Steady and transient behavior of perylene under high pressure*
Ting-Ting Wang(王亭亭) Yu Zhang(張宇) Hong-Yu Tu(屠宏宇) Lu Han(韓露) Ji-Chao Cheng(程基超)Xin Wang(王鑫) Fang-Fei Li(李芳菲) Ling-Yun Pan(潘凌云) and Tian Cui(崔田)
1State Key Laboratory of Superhard Materials,College of Physics,Jilin University,Changchun 130012,China
2School of Physical Science and Technology,Ningbo University,Ningbo 315211,China
Keywords: perylene,high-pressure,ultrafast spectroscopy
1. Introduction
The behavior of the excited state in molecule is largely affected by the surrounding environment such as temperature,pressure, and solvent,[1-6]of which the pressure can change the distances of atoms in molecules, and thus the probability of electron wavefunction overlap and electron delocalization. This change will directly affect their optical characteristics and electrons’ mobility.[7-10]While as a dye, perylene has excellent ability to accept electrons and cell permeability, and can be used as an intermediate in organic synthesis.It has great potential applications in biological imaging and high-performance polymer solar cells.[11-16]Furthermore,because of its simple and identical molecular composition,perylene is a candidate molecule to study the effect of pressure on absorption characteristics and electron mobility. So far,owing to the limitation of experimental techniques, most of the excited state dynamic properties under high-pressure are investigated in solutions,such as LDS698 solution,coumarin solution,etc.[17-22]However,the solvent of solutions can be solidified at a relatively low pressure,which is an anisotropic pressure. And coordination compounds also appear in the pressurization process,which may affect the dynamic process.[23]It is worth noting that there are differences between the photophysical properties of matter subjected to the anisotropic stress and the isotropic stress.[24]On the other side, most of theoretical calculations are based on a pure molecule system, in which neither anisotropic compressing nor coordination compounds is considered.[12-15,25,26]
In order to fill this gap,non-complexing,hydrostatic and isotropic pressure is applied to perylene to study the intrinsic behavior of electron transition process. Steady state results indicate that reduced inter-molecule distance enhances theπ-electron delocalization and thus creating the red-shifting and broadening of the absorption spectra. Transient state results show that the emergence of self-tapping exciton (STE)state andY-state complicates the excited state dynamic behaviors.While,the overall trend can be explained by the pressuredependent molecular bond length and the phenomenon ofπelectron delocalization.
2. Experiments
The perylene powder was purchased from Sigma-Aldrich (CAS: 198-55-0). Perylene’s molecular formula is C20H12,which is a polycyclic aromatic hydrocarbon with five rings.[27,28]The diamond anvil cell(DAC)with 400-μm culet size was used to generate pressures up to 19.5 GPa. The T-301 stainless steel gasket was pre-pressed to~65 μm in thickness and bored a concentric hole with a diameter of~170μm in the gasket. The gasket was squeezed between the two opposing diamond anvils. The hole of the gasket was used as a sample chamber to seal perylene powder, ruby, and pressuretransmitting media simultaneously. The pressure in DAC was calibrated by measuring the fluorescence of the ruby.[29]The pressure transmitting medium was silicone oil to guarantee hydrostatic pressure.[30]
2.1. UV-visible absorption spectra
The UV-visible (UV-vis) absorption spectra of perylene at different pressures were obtained by a home-made system forin-situhigh-pressure condition. The system was constructed by a spectrometer (Avantes, Netherlands, AvaSpec-2048x16, SensLine, 300 nm-1100 nm) and a halogen light source(Ocean Optics,USA,HL-2000,360 nm-2400 nm).
2.2. Transient absorption spectra
Thein-situhigh-pressure transient absorption system[31]was based on previous time-resolved technique.[32,33]A regenerative amplified Ti:sapphire femtosecond laser (Spectra-Physics, USA Spitfire, 800 nm, 35 fs) was used to generate a 35-fs, 800-nm laser beam with a repetition rate of 1 kHz.Then the beam splitter splits the 800-nm laser beam into two subbeams. One subbeam of relatively stronger passed through a 0.5-mmβ-BaB2O4(BBO)crystal to provide a 400-nm pump beam. The other 800-nm laser beam was focused into a cell filled with H2O/D2O to generate a supercontinuum serving as a probe beam. The delayed pump and probe beams were focused into DAC by an objective lens(S Plan Apo HL,20X/0.29). The signal was detected by PMT (Hamamatsu,Japan,PMTH-S1)and then sent into a lock-in amplifier(Stanford,USA,SR830,)for further processing.
3. Results and discussion
3.1. Steady state spectrum
In order to simply explore the effect of pressure on the optical behavior of perylene molecule and avoid the disadvantages of the solvent solidification under lower pressure and the effect of the complexing effect between the solvent and the solute under higher pressure, we chose the perylene powder as the sample loaded into the DAC. Figure 1 illustrates the UV-vis absorption spectra of perylene at ambient pressure and different pressures. There are three absorption peaks under ambient condition as indicated in Fig.1(a). They are 412 nm,438 nm, and 472 nm, respectively. Perylene is a conjugated aromatic molecule. As indicated in Fig. 2(a), each perylene molecule is composed of sp2-hybrid carbon atoms, and each carbon atom forms three covalent bonds. The fourth valence electron of each carbon stays in a 2p orbital formingπ-orbitals network perpendicular to the molecules’plane(ab)Fig.2(b).Perylene molecule has two electric dipole moments: one is thea-component (412 nm) that indicates the electric dipole moment parallel to the long molecular axis and the other is theb-component (438 nm) that indicates the electric dipole moment parallel to the short molecular axis, on theabplane.[34]The peak value 472 nm corresponds to theπ-orbital correlated transition.

Fig.1. Normalized UV-visible absorption spectra of perylene under(a)ambient condition and(b)different pressures.
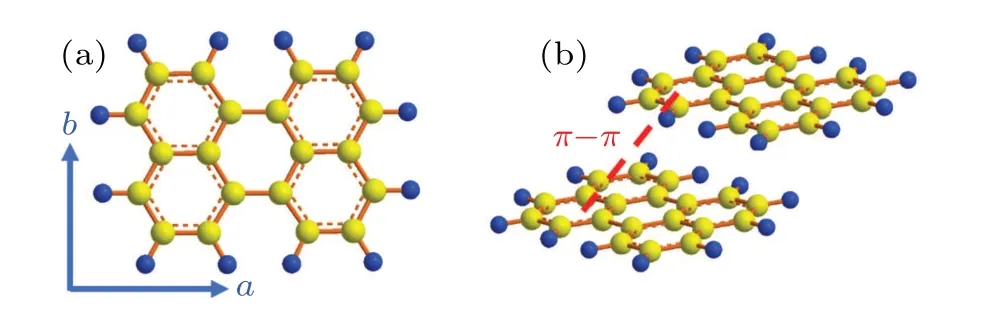
Fig.2. Molecular structure of perylene.
Figure 3 illustrates the anisotropic compressing of perylene molecules in response to pressure. The absorption peak at 412 nm and at 438 nm each display a slight red-shift with the pressure gradually increasing.[35,36]In comparison,the absorption peak at 472 nm shows a large red-shift and broadening, which indicates theJ-type stacking of molecules (see Fig. 2(b)). When the pressure reaches about 19.5 GPa, the range of steady-state absorption spectrum basically covers the entire visible light area. This variation of the absorption peak is consistent with the effect of pressure on the crystal structure.[25,26,37-39]Briefly, the red-shifts of three peaks are the results of reducing the lengths of bonds as well as inter-molecule distance, and thus enhancing the overlap between electron clouds. The increased overlap causes the conduction and valence band and diminished band gaps to disperse.[25,37]Therefore,the absorption peaks move to longer wavelength corresponding to small band gap. Furthermore,the pressure of response to the wavelength 472 nm is more sensitive than to the wavelengths 438 nm and 412 nm as shown in Fig. 3.[35,36]With the pressure increasing, the distances among adjacent molecules decrease, and electrostatic attraction between molecules with extendedπ-orbitals is more significant.[40]While,this attraction is more sensitive to pressure than to the bond length reducing in stacking molecules.As a consequence, the absorption peaks show different redshifts in degree as indicated in Fig. 2.[26,27,38,41]Based on the theoretical calculation, the evolutions of optical behavior in both steady state (Fig. 3) and transient state (Fig. 5)are divided into four pressure regions (ambient condition~1.7 GPa,2.7 GPa-6.8 GPa,8.2 GPa-14.3 GPa,and 16.9 GPa-21.1 GPa), which correspond to four compressing processes according to the response to lattice parameters.[26,35,36]The details will be described by combining with transient state information.

Fig. 3. Absorption peak wavelengths (412 nm: empty circles, 438 nm:empty stars,and 472 nm: empty triangles)versus applied pressure.
The exciton-phonon coupling constant related to the lattice relaxation energy is one of the important parameters describing the overall characteristics of the dynamics.[9,42,43]The exciton-phonon interaction in aromatic hydrocarbon crystals is strongly related to the molecular arrangement.[34]In order to observe inter-molecule distance effect on dynamics,we use the DAC device to apply an external pressure to the perylene,an aromatic hydrocarbon,thereby exploring the dynamics of the perylene at high-pressure by changing the relative arrangement of molecules and then changing their excitonphonon coupling situation in a wide range. From the UVvis absorption spectra, it can be seen that as the pressure increases, the absorption peak at 472 nm is more sensitive to the pressure response than the absorption peaks at 412 nm and 438 nm. Therefore,we choose band-edge to be 640 nm serving as a probe beam. To observe the exciton dynamics with inter-molecule distance decreasing, transient experiments are performed on the excited state. The decay dynamic behaviors of excited state at each pressure are indicated in Fig.4.
3.2. Transient state spectra
Figure 4 illustrates the dynamics of perylene molecules from ambient pressure to 21.1 GPa. Obviously, the decay process strongly depends on the applied pressure. A multi-exponential function [ariseexp(t/τrise)+a1exp(t/τ1)+a2exp(?t/τ2)+a3exp(?t/τ3)] is used to fit the dynamics curves of perylene, in which eachτriserepresent the rising time of excited state,τ1andτ2(τ'2) the intra-band relaxation(fast decay component with picosecond timescale), and occasionalτ3the inter-band relaxation (slow decay component with nanosecond timescale).

Fig.4.(a)Normalized transient spectra under different pressures and(b)decay under selected pressures(experimental data: empty circles; fitted data:solid line)for normalized dynamic curves of perylene.
Figure 5 illustrates the simulation results of decay data.Since the magnitude ofτ3is longer than the experimental limitation(2 ns),onlyτ1andτ2(τ'2)are discussed in the following. A positive signal is observed under each pressure,which suggests that the signal is generated mainly by the excited state absorption. Since both molecular structure and inter-molecule distance are modulated by pressure,rather different signals appear under high-pressure.
Region I lies between ambient pressure and~1.7 GPa as shown in Figs.3 and 5. The transient absorption increases through intraband trantionS3→S1withinτ1~0.8 ps and decays throughS1→STE state withinτ2~45.5 ps, then followed by a nanosecond scale decay due to the fluorescence process, Fig. 6. The STE and STE state are generated by reducing inter-molecule distance in the condense condition,which is formed by the interaction between excited states and lattice.[44]These results consist with the reported dynamic results in low pressure region (<0.5 GPa),[34,44]Both components turn slower with pressure increasing up to 1.7 GPa, inτ1~1.3 ps andτ2~105.4 ps. In this region,molecule interaction is the main factor for dynamics process. As illustrated by theoretical calculation, there is a relative steep compressibility in this region.[35]According to the absorption spectra in this region,the signal is correlated with the dynamics of STE state.[34]Depopulation from higher excited state to the STE state may experience different processes with pressure changing. Fasterτ1(<1 ps)is the result of direct population from higher excited states,and slowerτ1(>1 ps)is negative signal from vibrionic structure denoted asY-state,which is obviously under high-pressure(0.7 GPa),as indicated in Fig.6.[34,44]For the decay process,sayτ2,theY2relaxes to STE states after detrapping through a thermal activation process. This process is much slower than the direct relaxation process from the higher excited state to the STE state. The dramatically slowerτ1andτ2suggest that population ofY-states increases when pressure rising up to 1.7 GPa. While, the generation of STE requires a large energy because molecules become“tighter”with intermolecule distance reducing. The STE state moves to higher energy (0.13 eV under 1.2 GPa[34]) as indicated in Fig. 6.However,Y-state and exciton state redshift with the enhancement of wavefunction interaction among molecules under high pressure. At>1.2 GPa,Y-state luminescence is observable,which contributes to the negative signal at initial time.[34]

Fig.5. Lifetime of τ1 (empty circles), τ2 (empty triangles), and τ'2 (empty stars)as a function of applied pressures.
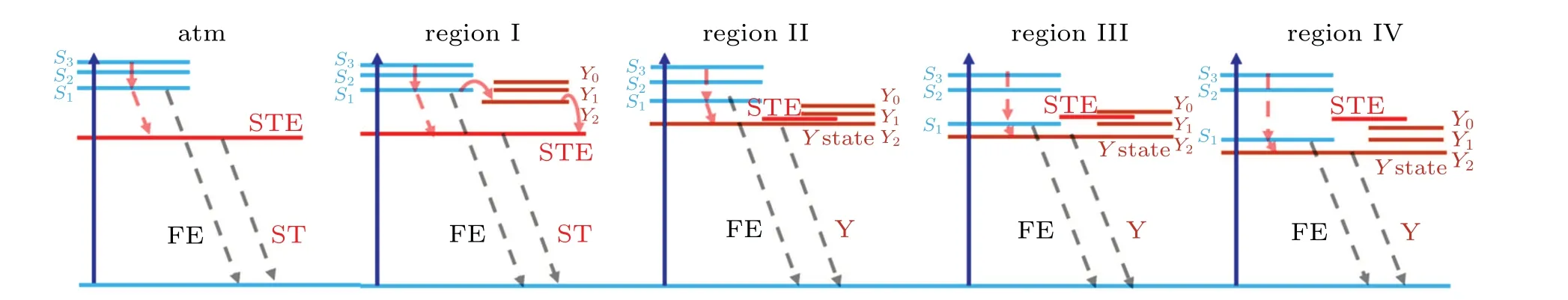
Fig.6. Pressure-dependent energy level evolution. S: excited states,STE:self-trapping exciton state,FE:free-exciton state,Y:Y-states.
Region II lies between 2.7 GPa-6.8 GPa. In this region,τ1>1 ps is rising component,τ'2in 2 ps-5 ps andτ2in 20 ps-50 ps decay component are detected. With pressure rising up to 2.7 GPa,negative signal disappears because STE states shift above theY2state and its relaxation toY2sate is impossible because of the trapping barrier. In this region,the anisotropic response of lattice to pressure is more obvious than in other regions.[25]Thus, a complicated electronic band shift is expected. The long-axis dipoles of molecules are perpendicular in theabplane. The compression is more effective on the long-axis dipole, which induces the lower exciton stateS1to drift dramatically.[34]Thus,rapid decayτ'2(in 2 ps-5 ps)generates as a result of reducing energy gap between excited stateS1and STE state. Meanwhile,S1state relaxes toY2state,τ2in 20 ps-50 ps,is much faster than that in region I because of reduced energy gap betweenS1andY2states. Bothτ2andτ'2turn fast with pressure increasing in region II,4.5 ps to 2.0 ps forτ'2,and 48.9 ps to 19.8 ps forτ2. This is also ascribed to reduced energy difference betweenS1andY2states. Since most of population(40%-80%)decays to the STE state as indicated by the large amplitude ofτ'2,luminescence fromY2state is not obvious in this region. Thus,no negative signal is observed.
Region III is between 8.2 GPa-14.3 GPa. In this region, the pressure effect on the lattice parameters tends to be isotropic.[25]As illustrated in the pressure-dependent absorption spectra (Fig. 1), the observing wavelength is near bandedge and thus the negative signals originating fromY-state luminescence states are overlapped. The STE state shifts upward to the middle ofY0andY1with inter-molecule distance decreasing. Meanwhile,S1shifts downward to lower energy,which is betweenY1andY2. SinceS1→Y1has larger transition rate as a result of closer energy gap,Y1luminescence signalis enhanced and contributes to the opposite signal in this region. Gentle change of 25 ps-35 ps (8.2 GPa-12.2 GPa)decay component indicates small energy gap modulation toS1state relaxing toY1state in this pressure region. However,this component turns slower with pressure increasing. Diffusion induced electron delocalization shows a great effect under such a high pressure,which can extremely prolong the excited lifetime.[3,11,20,44]This is also consistent with the rather broadened absorption band as indicated in Fig.1.While a knee point appears at 14.3 GPa. The amplitude of negativeY1signal decreases under this pressure. It means continuous shift ofS1to a state lower thanY1. The luminescence fromS1(FE) slows the decay component down to 154.3 ps. Then, the dynamic region evolves into the next pressure region.
Region IV is between 16.9 GPa-21.1 GPa. According to absorption under steady state(see Fig.1), the negative signal comes from the luminescence state, which deduces and disappears with pressure induced band gap dispersion. In this region,lattice response is totally isotropic to pressure as indicated by calculation,[25]which means that the inter-molecule distance is near the limitation with lattice turning harder. In this case,the effect of repulsion among atoms becomes strong,in which the barrier for electron diffusion emerges with the evolution of dielectric environment. Thus, relaxation ofS1state becomes faster due to less possibility of electron diffusion, 81.5 ps at 16.9 GPa to 57.7 ps at 19.5 GPa. With the pressure reaching to 21.1 GPa,the very fast relaxation component of 2.5 ps is the result of band gap dispersion,which may be deduced from the collapse of lattice structure.
As a summary, STE state andY-states appear with the pressure increasing and affect excited state dynamics process significantly. The evolution of effect is correlated with compressing properties of molecules,which is consistent with previous theoretical calculation.
4. Conclusions and perspectives
The optical behavior of perylene is studied by highpressure steady state and transient state spectra in an isotropic compressing and non-complexing conditions. With pressure increasing, the delocalization ofπ-orbital is more sensitive than the reducing of bond length as suggested by steady statespectra. While, the transient processes are strongly dependent on the pressure-affected positions of STE state andYstates. The results in both steady and transient state spectra can be explained by previous theoretical calculation based on anisotropic response. The experimental environment in this paper is consistent with the theoretical calculation, and only pure molecular system is considered without the influence of complexation. Therefore, these kinds of experiments can be widely used to verify the theoretical calculations and further practical basis.
- Chinese Physics B的其它文章
- Numerical investigation on threading dislocation bending with InAs/GaAs quantum dots*
- Connes distance of 2D harmonic oscillators in quantum phase space*
- Effect of external electric field on the terahertz transmission characteristics of electrolyte solutions*
- Classical-field description of Bose-Einstein condensation of parallel light in a nonlinear optical cavity*
- Dense coding capacity in correlated noisy channels with weak measurement*
- Probability density and oscillating period of magnetopolaron in parabolic quantum dot in the presence of Rashba effect and temperature*

