and newly differentiated neural cells continued to express Ankfy1. However, NG2 levels were lower than those in the progenitor cells (Figure 2A,C,D). After NSCs were differentiated for 5 daysin vitro, Ankfy1 was found to be expressed at low levels in different neural cell types, such as astrocytes (GFAP+cells;Figure 2A), neurons (Tuj1+cells;Figure 2C) and oligodendrocytes (Sox10+cells;Figure 2D). Since there are no previous studies of Ankfy1 in the nervous system, we investigated the function of this protein using a transgenic animal approach.
Development of Ankfy1 null mice
The chimera mouse was sourced and the colony raised in our animal facility. The gene-trap vector was inserted into intron 4, which is approximately 6 kb in length. Genomic PCR was performed to determine the exact insertion point. Heterozygote genomic DNA was used as the template DNA for PCR. The 3′-primer was chosen according to the sequence on the gene trap vector from bases 157–177. A series of 5′-primers were chosen based on the sequence of intron 4 at intervals of about 500 bases (starting positions 512, 1102, 1610, 2139, 2618, 3059, 3623, 4091, 4643, 5088, and 5611;Table 1). Only the first three 5′-primers produced PCR products (Figure 3A). The PCR products with the 5′-primer at position 512, 1102 and 1610 were approximately 1.7 kb, 1.1 kb, and 0.6 kb, respectively (Figure 3A). The PCR results consistently showed that the insertion site was around position 2000 in intron 4. The PCR results were sent for sequencing and the insertion site was determined at position 2067 (Figure 3B). Once the insertion site was located, PCR primers were designed forgenotyping the wild-type (WT) allele of Ankfy1 in the genetrapped mice.

Table 1 Sequences of the primers used to locate the insertion site of the gene-trap vector in the transgenic mouse genome
Ankfy1 null mice showed no phenotype change during development and in adults
Ankfy1 homozygotes were generated from hetero-heterozygote mating. However, the knockout (K/O) mice looked identical to the WT littermates and were viable and fertile with no obvious defects.
Since Ankfy1 is specifically expressed in NSCs/precursor cells, we carefully monitored neural development of the Ankfy1 null mice. At E13.5, the homozygote embryos were comparable in size to their WT littermates, with no differences in overall morphology. Simultaneously, we determined the expression of several precursor markers on the developing spinal cord, such as Glast, a radial glial marker, and Sox9, a glial precursor marker. There were no differences in gene expression patterns between the K/O and WT mice (Figure 4A). Because Ankfy1 was only expressed in the ventral part of the spinal ventricular zone, we assessed some ventral neuronal precursor markers, such as Olig2 (pMN marker, data not shown) and Nkx2.2 (p3 marker). There were no differences in gene expression patterns between the K/O and WT mice (Figure 4A; data not shown).
Neural development was further investigated at developmental stage E18.5 (Figure 4B) in Ankfy1 K/O mice. Similarly, the homozygous embryos at this later stage could not be distinguished from the WT littermates and there were no differences in expression patterns of NeuN, Olig2 and glutamine synthetase.
In adult mice, we found no gross defects in Ankfy1 K/O mice up to two-months-old. Furthermore, whole mouse necropsy showed that within all the major organs, neither the K/O nor heterozygote mice had any histological differences from WT mice (data not shown). One K/O mouse displayed some liver fibrosis, implying potential cirrhosis. However, this defect was not replicated in the other three K/O mice. In cases where the K/O mice were maintained beyond 2 months, no obvious defects were observed.
Genetic background determined Ankfy1 knockout mouse survival
The transgenic mouse was derived from the 129P2/OlaHsd ESC line. The chimera mice were mated with the C57/B6 mice for ten generations to obtain a transgene on a pure C57/B6 genetic background. During this process, homozygous mice could not be generated at the Mendelian ratio (Table 2). There were always some degenerated embryos (as early as E11.5) inside the heterozygous mothers after hetero-heterozygote mating (Table 2). Furthermore, litter sizes following hetero-heterozygote breeding were smaller in comparison with the normal C57/B6 litter size (Table 2).
After backcrossing with C57/BL6 mice for six generations, we were unable to obtain K/O embryos or pups. We checked as early as E11.5 in all cases and before E11.5 (E9–E10) in some cases, and could not find any homozygous embryos. Consistently, some degenerated embryos were found inside the heterozygous mothers after hetero-heterozygote mating. We tried genotyping degenerated embryos and found some of them were homozygotes for the Ankfy1 transgene.
We then switched the genetic background to Balb/C inbred mice. Again, as the genetic background became increasingly Balb/C, especially after six generations, the occurrence of K/O mice was negligible.
Discussion
Ankfy1 was selected from our genome-wide screening because of its specific expression in NSCs/precursor cells (Gray et al., 2004). The Ankfy1 expression patterns matched those of glial precursors. During early development stages (E11.5–13.5), Ankfy1 was specifically expressed in the ventral ventricular zone in the CNS, except in the forebrain. At later stages (E15.5–P5), Ankfy1-immunoreactive cells radiated out from the ventricular zone to the parenchyma. Double-staining confirmed its expression in glial precursors. From these data, we speculate that Ankfy1 may play important roles in NSCs and/or glial precursors.
Although only limitedin vitrostudies exist on Ankfy1, it is reported to be involved in different types of endocytosis (Schnatwinkel et al., 2004; Zhang et al., 2012). Furthermore, experiments suggest that it plays a minor role in macropinocytosisviaits association with Rab5 (Schnatwinkel et al., 2004; Zhang et al., 2012). Knockdown of Ankfy1 can cause an approximately 50 % decrease in macropinocytosis, but almost no effects in other types of endocytosis (Schnatwinkel et al., 2004; Zhang et al., 2012).
Macropinocytosis is thought to be important for membrane-receptor internalization and receptor tyrosine kinase pathways (Schmees et al., 2012; Nehru et al., 2013). Although overexpression of Ankfy1 can activate platelet-derived growth factor receptor-beta (PDGFRβ)-dependent signaling pathways, knockdown experiments have shown only a small effect on this pathway (Schmees et al., 2012; Nehru et al., 2013). Many studies have shown that macropinocytosis is important for several developmental processes, such as axon growth, cone development (Bonanomi et al., 2008), neural crest cell development (Padmanabhan and Taneyhill, 2015), and epithelial morphogenesis (Fabrowski et al., 2013). Macropinocytosis likely affects these processes through plasma membrane recycling.
Because of the interesting expression pattern of Ankfy1 in neural development, we studied the function of Ankfy1 using a transgenic animal model. However, Ankfy1 K/O mice showed no obvious defects in the CNS during development and in adults. There were no detectable defects on NSCs/ precursor cells or neuronal/glial cells. The K/O mice looked normal showing no difference from the WT littermates. A detailed histological examination revealed no histological defects in all major organs of the K/O mice at two-monthsold. Overall, our data suggest that the function of Ankfy1 during neural development is negligible, despite its specific expression in NSCs/precursors.

Figure 1 In situ hybridization showing expression patterns of Ankfy1 mRNA in the developing murine CNS.

Table 2 Genotypes of the Ankfy1 hetero-hetero mated offspring (from F1 to F6 backcrossing of C57/BL6 inbred)

Figure 2 Ankfy1 expression in different cell types; predominantly neural precursors in the central nervous system (fluorescent microscopy).
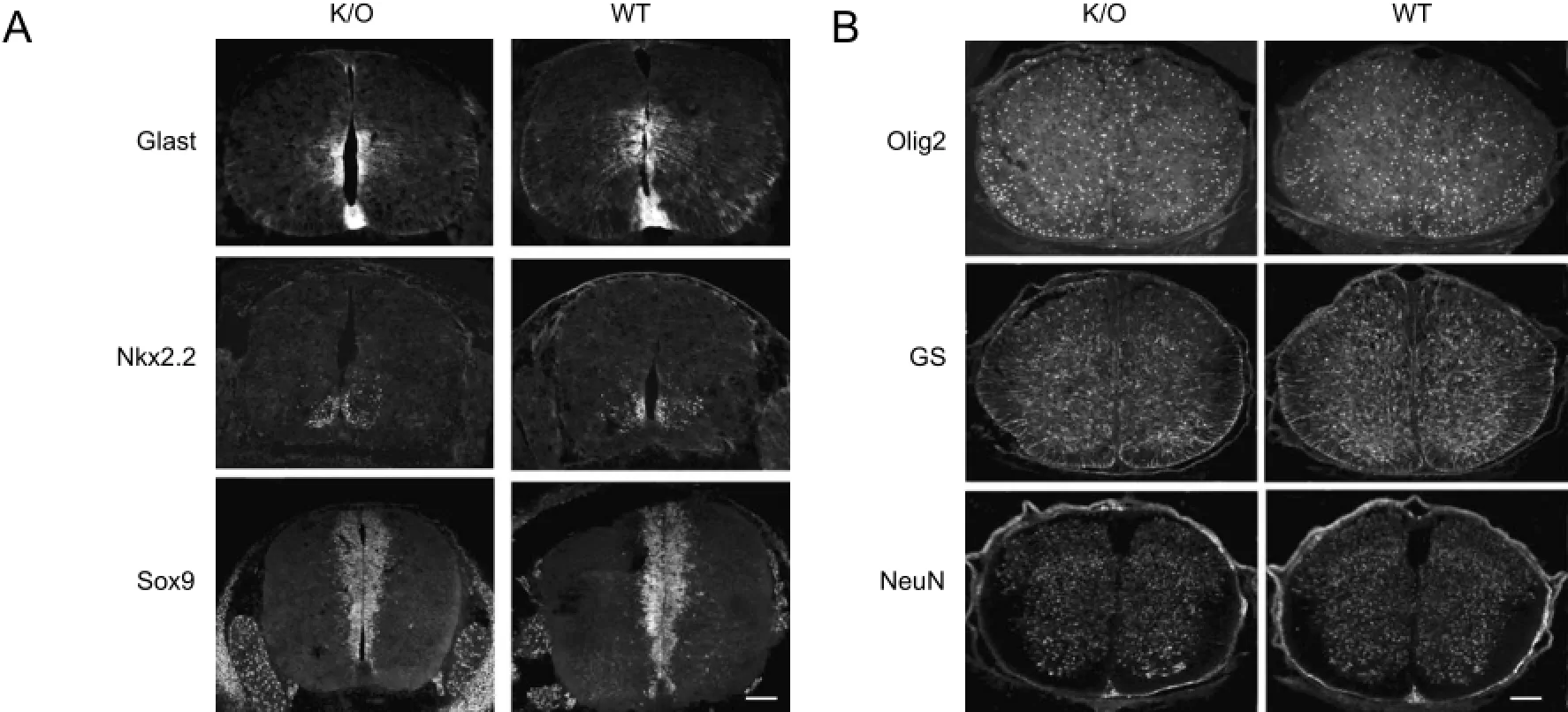
Figure 4 Expression patterns of several neural markers in the developing spinal cords of Ankfy1 null and control mice (immunofluorescence staining, fluorescent microscopy).
Ankfy1 was not expressed in the ventricular zone of the murine forebrain during development. Furthermore, inadult mice, we did not detect expression of Ankfy1 in the subventricular zone of the forebrain, where adult NSCs/precursors are located. Consequently, it is unlikely that Ankfy1 plays functional roles in adult neurogenesis.
To our surprise, Ankfy1 heterozygote mothers were unable to produce homozygous offsprings when they had a highly homogeneous genetic background. The gene-trap ESC line 129P2/OlaHsd was injected into C57/BL6 host blastocysts to generate chimeras. After six generations of backcrossing with C57/BL6 or Balb/C inbred mice, homozygotes were clearly absent. A few degenerated embryos were routinely found in pregnant heterozygous mothers as early as E11.5 (the earliest time point assessed). Even with less of a homogeneous genetic background (F1–F6), degenerated embryos occasionally appeared in heterozygous mothers after hetero-heterozygote mating. Furthermore, homozygotes were produced at a rate less than 1/4, the standard Mendelian ratio, and the litter sizes were smaller. These data showed that Ankfy1 protein affects early embryonic development, with a penetration level determined by genetic background. In the mixed genetic background, penetration was only partial; while in the more homogeneous background, penetration was 100%.
This phenomenon is not uncommon, despite being seldom reported. For example, Olig1 knockout mice with a mixed background are viable and fertile, showing a normal phenotype with only very mild delay in myelin formation. This defect is quickly self-corrected during late development stages (Lu et al., 2002). However, in a highly homogeneous C57/ BL6 genetic background, Olig1 mutant mice have a severe myelin deficit and die before the third week after birth (Xin et al., 2005). Studies on another neural protein, the Alzheimer’s amyloid precursor protein, showed similar results. The transgene Tg (amyloid precursor protein 695) merely induces the formation of amyloid plaques in the brains of outbred mice. However, it is lethal in inbred FVB/N or C57/BL6 mice (Carlson et al., 1997).
In summary, the membrane protein Ankfy1 is specifically expressed in NSCs/precursors during development. Ankfy1 K/O mice with a mixed genetic background showed no obvious phenotypic changes, particularly in respect of neural developmental defects. However, Ankfy1 K/O was lethal in early embryonic development in highly homogenous genetic backgrounds, such as C57/BL6 or Balb/C. These findings indicate that Ankfy1 plays important roles in early embryonic development and this function is highly regulated by genetic background. Our findings also showed that genetic background could play important and even dominant roles in determining the phenotype of transgenic mice.
Acknowledgments:We thank Dr. Charles Stiles (DFCI, Harvard Medical School) for the access to the DF/HCC Rodent Histopathology Core in Harvard Medical School and the Olig2 antibody. The mouse strain used for this research project, B6; 129P2-Ankfy1Gt (RRE069) Byg/Mmucd, identification number 015991-UCD, was obtained from the Mutant Mouse Regional Resource Center, a National Institutes of Health funded strain repository, and was donated to the Mutant Mouse Resource & Research Centers by Mutant Mouse Resource & Research Centers at University of California, Davis, CA, USA.
Author contributions:HF conceived and designed the study. CW, MD,MXR and LSC performed in situ hybridization and immunofluorescence staining. CW and MD performed cell culture. CW, HF and ZNL analyzed the data. CW and MD were responsible for mating mice. CW, HF and ZNL participated in the design and coordination of this study and helped to draft the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Bonanomi D, Fornasiero EF, Valdez G, Halegoua S, Benfenati F, Menegon A, Valtorta F (2008) Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones. J Cell Sci 121:3757-3769.
Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK (1997) Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6:1951-1959.
Fabrowski P, Necakov AS, Mumbauer S, Loeser E, Reversi A, Streichan S, Briggs JA, De Renzis S (2013) Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nat Commun 4:2244.
Fu H, Cai J, Clevers H, Fast E, Gray S, Greenberg R, Jain MK, Ma Q, Qiu M, Rowitch DH, Taylor CM, Stiles CD (2009) A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci 29:11399-11408.
Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, et al. (2004) Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 306:2255-2257.
Ito K, Ishii N, Miyashita A, Tominaga K, Kuriyama H, Maruyama H, Shirai M, Naito M, Arakawa M, Kuwano R (1999) Molecular cloning of a novel 130-kDa cytoplasmic protein, Ankhzn, containing Ankyrin repeats hooked to a zinc finger motif. Biochem Biophys Res Commun 257:206-213.
Kuriyama H, Asakawa S, Minoshima S, Maruyama H, Ishii N, Ito K, Gejyo F, Arakawa M, Shimizu N, Kuwano R (2000) Characterization and chromosomal mapping of a novel human gene. Ankhzn Gene 253:151-160.
Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109:75-86.
Nehru V, Voytyuk O, Lennartsson J, Aspenstrom P (2013) RhoD binds the Rab5 effector Rabankyrin-5 and has a role in trafficking of the platelet-derived growth factor receptor. Traffic 14:1242-1254.
Padmanabhan R, Taneyhill LA (2015) Cadherin-6B undergoes macropinocytosis and clathrin-mediated endocytosis during cranial neural crest cell EMT. J Cell Sci 128:1773-1786.
Schmees C, Villasenor R, Zheng W, Ma H, Zerial M, Heldin CH, Hellberg C (2012) Macropinocytosis of the PDGF beta-receptor promotes fibroblast transformation by H-RasG12V. Mol Biol Cell 23:2571-2582.
Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M (2004) The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol 2:E261.
Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR (2005) Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci 25:1354-1365.
Zhang J, Reiling C, Reinecke JB, Prislan I, Marky LA, Sorgen PL, Naslavsky N, Caplan S (2012) Rabankyrin-5 interacts with EHD1 and Vps26 to regulate endocytic trafficking and retromer function. Traffic 13:745-757.
Copyedited by Finnie E, de Souza M, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Hui Fu, Ph.D. or Zu-neng Lu, M.D., hueyfu@yahoo.com or lzn196480@126.com.
orcid: 0000-0003-0252-6009 (Hui Fu) 0000-0003-0001-3437 (Zu-neng Lu)
10.4103/1673-5374.194750
Accepted: 2016-08-24
- 中國神經再生研究(英文版)的其它文章
- The status of Nrf2-based therapeutics: current perspectives and future prospects
- Targeting neuronal nitric oxide synthase as a valuable strategy for the therapy of neurological disorders
- Six psychotropics for pre-symptomatic & early Alzheimer’s (MCI), Parkinson’s, and Huntington’s disease modification
- Applicability of tooth derived stem cells in neural regeneration
- Cortical spreading depression-induced preconditioning in the brain
- Neuroinflammation, neurodegeneration and regeneration in multiple sclerosis: intercorrelated manifestations of the immune response