Changes in interstitial fluid flow,mass transport and the bone cell response in microgravity and normogravity
Fei Wei,Kendal Flowerdew,Michael Kinzel,Luigi E.Perotti,Jackson Asiatico,Mahmoud Omer,Candice Hovell,Veerle Reumers and Melanie J.Coathup?
INTRODUCTION
The negative effects of spaceflight on human health are known to involve multiple biological stressors,including microgravity,radiation,loss of the light-dark cycle and confinement.Among these,microgravity exposure(between 10?3-10-5g)has been reported to have the strongest impact on human physiology and psychology.1While the history of human space flight has focused primarily on the development of research facilities located in Lower Earth Orbits,such as Skylab,Salyut,Mir,and most recently the International Space Station(ISS),there has been a recent shift toward pursuing territories farther afield,aiming at near-Earth asteroids,the Moon,and Mars.Acute skeletal loss has severe implications for long-term(>5 months)inhabitants of the ISS and will be a hindrance to space exploration,as up to one half of bone mass could be lost during a 3-year trip to Mars,resulting in mission-compromising low-energy bone fractures,complications from renal stones caused by skeleton-released calcium and an increased incidence of fragility fractures when returning to full or partial gravity.2This extreme and accelerated bone loss is 10-fold greater than postmenopausal osteoporosis,and it remains mechanistically elusive as to why there is such a magnitude of difference between bone loss on Earth and loss in microgravity.When in space,the lack of gravity eliminates a critical primary mechano-stimulus,and when combined with exposure to both galactic and solar cosmic radiation,additive injury to healthy human tissue function ensues.3–4An additional and detrimental effect may involve changes in dynamic fluid flow within tissue.Presently,there is only limited information about how changes in this key cell regulator may orchestrate bone turnover during longduration space travel.Gravity strongly affects fluid behavior by creating forces that drive and alter its motion.In the presence of gravity,fluid flow can also lead to altered phase interactions and processes that regulate gases.Controlling fluid flow in the absence of gravity creates both significant and novel challenges,where flow can be significantly complicated by temperature,capillary networks of different geometries,changes in fluid surface tension,droplets,and undesirable bubble formation.The near elimination of buoyancy,hydrostatic pressure and sedimentation cause adjustments to flow dynamics:liquids climb container walls,there is limited drainage of liquids,and liquids of different densities can stratify.5–8These are a few examples of the many complications induced by microgravity.Such complex multiphase and interfacial flow processes as well as phase separation and unavoidable alterations in particle biodistribution relate to the performance of biosystems.Currently,the impact of these changes within the porous system of bone,along with subsequent changes encountered at the cellular level,are not well understood.The aim of this review is to explore our current understanding ofinterstitial fluid motion and solute transport in two different conditions,normogravity and microgravity,and to determine how microgravity may influence the subsequent characteristics and behavior of bone tissue fluid when in space.We examine the hypothesis that microgravity may further deteriorate bone architecture through decreased fluid-induced mechanostimulation,altered mass transport and cytoskeletal changes.
NORMOGRAVITY
Interstitial fluid flow and mass transport within the soft tissue extracellular matrix
Interstitial fluid consists of a water solvent(92%)containing amino acids,sugars,salts,fatty acids,coenzymes,hormones,neurotransmitters,minerals,and cell waste products.9–10It accounts for 20%of the water in the human body and up to 12% of body mass.11Interstitial fluid exists either as fluid bound by physico-chemical forces to extracellular matrix(ECM)components(e.g.,heparan sulfate and other glycosaminoglycans)or as free fluid moving through the cellular biological medium.The size distribution of the components within the fluid ranges from small molecules and ions(<1 nm)to 10 nm proteins such as albumin,lipoproteins~20 nm in size,and fibrinogen(~30 nm).12In the body,electrostatic forces and energies(e.g.,ion pairs,hydrogen bonds)are essential for the interaction of virtually all biological macromolecules.13Due to the polar nature of water,the intercellular and intracellular interactions between water and hydrophilic and hydrophobic molecules,including polysaccharides,lipids,and proteins,are critical for healthy physiological processes.Furthermore,spatial control,ranging from the local control of protein activity and irreversible aggregation to the uptake of pathogens or the management of wastes,are similarly influenced by electrostatic forces and,as such,are essential to many cellular activities.14
A simple example of gravity’s impact on fluid flow is the creation of flows due to density differences(buoyancy-induced convection)as well as due to thermal convection.Gravitationally induced bulk convection is a type of natural convection caused by buoyancy variations that result from material properties other than temperature.With gravity,thermal convection occurs when heated fluids rise to the top along the gravity vector,which are then replaced by cooler fluids.Both bulk and thermal convection establish a fluid current in the body that is considered essential to driving mass transport and rapidly dissipating heat.15–16Within the interstitial space,the primary mass transfer mechanism is considered to be molecular diffusion,which is augmented by bulk convection.17–18The work of Swabb et al.19showed that,in soft tissues,the relative importance of convective versus diffusive mass transfer depends on the size of solute molecules,where larger solutes(i.e.,molecular weight>1 000 Da)were transported by convection-dominated mass transfer and smaller molecules were transported by diffusion.Notably,this study also showed that fluid velocity was regulated in part by the level of polysaccharides within the interstitium.In soft tissue tumor environments,osmotic and hydrostatic pressure gradients generated by physiologic processes such as drainage toward lymphatics,inflammation,muscle contraction and loading during ambulation were shown to drive flow via dynamic stress through the ECM.20–21Studies have demonstrated that fluid flow can reach velocities between 0.1 and 4.0 μm·s?1within the ECM of soft tissue.21–23Although slow,fluid flow nevertheless plays an important role in nutrient transport,soft tissue maintenance and remodeling,as well as the establishment and maintenance of the microenvironment,where limitations in the supply of vital nutrients lead either to tissue adaptation or necrosis.24
Interstitial fluid flow within the porous bone system
Bone is a natural composite material consisting of three primary phases:mineral(mainly hydroxyapatite),organic(~90% Type I collagen)and water.These phases are not interdependent but combine to determine the biomechanical properties of bone.The lacunae-canaliculi system(LCS)within bone tissue and its anatomical parameters vary according to bone type,location,age and health(Fig.1).The LCS is composed of larger lacunae(~10μm)and smaller canaliculi(0.1–0.5μm)inhabited by osteocytes,and this porous system facilitates the exchange of substances,with liquid flow providing nutrients,eliminating metabolic waste and generating fluid shear force to stimulate osteocyte viability and function.25–26Lacunae are roughly tri-axial ellipsoids in mature bone and globular in woven bone.27Canalicular length has been estimated as 23 to 50μm,with~41 to 115 canaliculi originating from each lacuna.28–29Spaces between crystallites of the mineral hydroxyapatite and collagen fibers also exist and are estimated to be~0.01 μm in size.30

Fig.1 A multilayered porous system in bone allows for dynamic fluid flow(green arrows)at the nano,micro-and macroscale.a A microCT image of healthy murine trabecular bone.The bone structure consists of micro-and macropores.b A schematic demonstrating osteocytes within the LCS.c Representative photomicrographs of a longitudinal section prepared from the distal femur of a healthy rat.The pores are inhabited by bone marrow containing blood vessels and multiple cell groups.The pores provide an environment for fluid movement(red arrows)and the generation of fluid-induced cellular mechanostimulation.d Representative images of osteocytes within the LCS.The osteocyte nucleus(blue)and cell processes(green)are observed within the canaliculi and allow communication between cells that are located at distant sites.Images were taken from cryo-sections prepared from healthy rat bone.Not presented are the vascular porosities within the Volkmann and Haversian canals,which provide an additional pore structure,as well as the collagen-hydroxyapatite porosities,which comprise the smallest pore size in bone
In addition to the LCS,cortical bone contains a vascular porosity via the larger-scale Volkmann canals and Haversian systems(~20 μm radius).Trabecular bone consists of a series of micropores ranging between 0.01–20 μm,with macropores 200–500 μm in size.Bone marrow is a cellular soft tissue located within the porous spaces of both cortical and trabecular bone that forms the environment for several cell types.The bone marrow is inhabited by blood vessels as well as multiple cell groups,including osteocytes,osteoblasts,macrophages,adipocytes,endothelial cells,and mesenchymal stem cells(MSCs)(Fig.2).Oxygen has low solubility in aqueous media and is limited by diffusion distance in most mammalian tissues such that cells are typically located within 100–200 μm from the nearest capillary to ensure efficient gas exchange.31

Fig.2 Scanning electron microscopy(SEM)images of cultured cells in vitro.a Human osteocytes are stellate in shape and are found within the lacunae-canalicular system.The cell body varies in size between 5 and 20μm in diameter and sits within the lacunae.Each cell body contains 40–60 cell processes,which are approximately 23–50μm in length.Cell processes occupy the canaliculi system and establish a communication network.The cell-to-cell distance is approximately 20–30μm.b Image demonstrating rounded M0 murine macrophages,approximately 10-20μm in size.When activated toward a pro-(M1)or anti-inflammatory(M2)phenotype,cytoplasmic extensions appear,and cell size increases with elongation of the cell body associated with the M2 phenotype(inset b).c Human mesenchymal stem cells(MSCs)are a heterogeneous cell population that are typically large,flat and spindle shaped.MSCs range between 15 and 40μm in size
In their pioneering work,Piekarski and Munro in 197726first theorized that in response to physiological loading,there is fluid flow within the complex interconnected LCS porous network within bone.Until the 1990s,investigators did not identify this network as a mechanosensory organ but instead hypothesized that fluid flow and the subsequent signaling response by cells was due primarily to the receiving of nutrients and removal of waste products.It was not until 1994 that Weinbaum et al.32introduced the hypothesis that bone cells sense mechanical load in response to fluid shear stress,initiating a signal for cellular excitation.It has since been established that the mechanically induced deformation of bone acts as a motive force for fluid displacement,generating fluid pressure gradients that drive interstitial fluid into the LCS and bony macrostructure.The forces generated act directly on bone cells,and this load-induced fluid flow is critical for mechanotransduction as well as enhancing convective solute transport within the macro-and microporosities.33As such,bone tissue would not survive without flow.
Advancements in our understanding of the cellular mechanoresponse to strains,fluid flow velocities and shear stresses are critical.However,these quantities are difficult to measure in situ.There have been many published numerical analyses of fluid flow and mass transport within the LCS as modeled in cortical bone.34–38Verbruggen et al.39employed fluid-structure interaction modeling to develop a complex 3D system that simulated the multiphysics of the mechanical environment of osteocytes in vivo.This study estimated that in a representative state of physiological activity,the average interstitial fluid velocity within the LCS and surrounding the osteocytes was~60.5 μm·s?1,with a maximum shear stress of~11 Pa.These results are similar to observations reported using experimental tracers in an in vivo mouse model(~60 μm·s?1and~5 Pa).40However,the bone marrow located within the larger pores that dominate the trabecular structure provides a specialized environment for fluid flow when compared to the liquid flows,pressure distribution,and principles of fluid shear stress within the microscopic LCS.When trabecular bone is subjected to mechanical loading,complexity is introduced through macropore deformation and the interaction between bone and the adjacent soft marrow tissue.Bone marrow is a highly viscous fluid that displays viscoelastic solid properties41and is subject to deformation by the surrounding pores as they change their shape and size under load.These structural changes introduce velocity and pressure gradients that cause cells in the marrow to move relative to one another and to be stretched and deformed,thereby imparting shear forces through intercellular or focal adhesions.42–43Notably,the fat composition and viscosity of bone marrow vary with age and location(40 to 600 mPa?s),and this may also influence the levels of fluid shear stresses generated.44It is therefore conceivable that the subsequent mechanical environment of the marrow within trabecular bone plays a critical role in directing cell activity,function,and fate.45However,there has been little insight into how marrow alters during loading and,subsequently,how the interstitial fluid-induced mechanostimulus to cells is modified.This complex multicellular environment has made the direct study of this microenvironment in situ challenging.Using a finite element model coupled with computational fluid dynamics(CFD),Birmingham et al.44estimated that the loadinduced fluid shear stresses in the marrow were between 0.02 Pa and 0.26 Pa under simulated physiological loading.More recently,Metzger and colleagues46used microscale CFD to model fluid velocity within the bone marrow component and demonstrated that during cyclic loading,the volumetric mean marrow velocity averaged~0.01 mm·s?1,applying a shear stress to cells that ranged from 1.67 to 24.55 Pa.Similarly,a numerical bone simulation model developed by Yao et al.47estimated that interstitial fluid flow induced a shear stress with a magnitude up to 30 Pa on the membrane of cells where parameters such as blood pressure,capillary density,capillary permeability,capillary orientation,interstitial pressure and interstitial porosity all affected the applied shear stress and the efficacy of substance exchange.
The role of the cytoskeleton
The mechanical aspects of cell life are central for cell motility,cell division,intracellular transport,and positioning of organelles,to name a few relevant phenomena.48Therefore,gravity and external mechanical stimuli,including tensile and compressive stresses,fluid-exerted shear,and hydrostatic pressure,all greatly influence the growth,development,and maintenance of healthy tissues and cells.49The cellular response to these forces can be described by 3 sequential events:mechanosensing involves the cell sensing changes in its local mechanical environment through changes to the cytoskeletal architecture;mechanotransduction involves the conversion of force-or geometry-induced changes into biochemical signals;and the mechanoresponse can be shortand/or long-term.Short-term responses include alterations to cell motility systems responsible for migration and surface adherence.50Long-term responses include modifications to the cell leading to altered cell survival or new deposition/remodeling of extracellular matrix.51The overarching term“mechanotransduction”refers to the set of mechanisms that enables the cell to convert a mechanical stimulus into biochemical activity.All osteogenic cells,from MSCs to osteoblasts to osteocytes,are mechanosensitive and therefore can sense and respond to applied force.52
The cell cytoplasm is not a simple liquid.The cell is a mechanical machine,and continuum mechanics of the fluid cytoplasm and the viscoelastic deforming cytoskeleton play key roles in cell physiology.48Cellular deformations are perceived via complex and intricate regulatory pathways,activating one or more putative mechanosensitive structures,which include adhesion molecules and adhesion complexes(transmembrane integrins,cadherins,and connexins),the cytoskeleton,primary cilia,lipid rafts,stretchactivated ion channels,G protein-coupled receptors,and the nucleus52(Fig.3).These interactions form crucial links in mechanical continuity that couple the inside of the cell to the outside environment.Virtually all bone cells express the necessary tools,including the primary cilium,53and their interdependent roles have previously been comprehensively reviewed.52In general,fluid shear stress can induce deformation of the bone cell membrane and alteration of membrane proteins,opening mechano-activated ion channels to allow the influx of cations,such as Ca2+,Na+,and K+,into the cell.52Stretch-activated ion channels include the DEC/ENAC family of cation channels(named after Caenorhabditis elegans degenerins and mammalian Na+channels),L-type(osteoblasts)and T-type(osteocytes)voltagesensitive calcium channels,and annexin V voltage-gated calcium channels.53Specifically,increases in osteoblast,osteocyte,54and MSC55membrane tension induce the opening of PIEZO1 channels.Mechanically activated nonselective Ca2+-permeable cation channels of the PIEZO family(PIEZO1 and PIEZO2)are recognized as the most important mediators of mechanotransduction and arecrucial for bone formation.56Mechanical stimulation enhances calcium flux into the cell,and the resulting calcium spikes mediate osteogenesis.
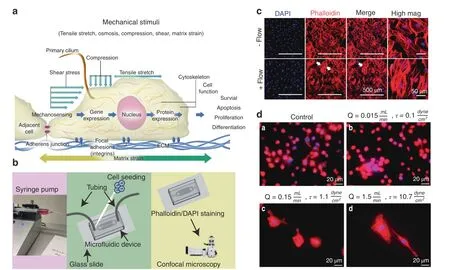
Fig.3 Gravity and external mechanical stimuli influence the growth,development,and maintenance of healthy tissues.a Cells sense their mechanical environment(e.g.,tensile stretch,compressive strains,and shear stimuli)via mechanisms involving cilia,adherens junctions,ion channels,and focal adhesion.b The controlled fluid environment provided by 2D microfluidic devices is a commonly used method to examine the cell response under different flows.c Representative micrographs of IDG-SW3 murine late osteoblasts/preosteocytes taken using confocal microscopy showing cell nuclei(DAPI(blue))and F-actin cytoskeletal filaments[phalloidin(red)].Cells were cultured within a microfluidic device.A flow rate of 0.15 mL·s?1 was applied,and the mechanosensitive actin filaments responded by realigning their structure,becoming more parallel in orientation(white arrows).Images show the fluid-induced response after 24 h of culture.d Representative micrographs of macrophages within microfluidic devices(24 h)and examined using fluorescence microscopy(×20 mag.).Images show red cytoplasmic actin filaments(phalloidin)and blue nuclei(DAPI).a Static-flow conditions and following the application of continuous fluid flow delivered at b 0.1 dyn per cm2,c 1.1 dyn per cm2 and d 10.7 dyn per cm2(physiological)fluid shear to cells.The cells were observed to respond differently to changes in fluid shear.M0(nonactivated)macrophages are characterized by their small size(~10μm)and abundant number,and this phenotype is indicated in the unstimulated static control group.Following the application of an extremely low fluid shear(0.1 dyn per cm2),the cells were fewer in number and slightly larger(~15–20μm),displaying a more M1-like,proinflammatory,osteodestructive phenotype.Notably,there were fewer nuclei,suggesting cell death.Remarkably,when exposed to higher shear rates,the cells become much larger(~80–100μm)and are round or spindle shaped,suggesting an osteoprotective M2 phenotype
The cytoskeleton is a mechanosensitive structure and consists of a network of actin,microtubules,and intermediate filaments,which provide shape and stability to cells and connect the ECM to the cell nucleus.50A recent study identified the cytoskeleton as an emerging key player in initiating mechanotransduction.57Actin and microtubule polymers are stiff filaments that constantly assemble and disassemble,with lifetimes on the order of seconds or minutes.The actin cytoskeletal components are comprised of filamentous F-actin,helical G-actin,and actin-binding proteins and act by sensing mechanical force with the subsequent generation of cytoskeletal contractile and protrusive forces.Microtubules are protofilaments containing α and β tubulin heterodimers.Presently,there is no evidence that microtubules function as mechanosensors.However,they do play an indirect role in the mechanoresponse by regulating force-controlled spindle organization,chromosomal alignment,and segregation during mitosis.50Additionally,cells under mechanical stress have been shown to demonstrate microtubule outgrowths along the periphery of the cell.Intermediate filament proteins form both homodimers and heterodimers and are considered the most stable cytoskeletal filaments,serving as sensors of mechanical force direction and strength.58Both actin filaments and intermediate filaments communicate with the external environment via adhesion complexes,which are bound to adhesion receptors.These adhesion complexes sense mechanical forces,relaying information to the cytoskeletal elements within the cell.58When focal adhesion complexes on the cell surface are stimulated by a mechanical signal,such as fluid shear stress,a number of pathways become activated within the cell.Specifically,there is a clustering of transmembrane integrins,integrin recruitment of proteins,and activation of signaling cascades,58thereby communicating stimuli external to the cell nucleus and resulting in responsive changes in gene expression.58–59Significant differences have been reported in terms of the cytoskeletal organization between cell types in bone.For example,MSCs comprise many thick actin bundles,while osteoblasts have fewer filaments and show a thin but dense meshwork of actin.52Fluid shear stressinduced osteogenic differentiation of MSCs is reported to occur via the critical contribution of the actin cytoskeleton.60When osteoblasts were stimulated by fluid shear stress,Pavalko et al.61reported reorganization of actin filaments,and Malone et al.62demonstrated that the response of osteoblasts to fluid shear stress was altered following disruption of the actin cytoskeleton;a similar response has been reported when the microtubule network is broken.63Chen and Jacobs64demonstrated that disruption of the microfilament assembly/disassembly process prevented the fluid flow-induced osteogenic differentiation of MSCs.Additionally,cilia,microtubule-based organelles projecting from the cell surface,have been found to convert fluid flow into a cell response without input from calcium channels or other stretch-activated channels.65In particular,cilia have been linked to osteogenic gene expression and protein secretion in osteocytes,osteoblasts,and mesenchymal stem cells;however,their mechanism of mechanosensation has not been clarified in bone.66
Fluid-induced biochemical events initiate intracellular signaling,and the mitogen-activated protein kinase(MAPK)/focal adhesion(FAK)signaling,Ras homolog gene family member A(RhoA)and its downstream effector Rho-associated protein kinase(ROCK)pathways,and the calcium and beta-catenin signaling pathways are considered to dominate events at the transcriptional level in the nucleus.52Notably,the Hippo-TAP/TAZ signaling pathway(Yes-associated protein(YAP);transcriptional coactivator with PDZ-binding motif(TAZ))has also recently been characterized as an important sensing pathway in bone.67–68In summary,these signaling pathways activate genetic programs,enabling cells to mount evolved responses to mechanical stimuli,thereby establishing cell-regulated feedback loops that maintain tissue homeostasis.69
Mechanical cues and bone cell activity
Dynamic loading of bone.In brief,the complexity and diversity of in vivo mechanical cues present distinct patterns of shear flow,tensile stretch or mechanical compression with various parametric combinations in magnitude,duration and frequency.70Therefore,the osteogenic response to high-or low-impact activity might be related to the response of bone cells either to a sudden increase(i.e.,higher rate)or decrease in fluid shear stress,respectively.The rate of loading appears to be critical in bone formation and maintenance;however,it is not well understood how bone cells respond to the rate of loading or what the actual physiological levels of“high”and“low”shear stress are(Fig.4).

Fig.4 A schematic showing the mechanical loads borne by bone in normogravity.με:microstrain,BMD:bone mineral density,ALP:alkaline phosphatase,OCN:osteocalcin.Several studies have suggested that the rate(determined by the frequency and amplitude)rather than the magnitude alone of the applied loading stimulus correlates with bone formation.75,247 This implies that bone formation is enhanced by dynamic loading,and therefore,both the magnitude(or amplitude)and the frequency of loading are important parameters.It has been shown that low magnitude[<10 με(<1 g;1 g=9.8 m·s?2)]and high frequency(10–100 Hz)loading stimulate bone growth,inhibiting disuse osteoporosis.248–249 Peak dynamic strain magnitudes within the physiological range of 1 500–3 000 microstrain(μ?)are reported to result in bone modeling and an increase in mass.Strain within the disuse range of 100–300 μ?activates osteoclastic activity and bone resorption.Strain levels above 3 000 μ?are considered overuse,and those above 5 000 are considered pathological overload250
Fluid shear stress(FSS).Cellular responses to shear flow that mimic physiological blood or load-induced interstitial fluid flow have been extensively investigated.In vitro methods duplicate these flows via(i)steady or laminar flow;(ii)pulsatile flow,which introduces changes in flow frequency;and(iii)oscillatory flow,which introduces changes in flow direction.Other factors that have been examined include magnitude,frequency,and length of application.64When investigating the effect of shear stress on human fetal osteoblast cell monolayers,Jacobs et al.71demonstrated that pulsing flow was a much greater stimulator than oscillating flow.The guidance of stem cells toward osteogenic differentiation in 3D bioreactors is also reported to depend on the flow regimen.Liu et al.72observed that intermittent flow(stress alternating from 4.2 dyn per cm2for 1 h to 0.34 dyn per cm2for 11 h)for 14 days significantly enhanced osteogenic gene expression relative to cells cultured in a continuous flow of 4.2 dyn per cm2or static control.A FSS of 4.2 dyn per cm2was produced by a flow rate of 4 mL·min?1for 1 h before being cultured under a fluid flow rate of 0.3 mL·min?1for 11 h.This study showed that a flow rate of 0.3 mL·min?1was too low to stimulate the cells and that the application of intermittent flow had a significant and positive impact on osteogenic differentiation,with the highest levels of ALP,OCN and collagen I(Col-I)expressed on Day 7.Osteocytes that were subjected to a 5-Hz pulse with a mean shear stress of 0.7 Pa,a pulse amplitude of 0.3 Pa and a peak shear stress rate of 8.4 Pa·s?1for 1 h produced a conditioned medium that inhibited the formation of osteoclasts,and the osteocytes were more responsive to flow than osteoblasts or periosteal fibroblasts via NO-dependent pathways.73Correia et al.74investigated the effect of steady and pulsatile medium perfusion on adipose-derived MSCs.The pulsating flow was applied in 12 h intervals,with the interstitial velocity fluctuating between 400 and 1 200 μm·s?1at a 0.5 Hz frequency for 2 h,followed by 10 h of steady flow.A 0.5 Hz frequency was used to resemble the dynamic force spectra applied to the human hip during slow walking.75The results from this study showed that pulsating fluid ranging between 400 and 1 200 μm·s?1was associated with fluctuating shear stresses(0.045–0.134 dyn per cm2)and improved early-stage bone formation in comparison to steady flow at 400 μm·s?1.Furthermore,the cell response to pulsatile fluid flow was progressively enhanced with the increase of steady flow during culturing.
The directionality of fluid flow was also shown to be important,with cells experiencing unidirectional flow exhibiting different characteristics from cells experiencing oscillatory fluid flow.Furthermore,the velocity of interstitial fluid flow is considered to play a key role in activating surrounding cells when bone is under stress.Physiological levels of fluid flow apply pressure onto the walls of the narrow channels within bone,creating shear stress and ranging in magnitude between 0.8 and 3 Pa(8–30 dyn per cm2).32,59Using a parallel plate flow chamber,Yi et al.76investigated protein releasefrom MSCs following the application of a steady flow producing 3 dyn per cm2of low shear stress for 6 h(Table 1).The results showed that MSCs responded to low shear stress,and 32 specific proteins were identified,of which 10 were upregulated.The effect of FSS on osteoblasts is reported to be detectable at 10 dyn per cm2,77which is at the lower end of the physiological range(8–30 dyn per cm2).32,59,78Riehl et al.79investigated the effect of physiologically relevant shear stresses at 2,15 and 25 dyn per cm2on MSCs and found that flow shear stress levels had a significant influence on MSC migration.The total displacements,confinement ratio,motility coefficient,and number of cells migrating with the flow over time showed an increasing trend with increasing shear stress.Grayson et al.80investigated the effect of interstitial flow velocity on cell phenotype and the formation of bone-like tissues in 3D engineered constructs.Flow velocities of 80,400,800,1 200 and 1 800 μm·s?1corresponding to estimated shear stresses ranging between 0.6 and 20 mPa were investigated,and the results demonstrated that velocities from 400 to 800 μm·s?1yielded the best overall osteogenic response to MSCs(based on OCN,OPN,bone sialoprotein(BSP)and Col-I expression).Using mathematical models,they determined that the lowest flow velocity of 80 μm·s?1would provide a sufficient oxygen supply(~0.205 mol·m?3)to maintain cell viability;however,this flow rate did not support osteogenesis.Fluid shear stresses ranging between 0.5 and 2 Pa(5–20 dyn per cm2)have been widely reported to beneficially impact osteoblasts in vitro,81such as through increased intracellular calcium production and an increased release of prostaglandins.82Furthermore,a study by Yu et al.83investigated the effect of fluid shear stress on the proliferation of MC3T3-E1 osteoblasts and showed that shear ranging within 1.5 to 52.6μPa promoted cell proliferation and differentiation with increased levels of runt-related transcription factor 2(RUNX2),whereas shear above 412 μPa inhibited growth.Studies have also augmented osteogenic activity via microflow within microfluidic chips.Leclerc et al.84studied the osteoblast response under 0,5,and 35 μL·min?1flow within a 3D microchannel and showed that ALP activity was enhanced 7.5-fold under a flow of 5 μL·min?1when compared with the static control.Jang et al.85designed a drug screening device and observed that a microfluidic flow of 0.2 μL·min?1with a shear stress of 0.07 dyn per cm2combined with a bone morphogenetic protein 2(BMP-2)cue significantly induced the osteogenic differentiation of MC3T3-E1 cells.FSS stimulation of osteoblasts has also been shown to increase cell adhesion by enhancing the affinity of intracellular integrins to extracellular matrix ligands as well as to biomaterial surfaces.86–87
Tensile strain.To respond to mechanical stimuli,a cell must first adhere to a surface through focal adhesion junctions.Human bone marrow-derived stem cells respond to active mechanical stimulation,where 2%-8% uniaxial strain through tensile stretching resulted in osteogenic differentiation or subsequent bending resulted in both osteogenic and chondrogenic differentiation88–89(Table 2).Tensile strains between 8%and 12%resulted in reduced proliferation as well as increased expression of RUNX2,ALP,Col-I and BMP-2,88,90–91suggesting the promotion of osteogenic differentiation at these strain levels.Studies have also shown that osteoblasts respond to tensile stretch and that<9% stretch strain promotes human osteoblast proliferation,which is strainmagnitude dependent.92–93However,at lower strain levels(<2.5%),the expression of BMP increased while suppressing the expression of transforming growth factor-β(TGF-β),as well as the activity of ALP and secretion of Col-I,92suggesting a reduction in osteoblast activity.Increased levels of osteoprotegerin(OPG),which is an inhibitor of osteoclastogenesis,have also been reported.94When higher strains of 15%were applied,proliferation increased with decreased expression of ALP and RUNX2,demonstrating an inhibitory effect on osteogenic differentiation and suggesting a higher threshold limit.95Interestingly,MSCs experiencing tension at these higher levels also exhibit reducedexpression of adipogenic,chondrogenic,and neurogenic markers such as Col-II,aggrecan,dystrophin related protein 2,and peroxisome proliferator-activated receptor-γ.96When tensile strains between 1 000 and 5 000 μ?were applied to osteoclasts at a frequency of 0.5 Hz,low-magnitude strain levels(2 000 and 2 500 μ?)suppressed osteoclastic fusion and activation,while high strains(5 000 μ?)promoted their fusion and activation.97These results show that varying strain levels have a direct role in regulating both bone-forming and bone-resorbing cells.

Table 1.Studies are presented in increasing order of shear stress and according to cell type
Compression.Compression is also a significant stimulus to bone cells.Dumas et al.98applied dynamic compression at various frequencies to MSCs seeded onto hydroxyapatite ceramic scaffolds.The results showed that compression at 3 Hz caused an upregulation of bone-specific proteins.In contrast,frequencies of 50 and 100 Hz reduced osteogenic differentiation and showed the cells to be responsive to both strain and strain rate(Table 3).Jagodzinski and colleagues99applied cyclic mechanical compression with a maximum strain of 10% to seeded MSCs under continuous perfusion and demonstrated an increase in the expression of RUNX2 and OCN,suggesting that the addition of perfusion to compression promoted osteogenic lineage commitment.Hydrostatic pressure can also encourage osteogenic differentiation.Both static(23 kPa)and dynamic hydrostatic pressures(10 to 36 kPa,0.25 Hz)were capable of inducing osteogenesis in rat bone marrow-derived MSCs.100Using CFD modeling,Anderson et al.101demonstrated that while the osteocyte cell body within the lacunae is exposed to the effects of hydrodynamic pressure,the cell processes within the canaliculi are exposed primarily to shear stress.The shear stress to the processes increases with increasing distance from the cell body.

Table 2.Tensile strain(osteogenic differentiation)

Table 3.Hydrostatic compression
MICROGRAVITY
Alterations in fluid motion
Spacecraft such as the ISS orbit at an altitude of approximately 400 km(250 miles)above the Earth’s surface.At this altitude,gravity is 90%as strong as on Earth’s surface.The spacecraft is in a constant state of freefall,resulting in apparent weightlessness.The difference in gravitational pull on the Earth’s surface and in space has a dramatic effect on surface tension and fluid dynamics.102Surface tension is the attraction of molecules within a fluid toward each other,and this attraction competes with gravity on the Earth’s surface.103Surface tension serves as a cohesive force drawing fluid together,while gravity compels it to dip in the center,eventually pulling the molecules apart.On Earth,gravity distorts the shape when a liquid is resting on or attached to a surface.Under the reduced gravity conditions experienced in space,both hydrodynamic shear and hydrostatic pressure are significantly reduced,and surface tension becomes the dominant force;as such,the molecules stay in tight spheres and films,maximizing intermolecular attraction.Furthermore,surface tension causes droplets of any liquid to form almost perfect spheres in the absence of gravity,and the movement of these fluid spheres and films is much slower when compared with movement on Earth.104
Mass transport and protein aggregation
The reduction in gravitationally induced fluid bioconvection may become critical in microgravity.As described earlier,convective fluid flow exists to dissipate metabolic products via mass transport,in particular the larger molecular weight solutes.As such,a reduced rate of fluid convection and a slower solute diffusion process in microgravity may contribute to impaired heat and biomolecule exchange,conceivably facilitating the preferential transport of smaller solutes.Although the pulsing blood pressure within the larger vessels in animal tissues and the muscle contractions that actively deform bone may serve to drive convective flow,a reduced level of fluid convection could still be an anoxic factor to cells localized within tissue where fluid flow is reduced due to microgravity.Few studies have investigated mass transport in normogravity versus microgravity.Liu et al.37developed a multiscale 3D fluid-solid coupled finite element model and estimated a 2-3 order of magnitude reduction in fluid flow and therefore solute transport within the LCS in microgravity.Furthermore,the transport of the simulated particle load differedbased on the load frequency,with solute transport increasing as the frequency increased.Using a similar computational model,Wang et al.38reported deficient mass transfer within the LCS in a microgravity environment,especially to osteocytes located at a distance from Haversian canals.The authors concluded that reduced mass transport may contribute to microgravity-induced osteoporosis.
In microgravity,particle sedimentation within fluids is significantly limited,and patterns due to changes in density or the tendency of particles to aggregate become more complex and difficult to predict.Mass transport plays a key role in crystal growth,and many in vitro protein crystallization experiments,where crystals of biological molecules are grown from supersaturated solutions,have been conducted to investigate the influence of microgravity.Certain proteins self-assemble into ordered supramolecular structures,such as crystals and filaments,under specific physiological and pathological conditions.105The aggregation process is influenced by two gravity-driven phenomena:sedimentation of the crystals and natural convection in the feeding solution.Among the proteins investigated were the enzyme lysozyme,the protein canavalin and the transport protein serum albumin.106From these early experiments,it was concluded that crystals grown in space were of a higher quality and generally of greater size than ground-based controls.More recently,Martirosyan et al.107demonstrated that when crystals containing different protein aggregate ratios were grown on the ISS,growth occurred principally by diffusional mass transport when compared with ground-based studies.The results showed the generation of additional nucleation events and the formation of altered crystal dimensions and different mean growth rates.It was speculated that the changes seen were due to lower transport rates for larger aggregates in this convection-limited environment.Furthermore,Bell et al.108investigated molecular self-assembly on the ISS and reported that lysozyme protein fibrils showed a distinctly different morphology.The fibrils formed in microgravity were shorter,straighter,and thicker than those formed in ground-based studies.A recent study by Matsushita et al.109demonstrated that microgravity suppressed amyloid fibril formation by reducing the protein?protein interaction via decreased fluid convection.The authors concluded that the cytotoxicity of amyloid fibrils may be reduced and that patients with amyloidosis,a protein metabolism disorder,may be more suitable than healthy people to live in space.These results were supported by Yagi-Utsumi et al.,105who reported significant differences in amyloid formation kinetics and fibril morphology between microgravity-grown and ground-grown Aβ(1-40)amyloids.Protein crystallization and fibrilization occurred much more slowly in microgravity,and the authors speculated that the absence of fluid convection combined with a slower diffusion rate resulted in a decreased fibril growth rate.The gravitational influence on molecular aggregation is still not fully understood,and it is unclear whether weak protein interactions are overcome in microgravity,leading to changes in aggregate concentration or in phase separation.
Biomolecules are unique in their properties,both in terms of size and complexity,and give rise to crystals and filaments that also have unique functions.110The in vitro studies described here highlight that mass transport,the mechanism of biologically relevant macromolecular protein crystal formation,and theself-assembly of fibrils in vitro are altered in microgravity compared with normogravity.However,it remains unclear what role,if any,altered biological macromolecule transport and aggregation may contribute to the tissue dysfunction seen in astronauts in vivo.Interestingly,without the gravity-induced loading vector as a guide,Tauber et al.111reported metabolic alterations in primary human macrophages following long-term exposure to microgravity.The results demonstrated that the transcription,translation and organization of cytoskeletal proteins were altered.Modification of their assembly and formation has also been reported by Tabony and Job.112–113Reduced transcription and translation of cytoskeletal and cytoskeletal-associated proteins in osteoblasts has also been reported.114–115Thus,it is plausible that microgravity-induced alterations in fluid flow may promote homeostatic dysfunction and contribute to underlying diseases initiated in space,including expedited bone loss.
Cytoskeletal changes
Osteoblast cell morphology is significantly altered in microgravity,with an increased cell area and volume and rounder shape reported.116–117Previous studies have theorized that the change in cell size is due to decreased mechanical stiffness and changes in tension within the actin filaments,which promotes cell expansion.118–119Nabavi et al.118reported enlarged nuclei when investigating the influence of microgravity on the nuclei of murine osteoblasts.An increased nuclear size is characteristic of programmed cell death,suggesting that microgravity conditions may promote osteoblast apoptosis.
In normogravity,microtubules self-organize in a pattern of periodic growth in the direction of gravity.112,120The influence of microgravity on microtubule structure was investigated by Nabavi et al..118Murine osteoblasts were exposed to 5 days of microgravity,and the results demonstrated that the bundles formed were significantly shorter and curved compared to the morphologies seen in normogravity(Fig.5).Similarly,Tabony et al.120observed that the microtubules became smaller,formed wave patterns and did not have the same ability to self-organize when compared with cells cultured in gravitational conditions.The authors suggested that the lack of gravity either stunted microtubule growth or created an environment that enhanced microtubule breakage.Hughes-Fulford and Lewis121showed that the number of filaments in the cytoskeleton was reduced following exposure to microgravity,and studies have described the formation of striped patterns.112,122Chen et al.123described a similar phenomenon in the formation of actin filaments under microgravity conditions.In the study,the actin cytoskeleton of rodent bone marrow-derived MSCs was reorganized and redistributed into abnormal patterns under both real and simulated microgravity conditions.After 4 days of microgravity exposure,the actin cytoskeleton of osteoblasts had collapsed,significantly impacting multiple downstream signaling pathways,most notably,inhibiting the BMP signaling axis.115,124Due to their collapse,the actin filaments were unable to regulate this process,impairing the mechanotransduction of external signals and limiting osteogenesis.Di et al.125investigated actin filament formation within murine osteocytes and described their reorganization and distribution toward the cell periphery under microgravity.Other studies have reported that exposure to microgravity decreased the expression of actin and actin-associated proteins,namely,Arp2/3 and RhoA,subsequently resulting in the disorganization of the actin cytoskeleton.126–127

Fig.5 The effect of microgravity on fluid flow within bone and the subsequent cell response reported in the literature.Bone tissue is a complex mechanical environment that provides a specialized habitat for numerous mechanosensitive cell types
A prominent effect of gravity-induced hydrostatic pressure is the adhesive compression of the cell against a rigid substrate or to other cells,where the greater the gravitational force is,the more focal adhesions form.128Studies have demonstrated loss of focal adhesions along the osteoblast surface after just 4 days in microgravity.129–130This loss may serve to hinder the cells’ability to sense changes in the external cell environment,consequently effecting cellular adherence,their migration capacity and viability as well as their response to fluid shear stress and any subsequent bone-forming124or bone-resorbing activity.118
One key cellular process that relies heavily on the cytoskeleton is the cell cycle.Both spaceflight and ground-based simulation studies support the theory of cell cycle arrest following microgravity exposure.129When the cytoskeleton is altered,such as it is in microgravity,cell growth can be blocked at either the G1 phase or G2/M checkpoint.Blocks during the G1 phase occur as a result of actin filament collapse,while G2/M checkpoint blocks are reported to occur as the result of microtubule polymerization complications.Finally,cilia,a key link between fluid flow and the cell response,have also been shown to disappear in osteoblasts under microgravity conditions.The microtubules are reported to be depolymerized within the cilia while in microgravity,which inhibits osteogenic differentiation,maturation,and mineralization of the bone cell.66
Alterations in fluid flow
Although body mass,extracellular fluid volume,and plasma volume are reduced during spaceflight and remain reduced upon landing,the changes in total body water are comparatively small.Microgravity may reduce fluid flow within bone via two mechanisms,cephalad fluid shifts and mechanical unloading,and these changes are proposed as mechanisms that in part cause bone loss in astronauts.
Cephalad fluid shifts.Under normal gravity conditions,a fluid pressure gradient exists extending from the head to the feet,with pressure being the greatest at the feet and lowest in the head.131–132When exposed to microgravity,this pressure gradient is lost,and the body experiences a uniform fluid pressure of approximately 30 mmHg.Loss of this physiological pressure gradient results in an increased pressure in the upper body and above the heart and a decreased pressure in capillaries below the heart.131As a result,blood flow is significantly reduced to the lower extremities with an increased flow to the head,chest,and upper extremities,compared to the body in normogravity.133–134The most easily visible manifestation of this is swelling of the face and thinning of the legs within a short time of exposure to microgravity.131The vasculature adapts,and blood vessels in the upper body undergo hypertrophy,while blood vessels in the lower body undergo atrophy.135Additionally,in microgravity,the overall blood volume decreases due to shifts in fluid toward the interstitial spaces.136Parallel to the nonuniform changes observed in fluid flow and pressure,bone loss during spaceflight is also not uniform within the body.131Collaren et al.137demonstrated that femoral and tibial perfusion was reduced within 10 minutes of beginning hindlimb suspension(HLS)in rats.The blood flow to these bones continued to decrease for the remainder of the 28 days of HLS.The cortical and cancellous masses in the femur and tibia both decreased over the 28-day course.These findings were reversed in the skull,mandible,clavicle,and humerus,which all demonstrated increased blood flow within 10 minutes of HLS and increased mass after 28 days.Interestingly,blood flow to these bones did not continue to increase but returned to normal 7 days after stopping HLS.
Mechanical unloading and reduced interstitial fluid flow.Few studies have investigated changes to interstitial fluid flow within bone when in microgravity,and it remains unclear what range of fluid dynamics stimulate mechanosensory cells to induce an osteoprotective or osteodestructive response.It is conceivable that the range of shear stress necessary to elicit a protective or destructive cell response will be cell-dependent.Due to their activity,exposure of osteocytes,macrophages,and osteoclasts to disadvantageous stresses may be more destructive at the tissue level than the exposure of MSCs and osteoblasts.However,the effects of such extremely low fluid shear stress on bone cells arenot fully understood.Using computational modeling,osteocytes,especially within the deeper layers of the lacunae and away from the Haversian canal,were estimated to undergo apoptosis due to a microgravity-induced reduction in fluid velocity and decreased fluid shear force stimulation,resulting in a reduction in bone mass.121Klein-Nulend et al.138theorized that decreased fluid flow caused by extended periods of unloading may have caused osteocytic disuse throughout the entire bone,leading to the accelerated osteoclastic resorption of bone.Amin139hypothesized that because microgravity causes a decrease in hydrostatic pressure,intramedullary pressures were also reduced,leading to decreased fluid shear forces to osteocytes and ultimately bone loss.Yang et al.140investigated the effect of fluid shear stress on murine osteocyte-like cells,and the results showed that nitric oxide(NO)and prostaglandin E2(PGE2),normally released following the application of shear stress under normogravity conditions,were inhibited in microgravity.Both NO and PGE2 are essential components in the tissue damage cascade,as they signal to enhance cell differentiation and growth.Additionally,this study also reported that three key bone formation biomarkers,ALP,OCN,and procollagen type I N propeptide(PINP),were suppressed or inhibited due to loss of shear stress under microgravity conditions.L-type calcium channels in osteoblasts are stimulated by fluid shear stress,which activates a number of bone formation signaling pathways.Sun et al.141reported that under microgravity conditions,these L-type calcium channels were inhibited in mouse osteoblast-like cells due to an upregulation in microRNA(miR?103),impairing new bone formation.Furthermore,Gao et al.142demonstrated that the osteogenic differentiation of MSCs was significantly reduced when the cells were exposed to extremely low fluid shear stress(0.01 dyn per cm2)compared to higher stresses.In osteoclasts,Gao et al.143showed that osteoclast precursor cells actively migrated toward regions of low-fluid shear stress,and in a later study,they demonstrated that the ratio of tartrate-resistant acid phosphatasepositive mature multinucleated osteoclasts was significantly higher under extremely low-fluid shear stress conditions.144
Although not in the context of bone,it has also been shown that 30 minutes of exposure to a low fluid shear stress(0.1 dyn per cm2)significantly promoted macrophage polarization toward a proinflammatory phenotype.145–146This may be critical,as macrophages and monocytes are immune cells able to directly regulate bone turnover through the release of either proinflammatory cytokines(e.g.,interleukin 1β(IL-1β),interleukin 6(IL-6),nitric oxide synthase,and tumor necrosis factor-α(TNF-α)),leading to bone loss,or anti-inflammatory cytokines(e.g.,interleukin 10,interleukin 13(IL-13),transforming growth factor-β)that promote bone formation and repair.147–148Spaceflight-associated immune system weakening ultimately limits the ability of humans to expand their presence in space.149Anemia and hematopoietic disorders are observed in astronauts,including leukocyte proliferation,a reduced number and activity of T-lymphocytes and natural killer cells,megakaryocyte loss and erythrocyte retention in the bone marrow compartment.150–151Furthermore,the percentage of monocytes and macrophages has been shown to increase under simulated microgravity conditions.152Interferon gamma(IFN-γ)is a potent proinflammatory activator of macrophages,153and IL-4,IL-12,and IL-17 are key activators of inflammation.154–156Notably,after spaceflight and~24 h prior to landing,blood samples collected from 19 astronauts displayed significantly increased plasma levels of IL-4,IL-17,IL-1β,IL-12,TNFα and IFN-γ.157–159The impact of microgravity on immune cell,blood lineage cell,and hematopoietic stem cell(HSC)dysfunction has not been extensively and systematically examined,149and the responses in vivo are largely unknown.In terms of CD34+HSCs,several studies have demonstrated their ability to enhance bone fracture repair.160–161However,the role of HSCs and their combined effect with other elements of the hematopoietic niches in the bone healing process remain largely unknown.162Microgravity has been shown to inhibit HSC proliferation,163potentially due to slower cell cycle progression,in addition to inhibiting HSC migratory abilities,but with no loss in HSC self-renewal capacity.164A microgravity-induced decrease in HSC differentiation to red blood cells has also been reported,165and Shi et al.149showed that microgravity significantly inhibited HSC differentiation to macrophages and impeded M1/2 polarization.The authors demonstrated that this effect involved the RAS/extracellular receptor kinase(ERK)/nuclear kappa-B ligand(NF-κβ)pathway and alterations in cellular metabolism.Hematopoietic stem cells are able to perceive both soluble signals and biomechanical inputs,including fluid mechanical stresses,from their microenvironment and are emerging as critical mechano-regulators of hematopoiesis.151The beating of the heart subjects HSCs to constant hemodynamic forces.In mice,circulating HSCs can experience shear stress that exceeds 600 dyn per cm2in regions of the aortic walls.166However,adult HSCs sheltered in the bone marrow may not be exposed to blood flow directly.The HSC response to fluid shear may occur through the conversion of mechanical signals to protein-level expression via the mechanoresponsive transcription factor YAP.Recently,it was shown that YAP activation and the upregulation of YAP target genes are sensitive to cyclic stretch,and for the first time,a connection between biomechanical cues and YAP in determining HSC fate has been confirmed.167Kruppel-like factor 2,168basic leucine zipper,151and cAMP response element-binding protein169may all also serve as other crucial transcription factors whose expression reflects the onset of fluid shear forces and prompts HSC differentiation.
Indubitably,several cells other than MSCs,HSCs,osteoblasts,osteocytes,osteoclasts,and macrophages have critical responsibilities during healthy bone regeneration and repair(e.g.,adipocytes,myocytes,and endothelial cells).These cells are also highly sensitive to microgravity and undergo morphological,functional,and biochemical changes in this environment.150,170–171Adipocytes contribute to regulating bone formation via the promotion or inhibition of osteoblast and osteoclast differentiation through the expression and secretion of white adipose tissue-derived peptides(including leptin,adiponectin,vesfatin and resistin)and adipocytokines(such as TNFα,IL-6 and IL-1β).172–173Notably,fluid flow has been shown to influence adipocyte maturation and activity.For example,when a physiological fluid shear stress of 10 dyn per cm2at 1 Hz was applied for 1 h to 3T3-L1 murine preadipocytes,adipocyte maturation was suppressed.174Interestingly,at a lower cyclical and continuous fluid shear stress of 0.77 dyn per cm2,human adipose stem cells(hASCs)displayed increased levels of osteogenic differentiation.175Furthermore,hASCs exposed to much lower shear stresses(0.007 9,0.031 3,and 0.078 6 dyn per cm2)and within 3D microfluidic devices showed decreased adiponectin secretion and increased free fatty acid secretion with increasing shear stress.176This study showed that adipogenesis markers were downregulated as the shear stress increased,suggesting that extremely low fluid shear stresses may favor adipose tissue formation.Interestingly,Kim and colleagues177applied a higher shear stress(19.8 dyn per cm2)to hASCs and reported the formation of endothelial cells.Together,these results suggest that fluid shear stress interactions with bone marrow adipocytes induce a mechanobiological response and that the responses can vary widely depending on the shear stresses applied.
Although not a focus in this review,the direct biochemical crosstalk between bone and skeletal muscle as a major driver of bone turnover is considered a novel research field.178During unloading,many skeletal muscle factors(myokines)increase(e.g.,IL-6,follistatin,olfactomedin1,and myostatin)and have detrimental effects on bone by supporting osteoclast formation and inhibitingosteoblast activity.179–180For the first time,Takafuji et al.181reported on the effects of fluid-driven mechanical stress on muscle-derived EV formation during muscle-bone interactions.Interestingly,the study demonstrated that application of a fluid flow shear stress of 6 dyn per cm2to C2C12 cells significantly enhanced muscle cellderived EV secretion that suppressed osteoclast formation and several osteoclast-related gene levels in both mouse bone marrow cells and macrophages.
Together,these studies demonstrate a lack of data;the further elucidation of the pathways involved in regulating bone turnover,including the role of adipocytes,macrophages,myocytes,and the HSC response to changes in fluid flow,is essential to more comprehensively understand healthy and pathological bone adaptation and regeneration in microgravity.The variable outcomes reported thus far indicate an intricate cellular response that demands careful systematic investigation to drive novel and critical therapeutic discoveries.
THE ROLE OF REACTIVE OXYGEN SPECIES IN THE MECHANORESPONSIVE CELL RESPONSE TO FLUID FLOW IN MICROGRAVITY
Bone marrow mainly includes two types of cells with respect to their origin:hematopoietic and mesenchymal.Reactive oxygen species(ROS)critically regulate the fate and function of stem cells in both the nonhematopoietic(e.g.,MSCs)and hematopoietic lineages(e.g.,HSCs),thus tightly controlling bone hemostasis and turnover.182Importantly,oxidative stress is implicated as a major causative factor in osteoporosis.183Exposure to microgravity during spaceflight missions causes excessive ROS production that contributes to cellular stress and damage in astronauts.184–185Oxidative stress causes protein and DNA damage,186induces cell senescence,187triggers osteoblast and osteocyte apoptosis and cell death,188suppresses osteoblastogenesis,and promotes adipogenesis.152,189Furthermore,osteoclasts are reported to be very sensitive to even low levels of ROS.190–192Reactive oxygen species have been shown to decrease osteoprotegerin(OPG)expression and increase RANKL and TNFα secretion,osteoclastic differentiation,193and ultimately bone resorption.193–194This occurs through ERK and NF-κβ activation.Ultimately,these factors disrupt bone regeneration and repair195–196and facilitate osteopenia and osteoporosis.197Notably,many HLS rodent models used to simulate microgravity have reported increased intracellular oxidative stress,including within macrophages,MSCs and osteoblast-like cells.198–199Furthermore,antioxidant treatment of HLS rats in vivo reduced intracellular ROS in macrophages.As a result,osteoclastic activity and bone density loss were significantly reduced,and bone structure and mechanical strength were preserved.198–200
The correlation between changes in fluid flow and ROS formation remains elusive.Several elements of the mechanotransduction cascade(e.g.,ion channels,integrins,cytoskeletal network,receptor kinases,and membrane lipids201)are redox-sensitive,and the effect of ROS is likely disparate in each cell type.However,extremely few studies have investigated varying fluid flow and the subsequent ROS generation within bone cells,and this concept remains largely unexplored.Studies have investigated skeletal muscle cells and MSCs and demonstrated that varying the levels of applied stretch directly influenced whether a pro-or anti-ROS response was observed.202–204Although not skeletally related,low fluid shear stress further increased endothelial intracellular ROS,205–206downregulated ROS scavengers in endothelial cells,207–208increased endothelial inflammatory(TNFα and IL-1β)cytokine release via NF-κβ,206,209–210and caused apoptosis.211In monocytes,flow-induced activation was shown to be regulated by ROS signaling,212and upon activation by low fluid shear stress,these cells differentiated into inflammatory macrophages when investigated in a renal fluid shear model.146Interestingly,Qin et al.213recently demonstrated that low fluid shear stress facilitated the phagocytosis of EVs by vascular endothelial cells in vitro and in vivo and suggested that areas of low magnitude shear stress may provide a theoretical basis for the development of EV-based nanodrug delivery systems in vivo.Similarly,new approaches in clinical treatment are advancing,including the development of numerous novel mechanosensing carriers such as liposomes or microaggregates able to sense changes in shear force and respond by releasing biomolecules during mechanics-targeted drug delivery.214–215Nevertheless,the studies described highlight the important need for further investigation into the role of fluid shear stress-induced ROS generation and its potentially direct relationship to the accelerated osteoporosis and dysfunction developed in microgravity.By exploiting fluid shear-mediated ROS pathways,there may be significant therapeutic potential for the treatment of diseases where oxidative stress plays a central role,such as osteoporosis.
FLUID FLOW WITHIN OSTEOPOROTIC BONE AND THE POTENTIAL ROLE OF ADIPOSITY IN NORMOGRAVITY AND MICROGRAVITY
Both osteoporosis on Earth and disuse-induced osteoporosis in microgravity progress by inducing a gradual transformation in bony macro-and microarchitecture where the interconnecting porous system is slowly resorbed.The porosity increases,and the pores enlarge with significant diversification in pore shape.These architectural alterations in pore number,size and shape,as well as an increased adiposity and viscosity within the bone marrow,not only affect the strength and stiffness of bone but also potentially modify fluid flow and the subsequent mechanical stimulus to cells(Fig.6).Fat accumulation has not been verified in astronauts;however,bone marrow adiposity increased in rodents during spaceflight,216–217and the causal relationship between increased adiposity and bone loss remains unclear.During spaceflight,functional hematopoietic tissue was replaced by marrow adipose tissue(MAT)216in rats.Although MAT adipocytes may play a supporting role in CD34+HSC proliferation218and in the regeneration of MSCs and hematopoiesis,219increasing MAT accumulation within bone is associated with a loss of HSCs,bone marrow dysfunction,high levels of ROS and proinflammatory cytokines,and the impairment of bone regeneration.220–221Microgravity-induced brown adipose tissue may further contribute to the heightened metabolic dysfunction reported.222–223Thus,MAT appears to have both osteoprotective and osteodestructive effects.Remarkably,Keune and colleagues216reported that compared with ground controls,rats flown in space had a 32%lower cancellous bone area and 306% higher level of marrow adiposity.The increased adiposity was due to an increase in adipocyte number(224%)and size(26%).Interestingly,the authors reported no change in bone formation over the 14-day spaceflight.Zhang et al.224demonstrated that under space microgravity,hMSCs favored adipogenesis over osteogenesis.These studies suggest that an overriding and dysfunctional response from MSCs,and potentially macrophages(via adipokine/proinflammatory cytokine release225)and osteoclasts,contributes to bone loss in microgravity.These alterations in bone architecture and marrow adiposity could limit new bone formation and favor bone resorption.33,226
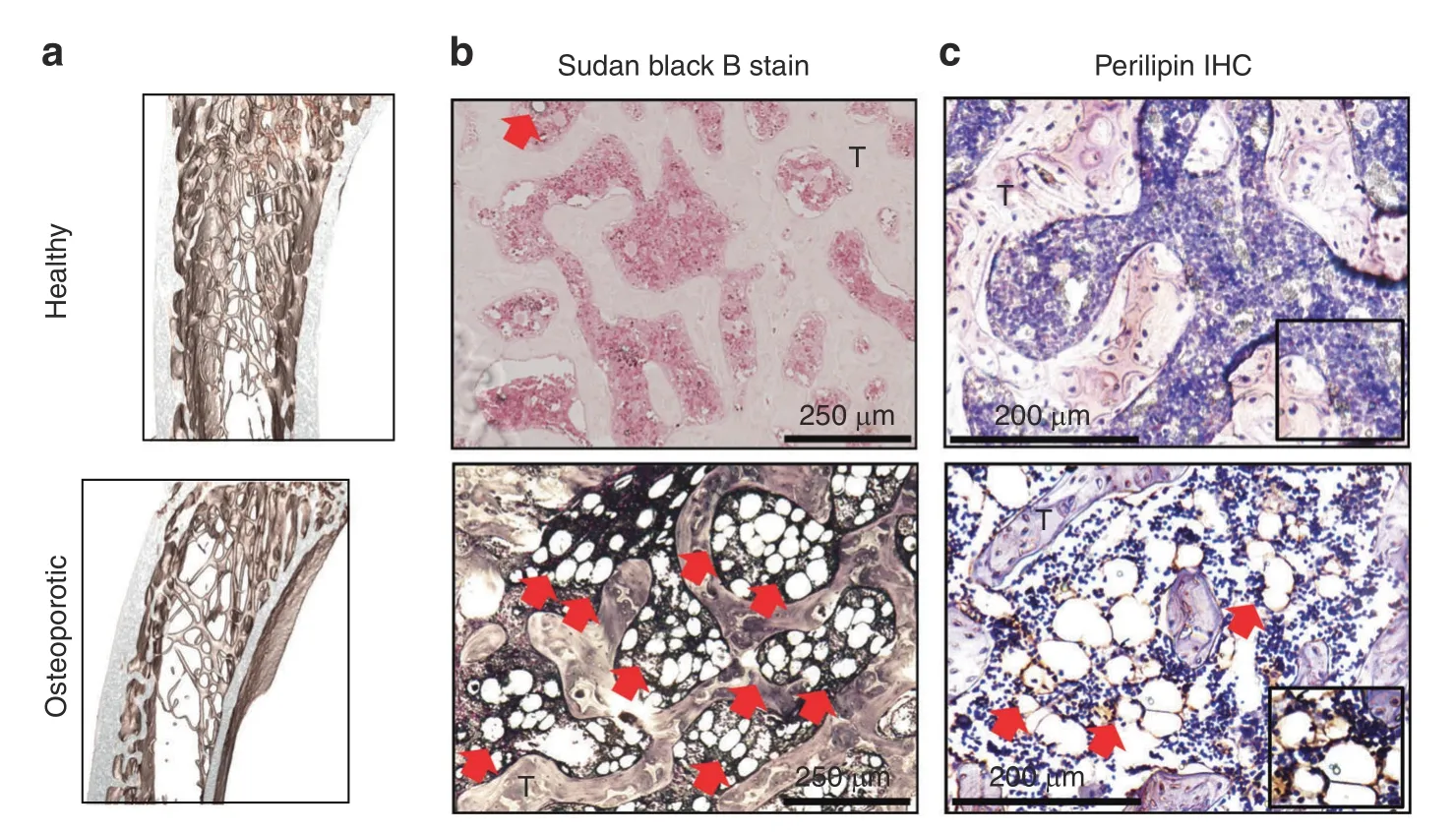
Fig.6 Images of healthy and osteoporotic bone in a rodent model developed in normogravity.A greater level of adiposity is observed within the osteoporotic architecture.a MicroCT images showing that the trabecular architecture is slowly resorbed,leading to alterations in pore number,size and shape.b Histological images demonstrate narrowing of the trabeculae(T)and the generation of an increased level of lipids in osteoporotic bone compared with healthy tissue.Sudan Black B stained phospholipids(gray)and intracellular lipids(black).c Perilipins are found exclusively on adipocytes,and using immunohistochemistry(IHC),a greater amount of positive staining is observed within the marrow of osteoporotic bone.Positive lipid staining is indicated by red arrows
As osteoporosis progresses,the mechanical environment within bone becomes increasingly complex.It is theorized that in normogravity,deformation of the pores during loading induces motion in the fluid-like marrow,resulting in the generation of pressure and velocity gradients.46Velocity gradients result in shear stress and tensile strain acting between the components of the marrow(Fig.7).Using a computational fluid-structure interaction CFD model,Birmingham et al.44demonstrated that in normogravity and under physiological loading conditions,the lower bone mass induced by osteoporosis resulted in an increase in fluid shear stresses to cells within the marrow.However,their results also estimated that due to the increased adipocyte formation that occurs as osteoporosis progresses,the viscosity of the bone marrow also decreases.This in turn reduced the shear stresses to cells,counteracting the increased architecturally induced stress measured.Furthermore,in one study,Metzger and colleagues227used CFD and cyclic compression to estimate the shear stress to cells in the marrow.The results demonstrated that shear stress levels were amplified as the osteoporotic architecture deteriorated,with over 90%of nonadipocyte cells experiencing higher levels of shear stress.However,the maximum shear stress decreased by 20% when the more viscous osteoporotic marrow content was modeled.Similarly,Vaugh et al.45developed a multiscale finite element model and demonstrated that a reduced bone volume resulted in an overall increase in bone deformation,leading to increased stimulation via microstrain to cells.Furthermore,an increased adipocyte content in the marrow resulted in lowering the microstrain levels to cells within the bone marrow,reportedly due to a shielding effect caused by the more compliant behavior of adipocytes.Despite this,the estimated levels of strain to cells remained much higher in the osteoporotic architecture;however,compensatory mechanobiological responses such as increased trabecular thickness and the axial alignment of trabeculae were effective in returning normal levels of microstrain to cells.In contrast,a poroelastic finite element analysis study by Gatti et al.33showed reduced interstitial fluid flow within the LCS in osteoporotic rats when compared with healthy architecture.Theinfluence of microgravity on fluid flow within bone has also been reported.Zhao et al.228developed a 3D axisymmetric fluid-solid finite element model of bone with a two-stage pore structure.The results demonstrated a reduced fluid flow rate of up to 32.19% and that fluid shear stress decreased from~2.0×10?4Pa in normogravity to 1.6×10?7to 6.0×10?8Pa within the LCS in a microgravity field;this represents a decrease of 99.92%and 99.97%,respectively.The results also estimated that the flow velocity increased with gravitational acceleration.Nevertheless,these studies suggest that marrow viscosity and changes in the bone fraction volume,both within the larger pores and smaller LCS system,can directly affect mechanobiological signaling within bone.These studies also estimate that fluid transmission and the shear stresses to cells within the LCS are significantly dependent on the gravitational fields.

Fig.7 a A schematic demonstrating alterations in the stresses derived by fluid flow in healthy and osteoporotic bone.As fluid flows within the porous bone network,it imparts pressure,shear stress and tensile strain on cells within the local environment.These fluid-induced stresses are influenced by the degree of pore curvature,surface topography,stiffness and level of adiposity.The increased marrow adipocyte content that occurs in microgravity may lower the fluid-induced microstrain levels to the cells within the bone marrow,and this effect may be further amplified in microgravity as fluid flow is estimated to be reduced by up to 99.97%.228 It is conceivable that a reduced interstitial fluid flow combined with increased adiposity contributes to the accelerated bone loss observed in microgravity.b Using CFD modeling,disparate levels of fluid velocity were measured within the structure of healthy versus osteoporotic rat bone.Notably,the contribution of adiposity was not modeled.While these models identify changes in fluid flow within trabecular bone under these two conditions,more complex analyses are essential to determine the role of increasing adiposity and its role,if any,in shielding cells against fluid shear stress and accelerating bone loss
IN-ORBIT EXPERIMENTATION
Experiments in orbit are rare and extraordinarily costly,with many logistical challenges to overcome,and as a result,studies that investigate the biological effects of spaceflight are limited.Although several forms of ground-based devices have been designed to simulate weightlessness and the effect of microgravity on cells,simulated gravity still differs from the real state of microgravity during spaceflight.This is of particular relevance when considering the influence of fluid-imposed stresses and strains on cells.Under normogravity conditions,cells cultured in standard static conditions experience atmospheric pressure and hydrostatic pressure from the surrounding culture medium.Ground-based simulators such as the 1,2 or 3D clinostat,rotating wall vessel,random positioning machine or use of diamagnetic levitation all directly or indirectly impact the fluid flow and/or stresses and strain imposed at the cellular level.Methods such as high-gradient magnetic fields(and subsequent force),vibration,centrifugal or rotational movement offer microgravity simulations that involve accelerated fluid motion where cells experience convection and flow-induced shear,friction,and other complex forces due to the movement of fluid molecules and cells against one another.229–230Such forces will alter the cell-strain response,making the interpretation of results confounded and limited.The use of parabolic flight to achieve microgravity introduces interrupted moments of zero gravity,each lasting approximately 22 seconds.The control of fluid flow and subsequent changes in cell strain cannot be assessed within such short periods.Biological studies in parabolic flight are further complicated by the frequent change in gravity,which will expose both fluid and cells to a broad range of gravitational forces,making it impossible to decipher changes due to microgravity alone.
An additional and significant challenge facing scientists is the description and quantification of the migration forces and velocities at the single-cell level that occur during the rapidsedimentation of cells.For example,the density difference between red blood cells and plasma is approximately 0.1 g·cm?3,which leads to sedimentation velocities of several μm·s?1for cells with a diameter of 7-8 μm.231Therefore,subtle hydrodynamic effects under either gravity or microgravity are difficult to measure.At the cellular scale,tissue fluidity and mass transport depend on the dynamics of the cells in fluid flow,specifically on their deformation and orientation and the electrostatic forces that attract them together.These dynamics are governed by cellular rheological properties,such as internal viscosity and cytoskeleton elasticity.In diseases in which cell rheology is altered or the microenvironment is changed,tissue fluid flow may be severely impaired.The nonlinear interplay between cell rheology and flow may generate complex dynamics,which remain largely unexplored.Computational fluid dynamic models and technologies able to mimic cells and their flow and behavior are emerging.With a better understanding of 3D-based fluid mechanics at the micrometer-length scale,a new generation of experimental tools that provide control over cellular microenvironments able to emulate physiological conditions with exquisite accuracy offer an exciting and promising solution.
DISCUSSION
In recent years,our scientific interest in spaceflight has grown exponentially and resulted in a thriving area of research,with hundreds of astronauts spending months in space.Hypothetically,alterations in fluid shear stress,compression and tensile strain may alter mechanotransduction by reducing the mechano-stimulus to cells.Studies reveal that exposure to microgravity is estimated to result in a 99.97%decrease in shear stress to cells in the LCS and that this extreme condition causes loss of cilia and focal adhesions,stunted growth of microtubules and collapse of actin filaments.This dysfunction may be further amplified and expedited through increased adiposity developing within the bone marrow,shielding the cells from essential levels of mechanostimulation.Furthermore,reduced fluid flow with limited bulk convection may reduce larger solute transport,impact crystal and filament formation and cause unwanted biomolecule folding or aggregation,potentially leading to the creation of chronic long-term bone disease.It is conceivable that an independent and/or synergistic combination of these components may ultimately lead to reduced osteogenesis,increased osteoclastic resorption and the significant loss in bone structure,volume and strength witnessed in humans and animals when in microgravity(Fig.8).
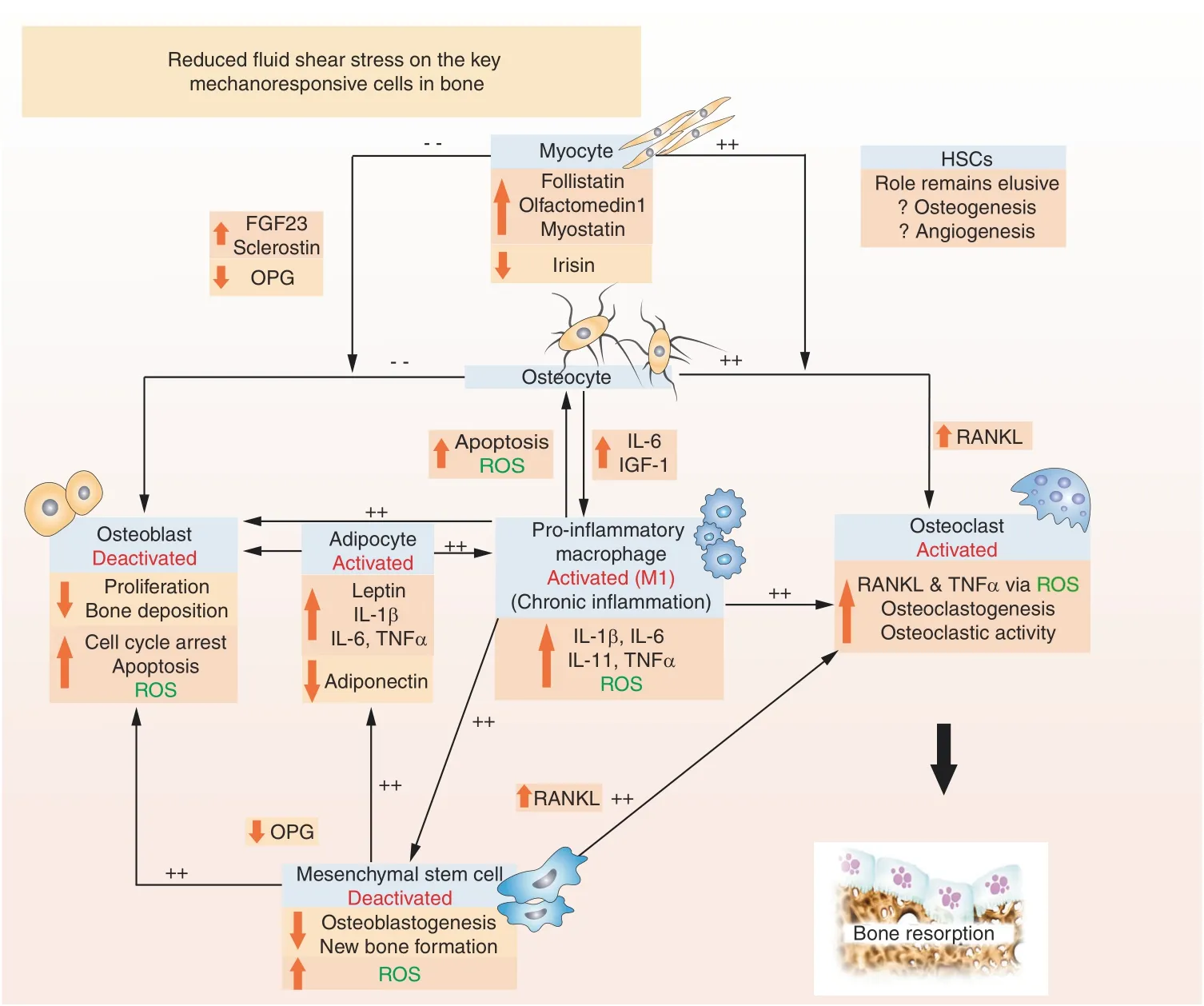
Fig.8 Based on the current literature,it is reasonable to speculate that extremely low fluid shear stresses induced via microgravity,alterations in osteoporotic bone architecture,and increased adiposity will together guide cells toward a phenotype that results in osteodestruction.Hypothesized mechanisms of bone loss due to extremely reduced fluid shear stress:osteocytes release more RANKL than OPG,thereby activating osteoclastic activity and a proinflammatory macrophage phenotype.The reduced OPG and increased proinflammatory cytokine release(e.g.,IL-1β,IL-6,and TNFα)and ROS suppress the osteogenic differentiation of MSCs as well as the proliferation and deposition of new bone by osteoblasts.Increased myostatin and decreased irisin expression further activate osteoclastic activity and inhibit osteoblastic activity.MSCs preferentially differentiate into adipocytes(via increased leptin and decreased adiponectin,for example)while secreting proinflammatory cytokines that contribute to maintaining the M1 macrophage phenotype.It is conceivable that the concerted response of osteocytes,osteoclasts,macrophages,adipocytes,myocytes,and MSCs,among other key cells not shown(e.g.,fibroblasts and endothelial cells),will serve to keep cells within a cycle of osteodestruction.The role of mechanoresponsive HSCs remains unknown.Experimentally unconfirmed associations are labeled in green
This review demonstrates that many questions remain.How and to what extent do changes in interstitial fluid flow and mass transport influence dysfunction?To what extent is reduced fluid velocity a contributing factor to bone loss within the osteoporotic microarchitecture?How soon does adiposity start to develop?It is known that it can take many years for bone mass to return when astronauts return to Earth,which suggests that the application of a“healthy”flow may not be able to restore dysfunctional cells to a normal phenotype.As commercial flights to space become accessible in the near future,many more humans will be exposed to space,conceivably with many in disparate states of health.A more sophisticated understanding of altered fluid flow and the role of gravity will undoubtedly accelerate new health safety strategies and solutions to the scientific challenges that remain,supporting both human exploration of space and human health on Earth.
ACKNOWLEDGEMENTS
This study was internally funded.Author J.A.’s work was supported by the National Aeronautics and Space Administration[grant No.80NSSC21M0309]issued through the NASA Office of STEM Engagement.
ADDITIONAL INFORMATION
Competing interests:The authors declare no competing interests.
- Bone Research的其它文章
- Current understanding of osteoarthritis pathogenesis and relevant new approaches
- Biomechanics and mechanobiology of the bone matrix
- Can intracellular Staphylococcus aureus in osteomyelitis be treated using current antibiotics?A systematic review and narrative synthesis
- Insights into skeletal stem cells
- Single cell analysis reveals inhibition of angiogenesis attenuates the progression of heterotopic ossification in Mkx? /?mice
- FABP4 secreted by M1-polarized macrophages promotes synovitis and angiogenesis to exacerbate rheumatoid arthritis

