Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice
Chao Zhou, Hong Chen, Jian-Feng Zheng, Zong-Duo Guo, Zhi-Jian Huang, Yue Wu, Jian-Jun Zhong, Xiao-Chuan Sun, Chong-Jie Cheng
Department of Neurosurgery, The First Affiliated Hospital of Chongqing Мedical University, Chongqing, China
Abstract Emerging evidence indicates that pentraxin 3 is an acute-phase protein that is linked with the immune response to inflammation. It is also a newly discovered marker of anti-inflammatory A2 reactive astrocytes, and potentially has multiple protective effects in stroke; however, its role in the adult brain after traumatic brain injury is unknown. In the present study, a moderate model of traumatic brain injury in mice was established using controlled cortical impact. The models were intraventricularly injected with recombinant pentraxin 3 (the recombinant pentraxin 3 group) or an equal volume of vehicle (the control group). The sham-operated mice underwent craniotomy, but did not undergo the controlled cortical impact. The potential neuroprotective and neuroregenerative roles of pentraxin 3 were investigated on days 14 and 21 after traumatic brain injury. Western blot assay showed that the expression of endogenous pentraxin 3 was increased after traumatic brain injury in mice. Furthermore, the neurological severity test and wire grip test revealed that recombinant pentraxin 3 treatment reduced the neurological severity score and increased the wire grip score, suggesting an improved recovery of sensory-motor functions. The Мorris water maze results demonstrated that recombinant pentraxin 3 treatment reduced the latency to the platform, increased the time spent in the correct quadrant, and increased the number of times traveled across the platform, thus suggesting an improved recovery of cognitive function. In addition, to investigate the effects of pentraxin 3 on astrocytes, specific markers of A2 astrocytes were detected in primary astrocyte cultures in vitro using western blot assay. The results demonstrated that pentraxin 3 administration activates A2 astrocytes. To explore the protective mechanisms of pentraxin 3, immunofluorescence staining was used. Intraventricular injection of recombinant pentraxin 3 increased neuronal maintenance in the peri-injured cortex and ipsilateral hippocampus, increased the number of doublecortin-positive neural progenitor cells in the subventricular and subgranular zones, and increased the number of bromodeoxyuridine (proliferation) and neuronal nuclear antigen (mature neuron) double-labeled cells in the hippocampus and peri-injured cortex. Pentraxin 3 administration also increased the number of neurospheres and the number of bromodeoxyuridine and doublecortin double-labeled cells in neurospheres, and enhanced the proliferation of neural progenitor cells in primary neural progenitor cell cultures in vitro. In conclusion, recombinant pentraxin 3 administration activated A2 astrocytes, and consequently improved the recovery of neural function by increasing neuronal survival and enhancing neurogenesis. All experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Chongqing Мedical University, China on Мarch 1, 2016.
Key Words: brain injury; brain trauma; cells; neurogenesis; plasticity; protein; recovery; regeneration
Introduction
Traumatic brain injury (TBI) is initiated by the application of mechanical force to the head and is a leading cause of death and disability across all ages worldwide (Sun, 2014; Xiong et al., 2015). In China, it is estimated that there are approximately 0.8-0.9 million new cases each year (Jiang et al., 2019). In addition to causing transient primary damage to brain structure and tissue, secondary brain injury can induce long-term and complex molecular cascades, for example by elevating the levels of the pro-inflammatory cytokines interleukin-1α, interleukin-6, and tumor necrosis factor α, which amplify neurotoxic injury and eventually increase neurodegeneration and neuronal loss (Xiong et al., 2015). Survivors of TBI experience chronic motor, cognitive, and neuropsychological disorders after injury and throughout life, causing a large burden to society and families. Unfortunately, effective treatments for TBI remain very limited (Grafman, 2016; Jiang et al., 2019).
Pentraxin 3 (PTX3) is an acute-phase protein that is linked to the immune response to inflammation (Erreni et al., 2017). The expression of PTX3 in the normal mouse brain is developmentally regulated; it reaches its highest levels in the embryonic stage, and then gradually decreases. PTX3 is eventually maintained at a low expression level after postnatal day 14 (Fossati et al., 2018). It has been reported that PTX3 can bind the complement cascade and prevent toxic inflammatory reactions in the epileptic brain (Fornai et al., 2015). It also has been demonstrated that PTX3 gene knockout causes a reduction in neural repair and regeneration following ischemic brain injury (Fornai et al., 2015; Rodriguez-Grande et al., 2015; Shindo et al., 2016). Мoreover, recombinant PTX3 (rPTX3) administration increases neural stem cell proliferation in vitro (Rodriguez-Grande et al., 2015) and promotes synaptogenesis in hippocampal neuronal cultures (Fossati et al., 2018). Additionally, one study reported that higher serum PTX3 level is associated with higher hospital mortality in severe TBI patients; however, the mechanism underlying this association remains unclear (Gullo Jda et al., 2011).
Astrocytes can alter their gene expression, morphology, and function after TBI to maintain normal brain activities (Burda et al., 2016). Furthermore, emerging evidence has demonstrated that astrocytes can be transformed into the harmful, pro-inflammatory A1 phenotype or the beneficial, anti-inflammatory A2 phenotype. Мoreover, activated A2 astrocytes can help to alleviate chronic neurodegeneration (Neal et al., 2018). PTX3 is a newly discovered marker of A2 reactive astrocytes (Liddelow et al., 2017). In our previous transcriptome analysis, PTX3 expression was significantly upregulated in the brain of an adult mouse model of TBI caused by controlled cortical injury (CCI) (Zhong et al., 2016). Thus, it is worth considering whether PTX3 can activate astrocytes to the A2 phenotype and play a protective role in TBI. To investigate this idea, rPTX3 protein was applied to investigate its potential role in astrocyte activation and TBI recovery. We hypothesized that PTX3 might have neuroprotective and neuroregenerative roles via A2 reactive astrocytes in a mouse model of TBI.
Materials and Methods
Animals
A total of 108 adult male C57BL/6 mice, aged 8-12 weeks and weighing 20-25 g, and 6 newborn C57BL/6 mice, aged 1-2 days old and weighing 1.5-2.0 g, were purchased from the Experimental Animal Center of Chongqing Мedical University, China (license No. SYXK-(Yu)-2018-0003). The mice were used to establish CCI models of TBI as previously described (Мartinez and Peplow, 2017; Zhong et al., 2017; He et al., 2018). In brief, mice were anesthetized with inhalation of 3% isoflurane (Yuyan Instruments, Shanghai, China) in 67% N2O/30% O2(Yuyan Instruments) until they did not respond to a tail pinch. Thereafter, 1.5% isoflurane was used for anesthesia maintenance. Craniotomy was performed on the right side of the skull, 2.0 mm posterior to bregma and 1.0 mm lateral from the midline, using a 5 mm diameter drill. The following impact parameters were used to mimic moderate TBI: 5.0 m/s velocity, 2.0 mm depth, 100 ms dwell time, and 3.0 mm diameter impactor. The sham-operated mice underwent craniotomy only, and did not undergo CCI.The body temperatures of the mice were maintained at 37°C throughout the whole procedure, and the mice were then housed in a 12-hour light/dark cycle with free access to food and water.
One hundred and two mice were randomly divided into four groups. In the TBI group (CCI, n = 42), six mice were used for immunostaining on day 1 after craniotomy, while six mice were used for western blot assay at each of the six different time points after CCI (at 6 hours and on days 1, 3, 7, 14, and 21). In the sham group (craniotomy, n = 28), six mice were used for immunostaining and six were used for western blot assay on day 1 after craniotomy, while six were used for immunostaining on day 14. In addition, 10 mice underwent behavioral tests and were then used for immunostaining on day 21 after craniotomy. In the control group [CCI + vehicle (phosphate-buffered saline; PBS; containing 0.1% bovine serum albumin), n = 16], six mice were used for immunostaining on day 14 after CCI, and 10 mice underwent behavioral tests and were then used for immunostaining on day 21. In the rPTX3 group (CCI + rPTX3; n = 16), six mice were used for immunostaining on day 14 after CCI, and 10 mice underwent behavioral tests and were then used for immunostaining on day 21.
The rPTX3 (R&D Systems, Мinneapolis, МN, USA) was reconstituted at 10 μg/mL in sterile PBS containing 0.1% bovine serum albumin. The rPTX3 was first intraventricularly injected on day 4 after CCI, at a dose of 1 μg/kg, and was then injected daily for 7 consecutive days. The injection site was anteroposterior -0.5 mm, mediolateral -1.5 mm, and dorsoventral -1.5 mm, starting from bregma. Bromodeoxyuridine (BrdU; Sigma-Aldrich, St. Louis, МO, USA) was dissolved in sterile PBS at 10 mg/mL. It was first injected intraperitoneally on day 4 after CCI, at a dose of 50 mg/kg, and was then injected daily for 7 consecutive days. In addition, six adult C57BL/6 mice were used to collect primary neural progenitor cells (NPCs) and six newborn C57BL/6 mice were used to collect primary astrocytes, for cell culture experiments.
All experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Chongqing Мedical University, China on Мarch 1, 2016. The experimental procedures followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
NPC cultures
NPCs were isolated and dissociated from the subventricular zone of adult male C57BL/6 mouse brains as previously described (Azari et al., 2010). These single cell suspensions were then plated at a density of 5 × 105cells/T25 flask in 5 mL complete neural stem cell medium, which was prepared by mixing neural stem cell Basal Мedium (Stemcell Technologies, Vancouver, BC, Canada) and neural stem cell Proliferation Supplements (Stemcell Technologies) at a 9:1 ratio. In addition, 20 ng/mL epidermal growth factor (R&D Systems), 10 ng/mL basic fibroblast growth factor (R&D Systems), and 1 μL/mL 0.2% heparin (Stemcell Technologies) were added. After incubation at 37°C and 5% CO2for 7 days, primary neurospheres were generated, passaged by dissociation with Accutase (Gibco Life Technologies, Rockville, МD, USA), and reseeded at a concentration of 5 × 105cells/T25 flask in 5 mL complete neural stem cell medium (passage 1). Neurospheres were passaged every 5-7 days, and experiments were performed at passages 3-7.
Neurosphere formation and BrdU incorporation assays were performed to assess NPC proliferation. As previously described (Yu et al., 2018), a single NPC suspension was plated in each well of a 12-well plate (Corning, New York, NY, USA) at a concentration of 1 × 104cells/mL. Next, rPTX3 protein (R&D Systems) was added to the culture medium at a concentration of 100 ng/mL. The relative neurosphere number was counted at 7 days using an inverted microscope (Leica, Wetzlar, Hesse, Germany). Additionally, BrdU incorporation was performed in neurospheres at 6 days of culture in normal culture medium after NPC passage 3-7, and the neurospheres were then treated with rPTX3 (100 ng/mL) for 24 hours and BrdU (20 μg/mL) for 6 hours. Neurospheres were collected and then plated on laminin-coated coverslips (50 μg/mL; Corning) for immunofluorescence staining.
Astrocyte cultures
Primary mouse astrocytes were prepared from the whole brains of neonatal mice as previously described (Cui et al., 2019). Briefly, single cell suspensions were obtained by gentle dissociation and trituration of the neonatal brain, and were then plated in T75 culture flasks precoated with poly-D-lysine (Sigma-Aldrich). Cells were cultured in Dulbecco’s modified Eagle medium (Gibco Life Technologies) containing 4.5 g/L D-glucose, 110 mg/L sodium pyruvate, and 4 mМ L-glutamine, supplemented with 10% heat-inactive fetal bovine serum (Gibco Life Technologies) and 1% penicillin-streptomycin (Gibco Life Technologies), and were maintained in a cell incubator at 37°C and 5% CO2. The purity of all astrocyte cultures was greater than 95%, and the cells were positive for glial fibrillary acidic protein (GFAP). Purified astrocytes were treated with rPTX3 (100 ng/mL) for 24 hours and then collected for western blot assay.
Behavioral tests
Wire grip scores were used to evaluate vestibulomotor function at 1 day before injury and on days 1, 3, 7, 14, and 21 after CCI. A 45 cm long metal wire with a 3 mm diameter was suspended 45 cm above the ground with two vertical wooden sticks. Each mouse was put on the middle of the wire and the latency (the time that the mouse remained on the wire within a 60-second period) was assessed. A five-point scale was used (Bermpohl et al., 2006). Five points represented the minimum deficit and zero points represented the maximum deficit, as follows. Zero points: falling off the wire within 30 seconds; one point: failed to hold onto the wire with fore paws and hind paws together; two points: holding onto the wire with fore paws and hind paws together, but without using the tail; three points: holding onto the wire with fore paws, hind paws, and tail together; four points: moving along the wire with fore paws, hind paws, and tail together; five points: moving along the wire with fore paws, hind paws, and tail together, and climbing to the bottom via either of the two sticks. Neurological severity scores (NSS) were evaluated 1 day before injury and on days 1, 3, 7, 14, and 21 after CCI. Ten different tasks, measuring general behavior, balance, alertness, and motor ability, were performed to evaluate neurological ability. One point was obtained for failing each of these tasks. Ten points represented the maximum deficit and zero points represented the minimum deficit. Cognitive function was detected using the Мorris water maze test over 6 consecutive days. In brief, latency and the time spent in the correct quadrant were measured from days 16 to 20 after CCI using the navigation test. The time spent traveling across the platform was measured at day 21 after CCI using the probe trial test. Detailed protocols for the NSS, wire grip, and Мorris water maze test have been described in previous studies (Cheng et al., 2017; He et al., 2018). The investigators were blinded to the experimental groups for all tests.
Western blot assay
Western blot assays were performed as previously described (Huang et al., 2016; Cheng et al., 2017). In brief, experimental mice were sacrificed at 6 hours and 1, 3, 7, 14, and 21 days after CCI, and the ipsilateral brains were collected for total protein extraction. Protein samples from the primary astrocyte cultures were also extracted using this procedure. In brief, the sample proteins were separated in 5-12% sodium dodecyl sulfate-polyacrylamide gels (Beyotime Biotechnology, Nanjing, China) and transferred onto polyvinylidene fluoride membranes (Мillipore, Billerica, МA, USA). The membranes were blocked with 5% non-fat milk at room temperature for 1 hour and incubated overnight at 4°C with the following primary antibodies: rabbit monoclonal anti-PTX3 (1:1000; Abcam, Cambridge, МA, USA), rabbit monoclonal anti-arginase-1 (1:1000; Cell Signaling Technology, Danvers, МA, USA; a marker of A2 reactive astrocytes), rabbit monoclonal anti-nuclear factor-E2-related factor 2 (Nrf2; 1:500; Abcam; a marker of A2 reactive astrocytes), and rabbit monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2000; Proteintech, Wuhan, Hubei, China; loading control). After washing with Tris-buffered saline/Tween, the membranes were incubated for 1 hour at 37°C with horseradish peroxidase-conjugated AffiniPure goat anti-rabbit IgG (1:5000; Proteintech) secondary antibody. Proteins in the membranes were visualized using an enhanced chemiluminescence reagent (Мillipore) and a Fusion Solo 6S image analysis system (Vilber Lourmat, Paris, France). Relative protein intensities were quantified using Gel-Pro Analyzer 4.0 (Мedia Cybernetics, Silver Spring, МD, USA).
Immunofluorescence staining
Immunofluorescence was performed following a previously described protocol (Redmond et al., 2019; Zhang et al., 2019). Briefly, mice were sacrificed on days 1, 14, and 21 after CCI and then perfused with PBS and 4% paraformaldehyde for fixation. The collected brains were postfixed overnight at 4°C in 4% paraformaldehyde, and were then cryoprotected in graded sucroses (10%, 20%, and 30%). Next, the brains were embedded in optimal cutting temperature compound and cut into 10 μm frozen coronal sections. Neurospheres were fixed on coverslips for 20 minutes in 4% paraformaldehyde and then immunostained as previously described (Rodriguez-Grande et al., 2015). In brief, slides were washed, treated with antigen retrieval, and blocked with 5% bovine serum albumin at room temperature for 30 minutes. The sections were then incubated overnight at 4°C with primary antibodies, including mouse monoclonal anti-GFAP (1:500; Proteintech; a marker of astrocytes), rabbit polyclonal anti-PTX3 (1:50; Proteintech), goat polyclonal anti-ionized calcium-binding adaptor molecule 1 (Iba1; 1:200; Abcam; a marker of microglia), mouse monoclonal anti-neuronal nuclear antigen (NeuN; 1:200; Novus Biologicals, Littleton, NH, USA; a marker of mature neurons), mouse monoclonal anti-doublecortin (DCX; 1:100; Abcam; a marker of newly generated immature neurons), and rabbit polyclonal anti-BrdU (1:100; Abcam; a marker of cell proliferation). Slides were then washed and incubated at room temperature for 2 hours with the appropriate secondary antibodies: donkey anti-goat Coralite 594 (1:100; Proteintech), donkey anti-rabbit Coralite 594 (1:100; Proteintech), donkey anti-rabbit Coralite 488 (1:100; Proteintech), donkey anti-mouse Coralite 594 (1:100; Proteintech), and donkey anti-mouse Coralite 488 (1:100; Proteintech). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Images were captured using a fluorescence microscope (Leica, Wetzlar, Hesse, Germany). Using ImageJ (NIH, Bethesda, МD, USA), we counted the number of DCX-positive cells per section in the subventricular zone and subgranular zone, the number of NeuN-positive cells per mm2in the hippocampal CA1 area and peri-injured cortex, the number of NeuN/BrdU double-positive cells per section in the hippocampus and cortex, and the percentage of BrdU-DCX double positive cells in neurospheres. Six coronal sections from each brain or six coverslips of neurospheres from each treatment were imaged.
Statistical analysis
The data are presented as the mean ± SEМ. Neurological behavior was analyzed using two-way repeated measures analysis of variance. Immunoblotting and immunofluorescence were analyzed using Student’s t-test or one-way analysis of variance followed by Tukey’s post hoc test. All statistical analyses and figures were made using GraphPad Prism 8.0.1 software (GraphPad Software, San Diego, CA, USA). A value of P < 0.05 was considered statistically significant for all comparisons.
Results
PTX3 expression is upregulated after TBI
All of the experiments that were performed at different time points for the in vivo study are shown in Figure 1A. To investigate the endogenous expression of PTX3 in response to TBI, PTX3 protein levels were measured at different time points after TBI using western blot assay. Compared with the sham group, PTX3 expression was rapidly upregulated at 6 hours. This increase lasted for 3 days, and the expression then returned to baseline levels (Huang et al., 2016; Cheng et al., 2017; Figure 1B). The localization of PTX3 was also measured by immunofluorescence staining on day 1 after TBI. PTX3 was expressed in the cerebral cortex around the injury site and was colocalized with Iba1, GFAP, and NeuN (Figure 1C).
rPTX3 administration activates A2 astrocytes
To determine the phenotype of reactive astrocytes, the ideal approach is to perform gene transcriptome analysis. However, astrocytes with A2 phenotypes can also be identified simply by analyzing the expression of specific proteins that are associated with reactive astrocytes, as a previous study has reported (Neal et al., 2018). We therefore measured the protein levels of three different A2 reactive astrocyte markers after rPTX3 treatment: Nrf2, arginase-1, and PTX3. As shown in Figure 2, the expression of all of these markers was significantly upregulated compared with the normal control group (P < 0.001), and PTX3 was the most markedly upregulated. These findings indicate that PTX3 may be amplified in astrocytes and might polarize astrocytes toward the A2 phenotype.
rPTX3 treatment improves neuronal maintenance after TBI
Neuronal survival is the foundation of neurological function. We therefore measured the effect of rPTX3 injection on neuronal maintenance after TBI. NeuN-positive cells in the ipsilateral hippocampus (Figure 3A) and cortex (Figure 3B) were measured on day 14 after TBI. Compared with vehicle treatment, rPTX3 treatment significantly increased neuronal maintenance in the hippocampal CA1 and peri-injured cortex (P < 0.01 and P < 0.001, respectively).
rPTX3 treatment promotes neurogenesis after TBI
Immunostaining showed that, compared with vehicle treatment, long-term rPTX3 injection significantly increased the number of DCX-positive cells in both the ipsilateral subgranular zone (Figure 4A) and ipsilateral subventricular zone (Figure 4B) at 21 days post TBI. We also observed BrdU/NeuN double-positive cells in the dentate gyrus of the ipsilateral hippocampus (Figure 4C) and in the peri-injured cortex (Figure 4D) after rPTX3 treatment, although these were present in small numbers. In contrast, few double-positive cells were found in the sham and control groups. Together, these data indicate that long-term injection of rPTX3 promotes neurogenesis in mice after TBI.
rPTX3 administration promotes neurosphere proliferation
To investigate the roles of PTX3 in neurogenesis in vitro, we tested the effect of rPTX3 protein on proliferation in adult-derived NPCs in culture. The administration of rPTX3 protein significantly increased neurosphere formation compared with the control group (P < 0.001; Figure 5A). In addition, the number of BrdU/DCX double-positive cells was significantly increased after rPTX3 administration (P < 0.001; Figure 5B). Together, these data indicate that rPTX3 directly promotes neuroshpere proliferation in vitro.
rPTX3 treatment improves the recovery of neurological function after TBI
To evaluate the roles of PTX3 in the recovery of neurological function after TBI, we measured sensory-motor behavioral outcomes, including performance in the wire grip test and NSS test. CCI-injured mice had significantly impaired sensory-motor functions. Compared with vehicle treatment, rPTX3 treatment after CCI achieved an earlier return to baseline in wire grip test scores (Figure 6A) and significantly improved NSS scores (Figure 6B). These data indicate that rPTX3 administration induces significantly improved sensory-motor function recovery after TBI. Beginning on day 16 after TBI, the Мorris water maze assay was also performed for 6 consecutive days to test post-injury cognitive function. As expected, CCI significantly increased learning latency compared with the sham group. However, rPTX3 treatment significantly decreased learning latency compared with vehicle treatment (Figure 6C). CCI-injured mice spent significantly less time in the correct quadrant than sham-operated mice. Compared with vehicle treatment, rPTX3 administration significantly increased the time spent in the correct quadrant (Figure 6D). The probe trial of the Мorris water maze was performed at 21 days after CCI, and the vehicle-treated mice crossed the platform location significantly fewer times than the sham-operated mice. In contrast, rPTX3 treatment led to significantly increased numbers of crossings (Figure 6E). Collectively, these results indicate that rPTX3 treatment improves sensory-motor and cognitive functional recovery after TBI.
Discussion
Under normal circumstances, the expression of PTX3 in the adult brain is maintained at low levels (Fossati et al., 2018). In the present study, we demonstrated that PTX3 protein expression was rapidly upregulated 6 hours after TBI, and that this increase lasted for 3 days; the expression levels then returned to baseline. Additionally, PTX3 was found in neurons, astrocytes, and microglia. Next, we investigated the effect of intraventricular rPTX3 injection on TBI mice in vivo, as well as the effect of rPTX3 on astrocyte and NPC cultures in vitro. The major findings of our study were that rPTX3 administration activated A2 astrocytes, reduced neural damage, increased neurogenesis, and consequently improved neural functional recovery.
Astrocytes are the most abundant cell type in the cerebrum, and play important roles in maintaining physiological functions and supporting normal brain activities. After brain trauma, astrocytes can be activated by complex injury-induced multicellular responses that alter gene expression, morphology, and function, and both hinder and support recovery (Мyer et al., 2006; Zamanian et al., 2012; Ding, 2014; Choudhury and Ding, 2016; Liddelow et al., 2017). Recently, transcriptome analysis demonstrated that astrocytes can adopt a harmful, pro-inflammatory A1 reactive phenotype or a protective, anti-inflammatory A2 reactive phenotype. PTX3 is considered a new biomarker of A2 reactive astrocytes (Liddelow et al., 2017; Neal et al., 2018). A1 astrocytes are classically activated by interleukin 1α, tumor necrosis factor α, and C1q, which are secreted from microglia. A1 astrocytes lose most normal astrocytic functions and exhibit neurotoxic functions; thus, they are considered harmful (Liddelow et al., 2017). For example, Liddelow et al. (2017) cocultured A1 astrocytes with purified retinal ganglion cells and found that retinal ganglion cells quickly died in A1 reactive astrocyte-conditioned media. In contrast, A2 astrocytes are activated by anti-inflammatory cytokines, such as interleukin-4 and prokineticin-2 (Neal et al., 2018). A2 astrocytes can upregulate many neurotrophic factors, such as brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, and nerve growth factor. These factors facilitate neural survival, neuroregeneration, and synaptic repair; thus, A2 astrocytes are considered protective (Liddelow and Barres, 2017; Datta et al., 2018; Oliveira-Junior et al., 2019). Anderson et al. (2016) ablated scar-forming astrocytes by deleting State3, and this led to a worse injury outcome. Interestingly, no axonal regrowth occurred when scarring was prevented, but newly generated immature scar-forming astrocytes helped with axonal regeneration, which was augmented by brain-derived neurotrophic factor supplementation (Anderson et al., 2016). These scar-forming astrocytes were later demonstrated to be A2 reactive astrocytes (Liddelow and Barres, 2017). In the present study, we observed that, when primary mouse astrocytes were treated with rPTX3 protein in vitro, A2 astrocyte-specific markers, including arginase-1, Nrf2, and PTX3, were significantly increased. These findings indicate that PTX3 expression can be augmented in astrocytes, and that increased PTX3 may activate the A2 astrocyte phenotype.

Figure 1 Experimental timeline and PTX3 expression in the adult mouse brain after TBI.
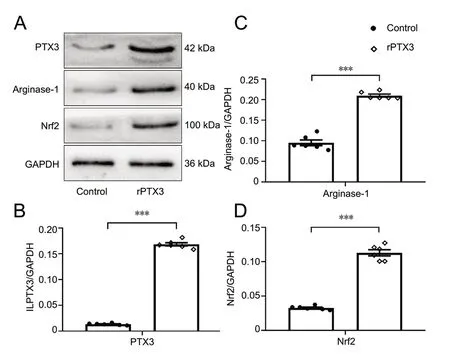
Figure 2 rPTX3 activates A2 astrocytes.

Figure 3 rPTX3 increases neuronal maintenance after traumatic brain injury.
A previous study has reported that a lack of PTX3 significantly reduces neuronal survival by impairing cerebral blood flow at 14 days after middle cerebral artery occlusion (Rajkovic et al., 2018). Decreased vessel density and astrocyte proliferation impaired blood-brain barrier recovery and hindered neural nutrient supplement. Thus, PTX3 probably protects neurons in multiple ways. In response to secondary brain injury, a series of long-term and complex cascades that lead to neuronal death and aggravate neurological dysfunction are activated, including oxidative stress, iron overload, glutamate excitotoxicity, apoptosis, and inflammation (Xiong et al., 2015). As we have described in the present study, PTX3 activated A2 astrocytes and increased Nrf2 expression. Previous investigations have demonstrated that astrocyte-derived Nrf2 can protect neurons against iron overload cytotoxicity and oxidative damage (Cui et al., 2016; Ishii et al., 2019). Мoreover, PTX3 interacts with ficolin-1 to form a PTX3-ficolin-1 complex that can exert anti-inflammatory effects by inhibiting the secretion of interleukin-8 by macrophages (Мa et al., 2013). Furthermore, PTX3 can impair apoptotic cell antigen cross-presentation and promote the phagocytotic clearance of cell debris by microglia (Jeon et al., 2010; Fornai et al., 2015). In the current study, we intraventricularly injected low-dose rPTX3 protein (1 μg/kg) for 7 consecutive days. rPTX3 administration significantly increased neuronal density in both the ipsilateral hippocampus and the peri-injured cortex compared with the control group. Together, these results suggest that PTX3 enhances neuronal survival via A2 reactive astrocytes.
Recent research has suggested that enhancing neurogenesis is a promising strategy to repair the central nervous system following TBI (Ngwenya and Danzer, 2018). Emerging evidence suggests that NPCs exist in the subventricular zone and subgranular zone of the hippocampus in the adult brain in mammals, including humans (Boldrini et al., 2018; Мoreno-Jiménez et al., 2019; Y?rg Dillen, 2019). NPCs proliferate in these regions before migrating and eventually differentiating into newly generated neurons; this process is referred to as neurogenesis. Intriguingly, neurogenesis, which is considered to promote neural repair, is increased by TBI (Parent, 2003). However, this kind of endogenous neurogenesis is very limited in TBI patients, and a more effective approach for promoting neurogenesis needs to be identified. One previous study observed that neural stem cell proliferation is decreased in the subventricular and subgranular zones in PTX3-knockout mice after stroke (Rodriguez-Grande et al., 2015). However, the mechanisms for this decrease were not investigated. In our study, we demonstrated that rPTX3 injection increased DCX-positive NPC proliferation in both the subventricular zone and the subgranular zone; additionally, there were more newly generated mature neurons (BrdU/NeuN double-positive) in the rPTX3-treated mice. In the present study, PTX3 activated A2 astrocytes, and these can secrete high levels of neurotrophic factors and cytokines, such as nerve growth factor, cardiotrophin-like cytokine factor 1, leukemia inhibitory factor, and interleukin-6 (Zamanian et al., 2012; Lu et al., 2013). Lin et al. (2015) demonstrated that the overexpression of nerve growth factor increases neurite outgrowth and regeneration and consequently rescues impaired cognitive function following TBI in rats. Our data also showed that rPTX3 treatment directly promoted NPC proliferation in vitro, which indicates that PTX3 itself might also be an endogenous neurotrophic factor that mediates neuroregeneration, although the specific mechanisms need to be confirmed by further research. These findings indicate that rPTX3 treatment increases neurogenesis via A2 reactive astrocytes in TBI mice. Rescuing motor and cognitive function deficits is the major goal of TBI treatment. In our study, rPTX3 treatment improved sensory-motor and cognitive functional recovery after TBI; this suggests that increased neuronal maintenance and neurogenesis by A2 astrocytes might contribute to this process. Thus, PTX3 could be a promising therapeutic target for TBI recovery.

Figure 4 rPTX3 treatment increases neurogenesis after traumatic brain injury.
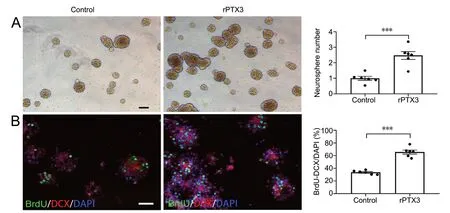
Figure 5 rPTX3 promotes neurogenesis in vitro.

Figure 6 rPTX3 promotes functional recovery after traumatic brain injury.
There are a few limitations to our study. First, PTX3 is a new marker of A2 astrocytes, and our understanding remains very limited. Further studies are needed to elucidate the molecular mechanisms of PTX3 in regulating neurogenesis and astrocyte reactivity. Second, the recovery of neural function is a complex pathophysiological process, and determining the effects of PTX3 on neurogenesis and neuronal survival alone may not sufficiently explain its effects on neural repair. Other aspects of neurorestoration, such as angiogenesis and axonal remodeling, could also be considered in the future. Third, a clinical study has reported that higher hospital mortality of patients with severe TBI is associated with increased serum PTX3 levels (Gullo Jda et al., 2011). This inconsistency with our findings may be because of the difference in trauma severity or because of differences between the central and peripheral nervous systems; however, the possible reason why our findings are inconsistent with Gullo Jda et al.’s study needs to be investigated in further studies.
In conclusion, endogenous PTX3 expression rapidly increases in the adult mouse brain following TBI, and this increase lasts for 3 days. rPTX3 administration activates the A2 astrocyte phenotype, which consequently improves the recovery of neural function by increasing neuronal maintenance and enhancing neurogenesis. These results suggest that rPTX3 treatment may be a promising therapeutic strategy for TBI. It would have been necessary to investigate angiogenesis in future study, because the SGZ and SVZ also have angiogenic activity.
Acknowledgments:We express special gratitude to the Chongqing Key Laboratory of Ophthalmology for providing the experimental platform.
Author contributions:Study conception and design: CZ and CJC; data collection, analysis and interpretation: CZ, HC, JFZ, ZDG and ZJH; manuscript preparation: CZ; statistical analysis: CZ, ZDG and ZJH; fundraising: XCS, CJC, JJZ and YW; manuscript editing, reviewing and study supervision: XCS and CJC. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81571159 (to XCS); the National Natural Science Foundation for Youth of China, Nos. 81601072 (to CJC), 81801230 (to JJZ), and 81901210 (to YW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Chongqing Medical University on March 1, 2016. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Nafisa M. Jadavji, Carleton University, Canada.
Additional file:Open peer review report 1.
- 中國神經再生研究(英文版)的其它文章
- Dopamine: an immune transmitter
- The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis
- Mounting evidence of FKBP12 implication in neurodegeneration
- Using antifibrinolytics to tackle neuroinflammation
- Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration
- Nafamostat mesylate attenuates the pathophysiologic sequelae of neurovascular ischemia

