Energies of Combustion and Specific Heat Capacities of Diaminofurazan, Dinitrofurazan and Diaminoazofurazan
LI Yan-feng, ZHAI Lian-jie, XU Kang-zhen, SONG Ji-rong, ZHAO Feng-qi
(1. School of Chemical Engineering, Northwest University, Xi′an 710069, China; 2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
1 Introduction
As one of the most simple furazan ring compounds, 3,4-diaminofurazan(DAF) features good thermal stability and big density, which make it efficient structural units in the development of high-energy-density materials(HEDMs)[1-5]. DAF has been an important precursor to a series of furazan-based energetic materials[6-10], 3,4-dinitrofurazan (DNF), 4,4-dimino-3,3-azofurazan(DAAzF) are two relatively simple derivatives of DAF, and their relationship are shown in Scheme 1. DNF was firstly reported at 1994[11], having a crystal density of 1.62 g·ml-1, a melting point of 15.8 ℃ and a boiling point of 168.8 ℃. DNF is a high energy explosive and has been exploited the high reactivity of the nitro-groups to nucleophile in the synthesis of a large number of energetic derivatives. DAAzF is another special rich-nitrogen insensitive energetic material for its big positive enthalpy of formation[12]. Many studies showed that the three furazan compounds have excellent properties and good application prospect[13-14].
Energy of combustion and specific heat capacity are twoimportant thermodynamic data and characteristic quantities closely related to energy and structure of material. We researched energies of combustion and specific heat capacities of the three furazan ring compounds to enrich thermochemical database and provide theoretical basis for further application. Meanwhile, the change rule of structure-property for the three compounds was also discussed.
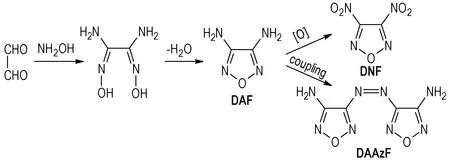
Scheme 1 Relationship of DAF, DNF and DAAzF
2 Experimental
2.1 Samples
DAF, DNF and DAAzF were synthesized by our research group in Xi′an Modern Chemistry Research Institute. Their purities are more than 99.5%(HPLC). DAF and DAAzF are solid, and DNF is liquid.
2.2 Energy of Combustion
Energy of combustion was determined with an IKA C5000 oxygen-bombcalorimeter (German) in adiabatic pattern. The calorimeter was calibrated with the standard substance benzoic acid having a purity of 99.99%, and each sample was tested with 6 times, The mean value (-26504±147) J ·g-1 [15]is very close to the standard value as (-26434±3 ) J ·g-1(T=298.15 K)[16], indicating that the measuring system is accurate and reliable. The uncertainty can be obtained by equationUc=ku, whereuis the standard uncertainty (the standard deviation of mean) the coverage factorkis 2, and level of confidence is 0.95.
2.3 Specific Heat Capacity
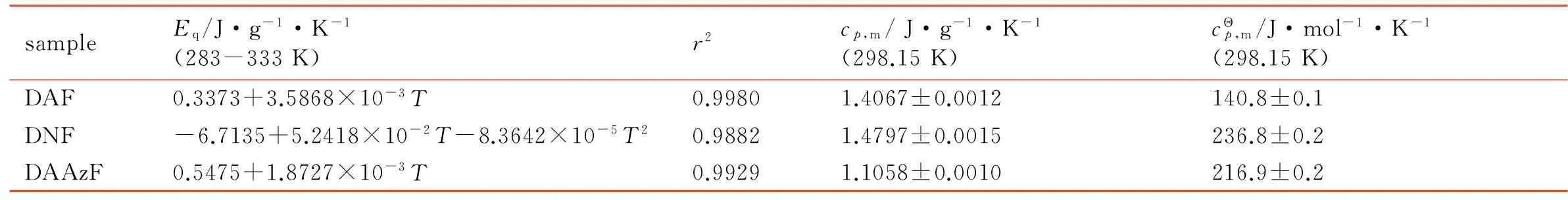
Specific heat capacity was measured using a Micro-DSCⅢ apparatus (SETARAM, France), with the operating temperature range of 283-333 K, temperature accuracy of 10-4K, heat flow accuracy of 10-4mW, and heating rate of 0.15 K ·min-1. The sample mass used for calorimetric measurement was about 200 mg. The reliability of enthalpy measurement was ensured by determinations of the enthalpy of dissolution of KCl (cr) in deionized water at 298.15 K. The result was (17.27±0.07) kJ·mol-1, which was very close to the literature value as (17.24±0.02) kJ·mol-1[17]. The equation of specific heat capacity for standard calcinedα-Al2O3obtained wascp(J·g-1·K-1)=0.184+1.997×10-3T(283 K Each sample was tested with 6 times, and the results are listed in Table 1. The constant-volume energies of combustion for DAF, DNF and DAAzF are (-13043±119), (-6863±37), (-12661±54) J·g-1, respectively. The energy of combustion tends to rise with the decrease of oxygen content in molecule, DAF(15.99%)>DAAzF(16.31%)>DNF(49.98%). DAF and DAAzF exhibit greater energy of combustion than DNF, indicating that amino group is an excellent burning group and markedly increase energy of combustion, but nitro group has the opposite influence. Due to the same number of amino group and the approximate nitrogen content (oxygen content), the difference of energy of combustion for DAF and DAAzF is small. Table 1 Determination results for the energies of combustion of the samples sampleNo.m/gΔT/KΔcU/J·g-1DAF10.157330.19831295420.157680.20061308430.157190.19841297340.157470.19971304050.157240.19741289960.157330.200213308mean13043±119DNF10.149000.1045686020.146500.1026683530.143300.1001679740.144000.1018689350.142500.0999693060.143670.10126863mean6863±37DAAzF10.143900.17761265220.144260.17911274530.143240.17571257640.143660.17661261050.143890.17741265260.103640.131712728mean12661±54 Note:mis the mass of the sample; ΔTis the temperature rising; ΔcUis the energy of combustion. M(s)+aO2(g)=bCO2(g)+cH2O(l)+dN2(g) (1) M= DAF: C2H4ON4,a=5/2,b=2,c=2,d=2; DAAzF: C4H4O2N8,a=4,b=4,c=2,d=4; Herein, it is necessary to illustrate that DNF is an oxygen-rich compound, whose oxygen content reaches 49.98%. It doesn′t need extra oxygen in combustion process according to equation (1). In order to obtain the standard molar enthalpy of combustion for DNF, another idealized reaction equation (2) was adopted. M(l)=aCO2(g)+bO2(g)+cN2(g) (2) M=DNF: C2O5N4,a=2,b=1/2,c=2; Meanwhile, in consideration of the rich nitro group in these compounds, idealized reaction equation (3) was employed. In the following reaction, NO2was considered as the gas product instead of usual N2for thermochemical calculation. M(s)+aO2(g)=bCO2(g)+cH2O(l)+dNO2(g) (3) M= DAF: C2H4ON4,a=13/2,b=2,c=2,d=4; DNF: C2O5N4,a=7/2,b=2,c=0,d=4; DAAzF: C4H4O2N8,a=12,b=4,c=2,d=8; (4) Δn=∑ni(products, g)- ∑ni(reactants, g) (5) where ∑niwas the total molar amount of gases in products or reactants. (6) If N2was considered as gas product, DAF has negative enthalpy of formation as (-57.1±11.9) kJ·mol-1, while DNF and DAAzF have positive enthalpy of formation as (295.3±6.0) kJ·mol-1and (327.8±10.5) kJ·mol-1. Compared with amino group, nitro group has a greater effect on positive enthalpy of formation. Diazotization structure also contributes to positive enthalpy of formation. Moreover, if NO2was considered as gas product, the value of enthalpy of formation of DAF is in close proximity to the literature value as 89.99 kJ·mol-1[22], which indicates that considering NO2as gas product is feasible. Enthalpies of formation for DAF, DNF and DAAzF are (80.6±11.9), (437.9±6.0) kJ·mol-1and (603.1±10.5) kJ·mol-1respectively, and the change rule is consistent with the above. Table 2 The thermodynamic values for the three furazan compounds at 298.15 K sampleM/g·mol-1-ΔcUm/kJ·mol-1-ΔcHΘma/kJ·mol-1-ΔcHΘmb/kJ·mol-1ΔfHΘma/kJ·mol-1ΔfHΘmb/kJ·mol-1DAF100.081305.3±11.91301.6±11.91306.6±11.9-57.1±11.980.6±11.9DNF160.051098.4±6.01087.3±6.01092.3±6.0300.3±6.0437.9±6.0DAAzF196.132483.1±10.52473.5±10.52483.1±10.5327.8±10.5603.1±10.5 of energy of combustion (J·g-1). The specific heat capacity of DAF is larger than that of DAAzF, indicating that diazotization structure may decrease the specific heat capacity of compound, but the values of DAF and DAAzF are still close to each other, which is consistent with the result of energy of combustion. Fig.1 Determination result of the continuous specific heat capacity of DAAzF Table 3 Determination results of continuous specific heat capacity at 298.15 K sampleEq/J·g-1·K-1(283-333K)r2cp,m/J·g-1·K-1(298.15K)cΘp,m/J·mol-1·K-1(298.15K)DAF0.3373+3.5868×10-3T0.99801.4067±0.0012140.8±0.1DNF-6.7135+5.2418×10-2T-8.3642×10-5T20.98821.4797±0.0015236.8±0.2DAAzF0.5475+1.8727×10-3T0.99291.1058±0.0010216.9±0.2 [1] LI Zhan-xiong, TANG Song-qing, OU Yu-xiang, et al. Synthesis status of furazano energetic Derivatives[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2002, 10(2): 59-65. [2] Talawar M B, Sivabalan R, Senthilkumar N, et al. Synthesis, characterization and thermal studies on furazan- and tetrazine-based high energy materials[J].JournalofHazardousMaterials, 2004, 113(1-3): 1125. [3] GE Zhong-xue, WANG Xi-jie, JIANG Jun, et al. Synthesis of 3, 4-dinitrofurazan[J].ChineseJournalofSyntheticChemistry, 2008, 116(3): 260-263. [4] Veauthier J M, Chavez D E, Tappan B C, et al. Synthesis and characterization of furazan energetics ADAAF and DOATF[J].JournalofEnergeticMaterials, 2010, 28(3): 229-249. [5] Chavez D E, Parrish D A, Leonard P. The synthesis and characterization of a new furazan heterocyclic System[J].Synlett, 2012, 23(14): 2126-2128. [6] Sheremetev A B, KulginaV O, Aleksandrova N S, et al. Dinitro trifurazans with oxy, azo, and azoxy bridges[J].Propellants,Explosives,Pyrotechnics, 1998, 23(3): 142-149. [7] Chavez D, Hill S, Hiskey M. Preparation and explosive properties of azo- and azoxy-furazans[J].JournalofEnergeticMaterials, 2000, 18(2-3): 219-236. [8] GE Zhong-xue, LAI Wei-peng, LIAN Peng, et al. Predicting on the detonation performances of poly-furazans with oxy bridges[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2008, 16(3): 280-284. [9] ZHANG Yu, WANG Bo-zhou, XU Kang-zhen, et al. Synthesis and characteristics of bis(nitrofurazano)furazan (BNFF), an insensitive material with high energy-density[J].Propellants,Explosives,Pyrotechnics, 2014, 39(6): 809-814. [10] ZHAI Lian-jie, WANG Bo-zhou, HUO Huan, et al. Synthesis, crystal structure and thermal behavior of 3,4-Bis(3-nitrofurazan-4-oxy)furazan[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2015, 23(1): 18-22. [11] Novikova T S, Melnikova T M, Kharitonova O V, et al. An effective method for the oxidation of aminofurazans to nitrofurazans[J].MendeleevCommun. 1994, 4(4): 138-140. [12] LI Yu-bin, HUANG Hui, LI Jin-shan, et al. HMX based low sensitive high explosives containing DAAzF[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2008, 16(3): 244-246. [13] Pagoria P F. A review of energy materialssynthesis[J].ThermochimicaActa, 2002, 384(1): 187-204. [14] Sikder A K, Sikder N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications[J].JournalofHazardousMaterials, 2004, 112(1-2): 1-15. [15] SUN Qian, LI Yan-feng, XU Kang-zhen, et al. Crystal structure and enthalpy of combustion of AEFOX-7[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2015, 23(12): 1235-1239. [16] Certificate of Analysis Standard Reference Material 39j Benzoic Acid Calorimetric Standard, NBS, Washington D C, 1995. [17] Marthada V K. The enthalpy of solution of SRM 1655 (KCl) in H2O[J].JournalofResearchoftheNationalBureauofStandards, 1980, 85(6): 467-471. [18] Ditmars D A, Ishihara S, Chang S S. Enthalpy and heat-capacity standard reference material: synthetic sapphire ((δ)-Al2O3) from 10 to 2250 K[J].JournalofResearchoftheNationalBureauofStandards, 1982, 87(2): 159-163. [19] Atkins P, Paula J D. Atkins′ Physical Chemistry (7th)[M]. Beijing: High Education Press, 2006. [20] Cox J D. CODATA recommended key values for thermodynamics, 1977 Report of the CODATA Task Group on key values for thermodynamics[J].JournalofChemicalThermodynamics, 1978, 10(10): 903-906. [21] HU Rong-zu, ZHAO Feng-qi, GAO Hong-xu, et al. Fundamentals and application of calorimetry[M]. Beijing: Science Press, 2011. [22] TIAN De-yu, ZHAO Feng-qi, LIU Jian-hong. Handbook of Energetic Materials and the Related Compounds[M]. Beijing: National Defense Industry Press, 2011.3 Results and Discussion
3.1 Energy of Combustion

3.2 Enthalpy of Formation


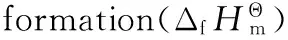


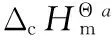
3.3 Specific Heat Capacity



4 Conclusions
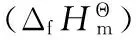

- 含能材料的其它文章
- Crystal Structure and Thermal Behavior of Potassium Dinitromethane
- Coatings of Activated Metal Hydride and Application in the Fuel-rich Propellant
- Synthesis and Properties of 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol and Its Ammonium and Hydroxylammonium Salts
- The Tensile Properties and Creep Performance of a Long-term Thermally Aged Plastic Bonded Explosive
- Thermal Behaviors of 1-Amino-2-nitroguanidine
- Synthesis and Thermal Properties of 1,1′-Dioxide-5,5′-azotetrazole Dipotassium Salt

