Ag-Cu Nanoparticles Supported on N-Doped TiO2Nanowire Arrays for Efficient Photocatalytic CO2Reduction
Xiao-nong WangJun MaYang-guang HuRan LongYu-jie Xiong
Hefei National Laboratory for Physical Sciences at the Microscale,Collaborative Innovation Center of Chemistry for Energy Materials(iChEM),School of Chemistry and Materials Science,and National Synchrotron Radiation Laboratory,University of Science and Technology of China,Hefei 230026,China
Key words:Photocatalytic CO2reduction,Schottky junction,Energy transfer,TiO2,Nanoparticles
I.INTRODUCTION
The energy and environmental issues associated with the consumption of fossil fuel have in fluenced our daily life.Carbon dioxide(CO2)largely contributes to the greenhouse gas among various emitted products.To solve both the environmental and energy issues,the conversion of CO2to valuable fuels such as methane(CH4)and methanol through photocatalysis has attracted wide interests[1?6].According to the fundamental principle,three steps are mainly involved in a process of photocatalytic CO2reduction:(i)absorption of incident photons by semiconductor to generate photoexcited electrons and holes,(ii)separation of photoexcited electrons and holes and their migration to the surface of photocatalyst,and(iii)CO2reduction by the electrons and oxidation reaction by the holes.For this reason,light absorption should be first engineered by selecting and modifying semiconductor in efforts to achieve high activity in photocatalytic CO2reduction.
Since the first report on photocatalytic CO2reduction in 1979[7],a variety of semiconductors have been investigated towards this application[5,8?11].Among various semiconductors,TiO2is a very promising candidate for photocatalytic reactions owing to its high stability and photocatalytic performance.However,the bandgap of TiO2at 3.2 eV makes it only absorb ultraviolet(UV)light,which accounts for 5%photons in the solar spectrum.In order to absorb visible light by TiO2,great efforts have been made to modify TiO2[12?15].In 2001,Asahi and co-workers reported that N-doped TiO2exhibits photocatalytic activity under visible-light illumination[12].The hybridization of N 2p orbitals in doped TiO2could narrow the bandgap,resulting in visible-light absorption.Since then,the N-doped TiO2has been widely investigated for visiblelight photocatalysis[16?18].
To further improve photocatalytic performance,the Schottky junction between metal and semiconductor has also been investigated to improve charge separation and transfer[19,20].By combining metal nanoparticles with semiconductor,photoexcited electrons can be transferred and trapped on the metal with suitable work function,which spatially separates the electrons from holes.In addition,the surface plasmon of noble metal nanoparticles(e.g.,Ag nanoparticles)may promote the creation and/or separation of electron-hole pairs through two different mechanisms[21?24]:(i)local electromagnetic field enhancement,and(ii)resonant energy transfer(RET)when the light absorption of semiconductor and the plasmonic band of metal nanoparticles sufficiently overlap.
Thus it should be a promising approach to the enhancement of photocatalytic CO2reduction by integrating N-doped TiO2with plasmonic metal nanoparticles.Upon the accumulation of sufficient photoexcited electrons on surface,the overall photocatalytic performance is still limited by active sites.It has been reported that the integration of cocatalysts(e.g.,PdCu[5],AuCu[25],and PtCu[26])with TiO2can provide active sites to enhance the photocatalytic conversion of CO2to valuable hydrocarbons.
In this article,we report a facile nanofabrication approach to combining dense Ag-Cu nanoparticles with N-doped TiO2nanowire arrays without the need of using surfactants.As compared with wet-chemical methods,this approach does not involve surfactants so as to make an intimate contact between metal and semiconductor,which would dramatically enhance the efficiency of electron transfer during photocatalytic CO2conversion.In this model,Ag nanoparticles offer a plasmonic band which sufficiently overlaps with the light absorption of N-doped TiO2,enabling RET to improve carrier creation and separation.Meanwhile,Cu nanoparticles provide active sites for CO2conversion[28?30].We specifically choose TiO2nanowires that have been widely investigated for solar energy harvesting and conversion process[16,31,32]as our material model.As compared with the disorderly dispersed nanowires,TiO2nanowire arrays exhibit superb performance in light trapping owing to their high aspect ratios[33,34].When light is introduced into the vertical arrays,multiple scattering would occur within the arrays,which effectively increases optical length and thus enhances light absorption.This design thus perfectly offers the improvement on light absorption by doping and light trapping,charge separation by Schottky junction and RET,and surface reactions by active sites,which all can enhance the performance of photocatalytic CO2conversion under full-spectrum irradiation.This nanofabrication technique should also provide a flexible approach to designing various hybrid structures by altering evaporation metals.
II.EXPERIMENTS
A.Chemical
Tetrabutyl titanate,hydrochloric acid,ethanol,and acetone were purchased from Sinopharm Chemical Reagent Co.,Ltd.The water used in the experiments was deionized.All chemicals were used as received without further purification.
B.Synthesis of TiO2nanowire arrays and N-doped TiO2 nanowire arrays
The TiO2nanowire arrays were prepared by a hydrothermal method.In a typical synthesis,the mixture of 12-mL deionized water and 12-mL hydrochloric acid was stirred for 5 min.Subsequently,0.4-mL tetrabutyl titanate was added into the mixture.After stirring for 15 min,the mixture solution was transferred to a 50-mL Te flon-lined stainless steel autoclave.A piece of cleaned FTO glass was then placed at an angle against the wall of the Te flon-liner with the conducting side facing down.The hydrothermal synthesis was conducted at 150?C for 3 h.After the reaction,the autoclave was cooled to room temperature naturally.The rutile TiO2nanowire arrays were prepared by simply annealing the sample in air at 450?C for 2 h,with a heating rate of 5?C/min.
The N-doped TiO2nanowire arrays were prepared by annealing the sample in a tube-type furnace in ammonia at 450?C for 2 h,with a heating rate of 1?C/min.
C.Integration of AgCu nanoparticles with TiO2nanowire arrays or N-doped TiO2nanowire arrays
An ultrahigh vacuum(UHV)electron-beam evaporation system(Shenyang Scientific Instruments,China,DZS-500)was used to deposit a layer of 5 nm Ag film and 5 nm Cu film(Ag5Cu5)on TiO2nanowire arrays or N-doped TiO2nanowire arrays.The evaporation rate was maintained at 0.03 nm/s under the pressure of about 1×10?4mbar.
D.Sample characterization
Scanning electron microscopy(SEM)images were taken on a FEI Sirion 200 field-emission scanning electron microscope operated at 5 kV.X-ray powder diffraction(XRD)patterns were recorded on a Philips X’Pert Pro Super diffractometer with Cu Kαradiation(λ=1.54178 ?).UV-Vis diffuse re flectance data were recorded in the spectral region of 300?800 nm with a Shimadzu SolidSpec-3700 spectrophotometer.X-ray photoelectron spectra(XPS)were collected on an ESCALab 250 X-ray photoelectron spectrometer,using nonmonochromatized Al-KαX-ray as the excitation source.
E.Photoelectrochemical measurements
The measurements were carried out on a CHI 660D electrochemical station(Shanghai Chenhua,China)in ambient condition under irradiation of a 300 W Xe lamp(Solaredge 700,China).The power density of full spectrum was set to be 100 mW/cm2,the ultraviolet(UV)light was measured to be 2.7 mW/cm2.Standard threeelectrode setup was used with the fabricated samples as photoelectrode,with a Pt foil as counter electrode,and the Ag/AgCl electrode as reference electrode.The three electrodes were inserted in a quartz cell filled with 0.5-mol/L Na2SO4electrolyte.The Na2SO4electrolyte was purged with Ar for 30 min prior to the measurements.The photocurrent responses of the prepared photoelectrodes(i.e.,I-V)were operated by measuring the photocurrent densities under chopped light irradiation with the light on/o ffcycles for each 10 s.
F.Photocatalytic CO2reduction
In a typical experiment,3-cm2photocatalysts including 0.03-mg Au and Cu were immersed into 30-mL deionized water with 5-mL triethanolamine as a sacrificial agent in a home-made quartz bottle,followed by saturation with high-purity CO2for 30 min.Subsequently,light irradiation was performed using a 300-W Xe lamp with full-spectrum light,UV light or visible light as the illumination source,respectively.The light source and power intensity were consistent for all the photocurrent measurements.The photocatalytic reaction was typically performed for 4 h.The amount of CH4,CO,and H2evolved was measured by gas chromatography(GC,7890A,Ar carrier,Agilent).H2was detected using a thermal conductivity detector(TCD),and CH4was measured by a flame ionization detector(FID).CO was converted to CH4by a methanation reactor,and then analyzed with FID.Three replicates were collected for each sample with relative error<10%.
III.RESULTS AND DISCUSSION
A.Sample characterization
Ag-Cu nanoparticles can make intimate contact with TiO2nanowire arrays during electron beam evaporation,which establishes the Schottky junction between the components.SEM images with different magnifications(FIG.1(a)and(b))show that the Ag-Cu nanoparticles have an average size of 20 nm and tightly contact the TiO2nanowire arrays.These nanoparticles are constructed as an immiscible Ag-Cu binary phase diagram instead of AgCu alloy[35].X-ray photoelectron spectroscopy(XPS,FIG.1(c))reveals the existence of N,Ag,and Cu elements in the sample,indicating the successful N doping.Moreover,X-ray diffraction(XRD,FIG.1(d))shows that all the peaks can be assigned to rutile TiO2(JCPDS No.21-1276),face-centered cubic(fcc)Ag,(JCPDS No.65-2871)and Cu(JCPDS No.04-0836).This verifies the formation of immiscible Ag-Cu binary phase(namely,Ag5Cu5).We thus name the sample with Ag-Cu nanoparticles supported on N-doped TiO2nanowire arrays as“Ag5Cu5/N-TiO2”.
In the sample preparation process,N element is doped into the lattice of rutile TiO2by annealing the TiO2nanowire arrays in NH3atmosphere.As shown in FIG.2,such N doping(N-TiO2)results in an extension of light absorption to the visible spectrum.By doping N into the TiO2,the light absorption range can be extended to 500 nm.This extended light absorption can thus sufficiently overlap with the plasmonic band of Ag5Cu5nanoparticles.The plasmonic band can be well resolved in the absorption spectrum of Ag5Cu5nanoparticles supported on the undoped TiO2nanowire arrays(Ag5Cu5/TiO2).As compared with bare TiO2,

FIG.1 SEM images of Ag5Cu5nanoparticles combined with N-doped TiO2nanowire arrays(Ag5Cu5/N-TiO2)at(a)low and(b)high magnification.(c)XPS spectra of Ag5Cu5/NTiO2.(d)XRD pattern of Ag5Cu5/N-TiO2.
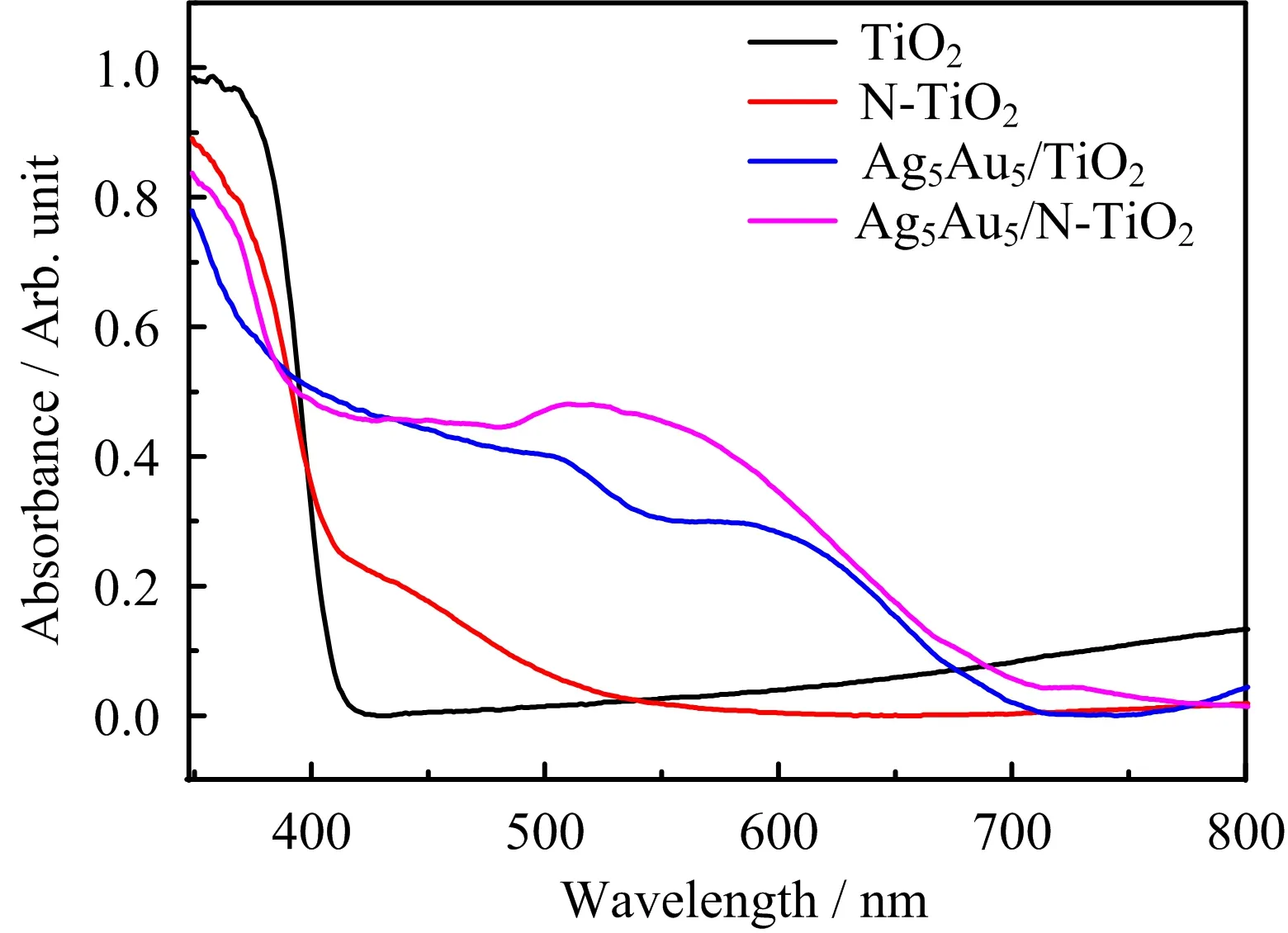
FIG.2 UV-Vis diffuse re flectance spectra of TiO2nanowire arrays,N-doped TiO2nanowire arrays,Ag5Cu5/TiO2 nanowire arrays and Ag5Cu5/N-doped TiO2nanowire arrays.
the light absorption in the visible spectrum should result from the plasmonic effect of Ag5Cu5nanoparticles.Thus we anticipate that the N-doped TiO2and Ag5Cu5nanoparticles can offer an overlapping light absorption in the sample of Ag5Cu5/N-TiO2.Since the RET process requires sufficient overlap between semiconductor and metal nanoparticles,such a match in the spectral range can help enhance carrier creation/separation and thus photocatalytic performance.The apparent enhancement around 400?550 nm for the Ag5Cu5/N-doped TiO2sample results from the match between the Ag5Cu5and N-doped TiO2.
B.Photoelectrochemical(PEC)performance

FIG.3 Photocurrent-potential curve of(a)TiO2nanowire arrays and N-doped TiO2nanowire arrays under visible-light irradiation,(b)N-doped TiO2nanowire arrays and Ag5Cu5/N-doped TiO2nanowire arrays under UV-light irradiation,(c)N-doped TiO2nanowire arrays and Ag5Cu5/N-doped TiO2nanowire arrays under visible-light irradiation,and(d)N-doped TiO2nanowire arrays and Ag5Cu5/N-doped TiO2nanowire arrays under full-spectrum light irradiation.
Upon the completion of sample synthesis and fabrication,we investigate the PEC performance of our samples with the illumination source of UV light,visible light,and full-spectrum light,respectively.As shown in FIG.3(a),TiO2can barely generate photocurrents under visible-light illumination.This situation can be improved by doping TiO2with nitrogen.The doping of N atoms leads to the orbital hybridization of N 2p and TiO2valence band,and thus narrows the bandgap of TiO2.The resulted visible light absorption gives a relatively apparent photocurrent response under visiblelight illumination.
We further evaluate the effects of Ag5Cu5nanoparticles on the photocurrent response of N-doped TiO2nanowire arrays.Ag5Cu5nanoparticles potentially can play dual roles in photocurrent enhancement?Schottky junction and plasmonic RET.To appreciate the promotion by Schottky junction,we collect photocurrents under UV-light illumination.As shown in FIG.3(b),the photocurrents of N-doped TiO2nanowire arrays can be enhanced 5 times by the addition of Ag5Cu5nanoparticles.Given that plasmonic effect is excluded under UV irradiation,this enhancement should result from the function of Schottky junction.The Schottky junction traps electrons on Ag5Cu5nanoparticles,and thus reduces the recombination of the electron-hole pairs in the N-doped TiO2.
Furthermore,theRET processisassessedunder visible-light illumination,in which the plasmonic property of Ag5Cu5nanoparticles can be activated.As shown in FIG.3(c),the Ag5Cu5/N-doped TiO2nanowire arrays give a 3.8 times stronger photocurrent response than the N-doped TiO2.This enhancement results from the surface plasmon of metal nanoparticles whose band matches with the light absorption of N-doped TiO2.The RET process enhances the carrier creation and separation,which can boost the PEC performance under visible-light illumination.Taken together,the integration of N-doped TiO2with Ag5Cu5nanoparticles can significantly enhance the full-spectrum performance as shown in FIG.3(d).As compared with the N-doped TiO2,the photocurrent of Ag5Cu5/N-doped TiO2is enhanced about 6.4 times,owing to the synergetic effect of Schottky junction and RET process.
C.Photocatalytic CO2reduction

FIG.4 The average rates of photocatalytic(a)CH4,(b)CO,and(c)H2production for TiO2nanowire arrays,N-doped TiO2 nanowire arrays,and Ag5Cu5/N-doped TiO2nanowire arrays under UV-light,visible-light,and full-spectrum irradiation,respectively.(d)Schematic illustrating the major process in the photocatalytic reactions.Performance of Ag5Cu5/N-doped TiO2nanowire arrays for(e)CH4and CO evolution and(f)H2evolution in 3 successive 4 h cycles under full-spectrum irradiation.
Upon acquiring the giant enhancement on photocurrent response,we are in the position to evaluate the performance of our samples in the photocatalytic CO2reduction with triethanolamine as a sacrificial agent in H2O.FIG.4(a?c)shows the average rates of photocatalytic CH4,CO,and H2production by TiO2nanowire arrays,N-doped TiO2nanowire arrays and Ag5Cu5-supported N-doped TiO2nanowire arrays under different light illumination,respectively. The N-doped TiO2nanowire arrays integrated with Ag5Cu5nanoparticles exhibit significantly higher photocatalytic activity than bare TiO2and N-doped TiO2in the production of total products,demonstrating the importance of synergetic Schottky junction and RET effects to photocatalysis.Specifically,the production rate of CH4by the Ag5Cu5/N-doped TiO2nanowire arrays is 720 μmol·g?1·h?1,which is about 6 times that of the TiO2nanowire arrays(FIG.4(a)).The production rates in full spectrum are higher than the sum of those under UV and visible illumination for the Ag5Cu5/N-doped TiO2nanowire arrays,indicating the synergetic effects.
Based on the experiment results,we can summarize the major processes in the photocatalytic CO2reactions as shown in FIG.4(d).The UV light and partial visible light can photoexcite electrons and holes in N-doped TiO2.The electrons are then separated from holes and become trapped by the metal nanoparticles through the Schottky junction.Moreover,the visible light can induce the surface plasmon of Ag5Cu5nanoparticles whose band has a spectral overlap with the light absorption of N-doped TiO2,which enhances the creation and separation of electron-hole pairs through the RET process.Under the full-spectrum illumination,the Schottky junction can be synergized with the RET process,thereby further enhancing the photocatalytic performance in CO2reduction.We have performed the photocatalytic reaction for 3 cycles using the same Ag5Cu5/N-doped TiO2sample,each of which lasts 4 h under full-spectrum irradiation.As shown in FIG.4(e)and(f),the sample shows excellent performance stability in the recycling test.
IV.CONCLUSION
In conclusion,we have prepared the N-doped TiO2nanowire array through a hydrothermal method,followed by the deposition of Ag5Cu5nanoparticles through electron beam evaporation.The doped N can expand the absorption range of TiO2to visible light,matching the surface plasmonic resonance of Ag5Cu5nanoparticles.The Schottky junction between semiconductor and metal can be synergized with the RET process in this work,enhancing the photocatalytic performance in CO2conversion.Another contribution from Ag5Cu5nanoparticles is the role of Cu as active sites for CO2reduction.This work provides a method for largescale photocatalyst fabrication towards future practical applications.
V.ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China(2017YFA0207301),National Natural Science Foundation of China(No.21725102,No.21471141, No.21601173), CAS Key Research Program of Frontier Sciences (QYZDB-SSWSLH018),CAS InterdisciplinaryInnovation Team,Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology(No.2016FXCX003),Anhui Provincial Natural ScienceFoundation (No.1608085QB24),and Chinese Universities Scientific Fund(WK2310000067).
[1]S.Ma,M.Sadakiyo,M.Heima,R.Luo,R.T.Haasch,J.I.Gold,M.Yamauchi,and P.J.A.Kenis,J.Am.Chem.Soc.139,47(2017).
[2]H.Zhang,J.Wei,J.Dong,G.Liu,L.Shi,P.An,G.Zhao,J.kong,X.Wang,X.Meng,J.Zhang,and J.Ye,Angew.Chem.Int.Ed.55,14310(2016).
[3]W.Tu,Y.Zhou,and Z.Zou,Adv.Mater.26,4607(2014).
[4]J.L.White,M.F.Baruch,J.E.Pander,Y.Hu,I.C.Fortmeyer,J.E.Park,T.Zhang,K.Liao,J.Gu,Y.Yan,T.W.Shaw,E.Abelev,and A.B.Bocarsly,Chem.Rev.115,12888(2015).
[5]R.Long,Y.Li,Y.Liu,S.Chen,X.Zheng,C.Gao,C.He,N.Chen,Z.Qi,L.Song,J.Jiang,J.Zhu,and Y.J.Xiong,J.Am.Chem.Soc.139,4486(2017).
[6]Y.Q.Feng,H.Y.Cheng,J.Han,X.Z.Zheng,Y.Y.Liu,Y.Yang,and L.W.Zhang,Chin.Chem.Lett.28,2254(2017).
[7]T.Inoue,A.Fujishima,S.Konishi,and K.Honda,Nature 277,637(1979).
[8]L.Tan,W.Ong,S.Chai,and A.R.Mohamed,Chem.Eng.J.308,248(2017).
[9]Y.Wang,N.Huang,J.Shen,P.Liao,X.Chen,and J.Zhang,J.Am.Chem.Soc.140,38(2018).
[10]M.F.Kuehnel,K.L.Orchard,K.E.Dalle,and E.Reisner,J.Am.Chem.Soc.139,7217(2017).
[11]G.Liu,X.Meng,H.Zhang,G.Zhao,H.Pang,T.Wang,P.Li,T.Kako,and J.Ye,Angew.Chem.Int.Ed.56,5570(2017).
[12]R.Asahi,T.Morikawa,T.Ohwaki,K.Aoki,and Y.Taga,Science 293,269(2001).
[13]I.Justicia,P.Ordejon,G.Canto,J.L.Mozos,J.Fraxedas,Battiston,G.A.Battiston,R.Gerbasi,and A.Figueras,Adv.Mater.19,1399(2002).
[14]X.Liu,G.Zhu,X.Wang,X.Yuan,T.Lin,and F.Huang,Adv.Energy Mater.6,1600452(2016).
[15]Q.Wu,F.Huang,M.Zhao,J.Xu,J.Zhou,and Y.Wang,Nano Energy 24,63(2016).
[16]G.Wang,X.G.Xiao,W.Li,Z.Lin,Z.Zhao,C.Chen,C.Wang,Y.Li,X.Huang,L.Miao,C.Jiang,Y.Huang,and X.Duan,Nano Lett.15,4692(2015).
[17]J.H.Pan,G.Han,R.X.Zhou and S.Zhao,Chem.Commun.47,6942(2011).
[18]G.Liu,L.C.Yin,J.Wang,P.Niu,C.Zhen,Y.Xie,and H.M.Cheng,Energy Environ.Sci.5,9603(2012).
[19]A.L.Linsebigler,G.Lu,and J.T.Yates Jr.,Chem.Rev.95,735(1995).
[20]W.J.Wang,Y.Wang,Q.Xu,H.X.Ju,T.Wang,Z.J.Tao,S.W.Hu,and J.F.Zhu,Chin.Chem.Lett.28,1760(2017).
[21]S.Linic,P.Christopher,and D.B.Ingram,Nat.Mater.10,911(2011).
[22]X.Wang,C.Liow,D.Qi,B.Zhu,W.R.Leow,H.Wang,C.Xue,X.Chen,and S.Li,Adv.Mater.26,3506(2014).
[23]L.Weng,H.Zhang,A.O.Govorov,and M.Ouyang,Nat.Commun.5,4792(2014).
[24]J.Li,S.K.Cushing,P.Zheng,T.Senty,F.Meng,A.D.Bristow,A.Manivannan,and N.Wu,J.Am.Chem.Soc.136,8438(2014).
[25]S.Neatu,J.Macia-Agullo,P.Concepcion,and H.Garcia,J.Am.Chem.Soc.136,15969(2014).
[26]X.Zhang,F.Han,B.Shi,S.Farsinezhad,G.P.Dechaine,and K.Shankar,Angew.Chem.Int.Ed.51,12732(2012).
[27]M.Aresta,A.Dibenedetto,and A.Angelini,Chem.Rev.114,1709(2014)
[28]S.Posada-Perez,P.J.Ramirez,J.Evans,F.Vines,P.Liu,F.Illas,and J.A.Rodriguez,J.Am.Chem.Soc.138,8269(2016).
[29]Z.Cao,D.Kim,D.Hong,Y.Yu,J.Xu,S.Lin,X.Wen,E.M.Nichols,K.Jeong,J.A.Reimer,P.Yang,and C.J.Chang,J.Am.Chem.Soc.138,8120(2016).
[30]K.P.Kuhl,E.R.Cave,D.N.Abram,and T.F.Jaramillo,Energy Environ.Sci.5,7050(2012).
[31]G.Ai,H.Li,S.Liu,R.Mo,and J.Zhong,Adv.Funct.Mater.25,5706(2015).
[32]X.Sheng,D.He,J.Yang,K.Zhu,and X.Feng,Nano Lett.14,1848(2014).
[33]J.Wang,D.N.Tafen,J.P.Lewis,Z.Hong,A.Manivannan,M.Zhi,M.Li,and N.Wu,J.Am.Chem.Soc.131,12290(2009).
[34]Z.Jiang,F.Yang,N.Luo,B.T.T.Chu,D.Sun,H.Shi,T.Xiao,and P.P.Edwards,Chem.Commun.47,6372(2008).
[35]M.Jabbareh and F.Monji,Calphad 60,208(2018).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Location Effect in a Photocatalytic Hybrid System of Metal-Organic Framework Interfaced with Semiconductor Nanoparticles
- Experimental and Theoretical Study on Dissociative Photoionization of Cyclopentanone
- Electrochemical Study on Hydrogen Evolution and CO2Reduction on Pt Electrode in Acid Solutions with Different pH
- Effect of a Single Repeat Sequence of the Human Telomere d(TTAGGG)on Structure of Single-Stranded Telomeric DNA d[AGGG(TTAGGG)6]
- Single Pt Atoms Supported on Oxidized Graphene as a Promising Catalyst for Hydrolysis of Ammonia Borane
- Enhanced Oxygen Reduction on Graphene via Y5Si3Electride Substrate:a First-Principles Study
