Indocyanine green dye and its application in gastrointestinal surgery: The future is bright green
Zavier Yongxuan Lim, Swetha Mohan, Sunder Balasubramaniam, Saleem Ahmed, Caroline Ching Hsia Siew,Vishal G Shelat
Abstract Indocyanine green (ICG) is a water-soluble fluorescent dye that is minimally toxic and widely used in gastrointestinal surgery. ICG facilitates anatomical identification of structures (e.g., ureters), assessment of lymph nodes, biliary mapping, organ perfusion and anastomosis assessment, and aids in determining the adequacy of oncological margins. In addition, ICG can be conjugated to artificially created antibodies for tumour markers, such as carcinoembryonic antigen for colorectal, breast, lung, and gastric cancer, prostate-specific antigen for prostate cancer, and cancer antigen 125 for ovarian cancer. Although ICG has shown promising results, the optimization of patient factors, dye factors, equipment, and the method of assessing fluorescence intensity could further enhance its utility. This review summarizes the clinical application of ICG in gastrointestinal surgery and discusses the emergence of novel dyes such as ZW-800 and VM678 that have demonstrated appropriate pharmacokinetic properties and improved target-tobackground ratios in animal studies. With the emergence of robotic technology and the increasing reporting of ICG utility, a comprehensive review of clinical application of ICG in gastrointestinal surgery is timely and this review serves that aim.
Key Words: Fluorescence imaging; Gastrointestinal surgery; Ⅰndocyanine green
INTRODUCTION
Indocyanine green (ICG) was first developed during World War II for colour imaging, and later in the 1950s, used in the medical field to quantify cardiac and renal function. It is a minimally toxic, water-soluble fluorescent dye that is rapidly taken up by the liver and excreted into the bile ducts within minutes after injection, making it ideal for such applications[1,2]. ICG is a favourable contrast agent forin vivoapplication due to its 820 nm near-infrared (NIR) emission wavelength, minimising interference from blood and tissue autofluorescence at 500-600 nm[3]. After intravenous injection, ICG binds to plasma proteins and has a half-life of three minutes. As the lymph is rich in protein content, lymphatics and lymph nodes (LNs) can be easily mapped after ICG injection. In general, ICG is safe at doses below 0.5 mg/kg body weight, however adverse reactions like nausea, pyrexia, and anaphylaxis may occur[1-3].
As early as 1959, ICG quantification was used to assess hepatic function. Given ICG’s affinity for the blood, ICG levels in the blood corresponded directly with hepatic function[2]. It was also used to determine cardiac output, and for videoangiography for assessment of choroidal neovascularization[4,5].
Recently, fuelled by the emergence of robotic technology, ICG has gained widespread usage in the identification of tumours, lymphatic mapping, and evaluation of organ perfusion and anastomosis[6]. With its increasing application in general surgery, novel uses for ICG are continuously being uncovered. Therefore, the present review aims to provide a summary and critical analysis of the established applications of ICG in general surgery, as well as emerging avenues for future research and development.
METHODOLOGY
An electronic search of PubMed (MEDLINE), Embase (Ovid), and Google Scholar was performed for the concepts of (“Indocyanine Green” [MeSH Terms]), (“Esophagus” [MeSH Terms]), (“Stomach” [MeSH Terms]), (“Liver” [MeSH Terms]), (“Gallbladder” [MeSH Terms]), (“Pancreas” [MeSH Terms]), (“Adrenal Glands” [MeSH Terms]), (“Spleen” [MeSH Terms]), (“Intestine, Small” [MeSH Terms]), (“Colon” [MeSH Terms]), (“Rectum” [MeSH Terms]), (“Peritoneum” [MeSH Terms]), (“Blood Vessels” [MeSH Terms]), (“Abdomen” [MeSH Terms]), (“General Surgery” [MeSH Terms]) in January 2023. Relevant articles published in English were identified and summarised to produce an up-to-date review on the history, present and future use of ICG in abdominal surgery. We discuss clinical application of ICG in individual organs with a cranial to caudal approach of human anatomy.
RESULTS
Oesophagus
Lymphatic mapping in oesophageal cancer: Oesophageal cancer is a biologically aggressive disease with poor prognosis despite treatment, endoscopic or surgical, with the intent to cure[7]. Lymphadenectomy significantly improves accuracy of tumour staging and impacts long-term survival of patients with oesophageal cancer. However, at present, most lymphadenectomies are performed based on anatomical territory understanding and surgeons’ experience and expertise with wide variation in the extent of nodal harvest. Current American Joint Committee on Cancer (AJCC) guidelines recommend the removal of ≥ 20 LNs for T2 disease, or ≥ 30 for T3 and T4 disease, while National Comprehensive Cancer Network guidelines recommend the removal of at least 15 LNs to ensure adequate nodal staging[8,9].
Studies have proposed the use of radiocolloid tracers for sentinel LN (SLN) mapping, but these largely require open procedures with back table dissection of the specimen and radiation exposure[10]. Radioisotope methods are unable to predict locations of primary SLNs perioperatively with high accuracy. This can be attributed to poor spatial resolution and low detail regarding surrounding anatomy, for reasons including the shine-through phenomenon, where the radiation flare of the primary tumour outshines the SLN near to the primary tumour[11,12]. A feasibility study by Yuasaet al[12] proposed the use of NIR fluorescence imaging (FI) using ICG, together with preoperative computed tomography (CT) lymphography for SLN localisation[10]. This involved the injection of ICG in 2 regions around the tumour after thoracotomy, and the oesophagus and LNs that fluoresced were harvested.
A first in human pilot trial by Hacheyet al[10] demonstrated the feasibility of using NIR guided lymphatic mapping as the sole modality for SLN identification in minimal access oesophagectomy. Regional LNs distinct from the oesophagus specimen were identified in 66.7% (6/9) of the patients where ICG diluted in human serum albumin (HSA) was used, as compared to 40% (2/5) of the patients with ICG only. In both groups, ICG was injected peritumourallyvia4-corner submucosal injections adjacent to each lesion[10]. The dilution of ICG with HSA increases the quantum yield, which is the efficacy at which fluorescent molecules convert absorbed photons into emitted photons, and also the SLN retention[13,14]. Furthermore, the combination of ICG with neomannosyl HSA, which targets the macrophage mannose receptor CD206, was trialled by Kimet al[15]. This combination was used on porcine models for oesophageal SLN identification and demonstrated higher fluorescence signal, LN retention and allowed for more precise real-time SLN detection in surgery. The use of ICG for lymphatic mapping may allow for targeted lymphadenectomy, decreased operative time, and hence decreased postoperative complications while ensuring the completeness of resection and improving cancer-free survival[16].
Evaluation of oesophago-gastric anastomosis: The evaluation of gastrointestinal-oesophageal anastomosis is the most common application of ICG FI for oesophageal pathologies. There is significant postoperative morbidity and mortality associated with anastomotic leak (AL) post-oesophagectomy. A major factor contributing to oesophago-gastric AL is ischaemia at the tip of the gastric conduit, due to insufficient perfusion from the isolated right gastroepiploic artery[17]. Figure 1 below illustrate this. Therefore, the use of ICG FI intra-operatively to assess perfusion can be valuable as it allows for live monitoring of conduit perfusion, early detection of reversible conduit ischaemia, and hence better selection of the optimal site for anastomosis. Other optical techniques such as optical coherence tomography and NIR spectroscopy have been assessed by authors, but ICG remains the most widely used given the safety, reliability, and ease of use[18].
However, the use of ICG fluoroscopy for assessment of perfusion does not provide surgeons with a quantitative assessment of perfusion but is instead estimated based on the time from initial ICG enhancement at the root of the gastroepiploic artery until gastric tube tip. Nomaet al[19] suggested that anastomosis be performed proximal to the point of fluorescence reached in 30 s, or the 90 s rule established by Kumagaiet al[20]. Nomaet al[19] reported significant reduction in leakage rate and duration of postoperative intensive care unit (ICU) stay for the ICG group, with no increase in other complications such as pneumonia. In a meta-analysis including 5 studies and 616 patients, Slooteret al[21] concluded that ICG reduces the risk of AL and graft necrosis [odds ratio (OR) = 0.30, 95% confidence interval (CI): 0.14-0.63]. Based on this, we computed the number needed to treat (NNT) for ICG to reduce 1 case of AL or graft necrosis as 6.6 oesophagectomies.
Identification of chylothorax post-oesophagectomy: Besides the use of ICG in oesophageal surgery for assessment of perfusion, a new and upcoming use of ICG is for the detection of chyle leak post-oesophagectomy. The incidence of chylothorax ranges from 1.1%-21% in oesophagectomy patients, with extensive LN dissection anden blocresection of the thoracic duct for oncological reasons as risk factors[22]. Traditionally, the ingestion of milk immediately before surgery, or the intraoperative administration of milk into the duodenum were techniques used to identify the site of chyle leak[23].
Kaburagiet al[24] however reported the successful use of intraoperative ICG fluorescence lymphography for the identification of the chyle leak, and to confirm ligation of the thoracic duct transabdominally. Kamiyaet al[23] similarly achieved this through the injection of 1.5 mL of ICG subcutaneously at the inguinal region bilaterally, and obtained fluorescence images of lymph flow 14 min after injection using a NIR camera. This is in contrast to other techniques such as lymphoscintigraphy, which can identify chyle leak, but cannot delineate the exact site of leak without the use of a single-photon emission computerized tomography scan[25]. Management of the chyle leak reduces the need for postoperative nutritional interventions, infectious morbidity, and reduces the length of hospital stay[22].
Stomach
ICG guided LN dissection: Gastrectomy with D2 lymphadenectomy is a technically demanding surgery requiring experience and expertise to achieve radical lymphadenectomy. With advances in minimal access technology, adoption of training curricula and fellowship programs, laparoscopic gastrectomy is routine in many institutions. ICG can help to improve LN harvest while minimizing complications. Chenet al[8] reported a randomized control trial with 266 gastric cancer patients comparing ICG use in gastrectomy with conventional gastrectomy. The ICG group had significantly greater LNs retrieved compared to the non-ICG group (49.6 LNsvs41.7 LNs respectively;P< 0.001). In addition, in a matched cohort study of 37 patients who underwent robotic gastrectomy with D2 LN dissection demonstrated higher mean total number of harvested LNs in the ICG group than the control (50.8vs40.1,P= 0.03)[26]. Higher nodal yield aids accurate staging and potentially contributes to improved survival outcomes. The iGreenGO study is a prospective multicentre study which seeks to determine if the use of ICG necessitates a change in surgical conduct, such as performing more extensive dissection after the surgeon has already completed D2 lymphadenectomy without ICG aid[27]. ICG remains a useful surgical adjunct for a surgeon early in their learning curve and for advanced gastric cancers.Sentinel LN mapping: The stomach has a complex lymphatic drainage system. Gastrectomy with D2 lymphadenectomy remains the gold standard for resectable gastric cancer, however this has higher morbidity than D1 lymphadenectomy therefore may be excessive in clinical T1/T2 N0 gastric cancers where LN metastasis maybe limited. SLN mapping may be a solution to this conundrum where radical lymphadenectomy may be carried out only if SLN is positive. In a prospective multicentre trial by Kitagawaet al[28], 397 patients underwent SLN biopsy (SLNB), and the method showed high accuracy in detecting sentinel nodes and metastatic SLNs, with a false negative rate of 1%. Future studies should compare long-term oncologic outcomes of SLN guided surgeryvsconventional surgery, but this has the potential to change surgical management of gastric cancer as what SLNB has done for breast cancer surgery.
Localisation of gastric tumour to guide resection in early gastric cancer: Early gastric cancer may not be visible to the surgeons on the serosal surface. Injection of ICG submucosally around the tumour will emit fluoresce on the serosal surface and aid to ensure adequacy of resection margins when performing subtotal gastrectomy. In a retrospective study including more than 500 patients with early gastric cancers in the body of the stomach, Choet al[29] demonstrated that ICG diffusion area along the gastric wall secured a resection margin of > 28 mm.
Leak tests after sleeve gastrectomy and other anastomosis based bariatric surgeries: ICG has been used by bariatric surgeons for leak test after sleeve gastrectomy and other bariatric surgeries. ICG is instilledvianasogastric or orogastric tubes after the sleeve gastrectomy or after anastomosis is completed. Kalmaret al[30] reported a sensitivity of 100.0% and specificity of 98.3% for ICG based leak tests. Hagenet al[31] reported a series of 95 patients who had Roux-en-Y gastric bypass who had leak tests with air and with a mix of methylene blue and ICG. In their series, no patients had a positive leak test with air, no patients showed methylene blue excretion, and an ICG leak was observed in 4.2% (4/95) patients, suggesting that ICG maybe more sensitive for small ALs. These results need to be validated by others.
ICG in revisional bariatric surgery: ICG has proven it’s utility in revisional bariatric surgery. Anatomy of the stomach is distorted in cases of previous gastric surgery especially if complications such as ulcers or perforations have occurred. In addition, in cases where records of previous surgeries are also not available makes deciphering the exact procedure the patient had underdone, vascular pedicles takenetc. challenging. This makes the surgery technically challenging with potential for increased morbidity. ICG helps to highlight areas of poor vascularity, identify old staple lines to enable better surgical planning to prevent crossing of staples lines, leaving blind gastric pouches and performing anastomosis in areas of good vascularity[32].
Liver
Tumour visualization: Hepatectomy remains the gold standard in treatment of liver malignancies and some benign masses. However, the key to a successful oncological resection is negative margins, which requires clear segment demarcations based on vascular and lymphatic supply[33].
ICG is typically administered intravenously several hours or days before surgery and will be taken up by hepatocytes, which illuminate under an infrared source. ICG is then excreted in the bile and disappears from healthy hepatocytes within a few hours before the surgery begins. However, as the cancerous hepatocytes are underactive and metabolize the ICG slowly, these will be the only areas that illuminate during the operation. Figure 2 shows the use of ICG for the resection in a patient with hepatocellular carcinoma.
In non-hepatocellular cancers, the areas around the tumour will retain the ICG instead. This is termed tumour and peritumoural fluorescence and helps differentiate between hepatocellular and non-hepatocellular cancers intraoperatively[34]. However, since ICG is metabolized by the liver, further studies need to be conducted with regards to dose adjustment for cirrhotic patients, who constitute a large proportion of liver cancer patients[35].
In addition, in a prospective study of 54 patients who underwent robotic assisted liver resections with ICG demonstrated that ICG use decreased operative time and achieved more resections with no histopathologically proven macro- or microscopic tumour residual[36].
Liver function assessment: Proper patient selection is vital for hepatectomies as even healthy patients without underlying liver disease can have severe postoperative liver dysfunction. For patients with pre-existing liver disease, even a minor resection could lead to posthepatectomy liver dysfunction or failure. ICG clearance has been noted as a valuable tool to identify patients that are at risk of developing posthepatectomy liver failure (PHLF)[37,38].
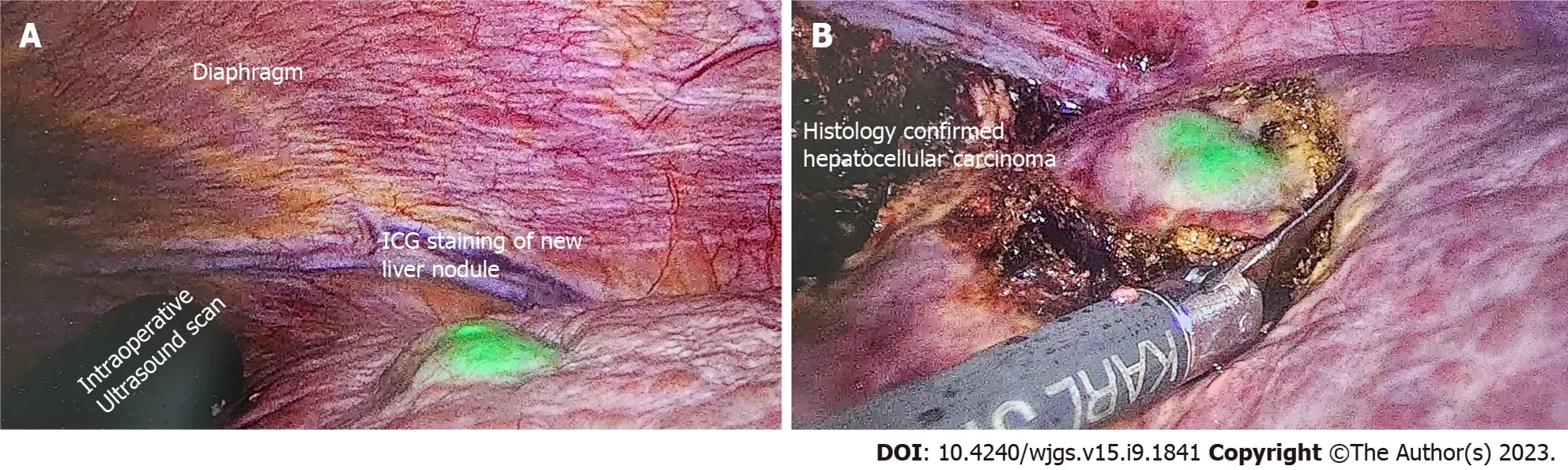
Figure 2 A 84-year-old patient with imaging showing 7 cm hepatocellular carcinoma was scheduled for elective laparoscopic right posterior sectionectomy. Indocyanine green dye was injected 10 d before the surgery date. A: Cirrhotic liver with a new liver lesion detected by positive indocyanine green (ICG) staining; B: Excision of this nodule with adequate margins as guided by ICG. Postoperative histology confirmed the new nodule to be primary hepatocellular carcinoma. ICG: Indocyanine green.
The ICG retention test after 15 min (ICG-R15) is used conventionally. A single bolus of ICG is administered intravenously, and venous blood samples are drawn and read with a pulse spectrophotometer at 15 min[39]. Literature suggests that ICG-R15 of more than 14% is prognostic of PHLF[38-40]. A study by Schwarzet al[37] comprising 698 patients similarly showed that patients with impaired ICG clearance were twice as likely to have postoperative liver dysfunction. A recent retrospective study however highlighted that in patients treated with associating liver partition and portal vein ligation for staged hepatectomy, ICG-R15 overestimated the true liver function increase post-operatively[41]. These results remain to be validated, and are essential in tailoring treatment to prevent PHLF.
Liver cyst: Several studies have reported the use of ICG FI for liver cyst fenestrations performed laparoscopically. Uneet al[42] reported the successful implementation of ICG FI to allow for clear distinguishment of cyst from liver parenchyma to guide resection. Hanakiet al[43] also reported that ICG FI allowed for visualisation of small bile ducts located within the cyst wall to decrease the risk of bile leaks and prevent iatrogenic bile duct injury (BDI). Authors injected ICG intravenously 1-h prior to surgery. In addition, ICG can be administeredviaendoscopic nasal biliary drain during hepatic cyst deroofing procedures to allow for immediate visualisation, and can also allow for assessment of minor biliary leakage from resection margins or staple lines, preventing postoperative biliary leakage[44]. Figure 3 illustrates the use of ICG FI for liver cyst deroofing.
Gallbladder
Biliary mapping during laparoscopic cholecystectomy: Laparoscopic cholecystectomy is one of the most frequently performed operations worldwide. BDI is an uncommon but significant complication associated with cholecystectomy as it reduces patients’ quality of life and exposes surgeon to litigation[45]. The common cause of BDI are misidentification of anatomy, severe scarring and fibrosis due to chronic pathology and surgical experience. In estimated 10%-15% patients, it is not possible to obtain critical view of safety to expose Calot’s triangle and a surgeon has to determine the next course of action that may include calling for help[46] and conversion to a bail-out procedure like subtotal cholecystectomy[47]. ICG NIR fluorescence instead provides detailed and real time anatomical mapping of the biliary structures to reduce BDI risk[48]. Yonget al[49] highlighted in his case study of a 40-year-old male undergoing laparoscopic cholecystectomy, that the cannabidiol (CBD) and cystic duct were only discernibleviaICG FI and not at all under white light.
While intraoperative cholangiography remains the gold standard for laparoscopic cholecystectomies, intraoperative ultrasound and ICG NIR FI are often considered as good alternatives. ICG NIR FI has been found to only be useful in discerning the extrahepatic biliary tree, while intraoperative cholangiography is useful for evaluating the intrahepatic biliary tree[50]. However, ICG NIR FI is superior in terms of causing less radiation exposure[49]. Figure 4 below demonstrates the use of ICG in laparoscopic cholecystectomy.
ICG can be administered through either the intravenous or intrabiliary route. For the intravenous route, ICG is administered 30 min before the surgery. Since ICG is metabolized by the liver and excreted in bile, the biliary structures are visualized intraoperatively immediately after dissection of the Calot’s triangle[51,52]. For the intrabiliary route, the gallbladder is punctured with cholangiogram or pigtail catheter mid-surgery, and the bile is aspirated and mixed with ICG solution, and then re-injected into the gallbladder[45]. Currently, the intrabiliary route is proven to be more efficacious in mapping the biliary tree. In a retrospective study of 24 patients by Shibataet al[53], ICG was administered intravenously in 12 patients and intrabiliary for 12 patients. The biliary tree was well-identified in 100% (12/12) of the patients in the intrabiliary group, as compared to only 83.3% (10/12) of the patients in the intravenous group. Ambeet al[52] reported no statistically significant differences in the duration of operation, length of stay in hospital, and risk of BDI when comparing between ICG guided and non-ICG guided laparoscopic cholecystectomy. For this study, the median duration of operation was 53vs54 min in the group with and without ICG respectively. Median length of stay was 2 d and no BDI occurred for both groups.
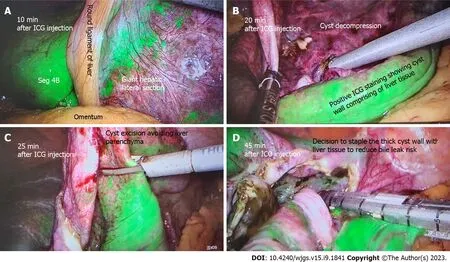
Figure 3 A 60-year-old patient undergoing elective liver cyst deroofing for a symptomatic solitary benign epithelial liver cyst was injected with 7 mL of indocyanine green dye after insertion of camera port. A: Liver enhancement at 10 min; B: After 20 min of injection shows the dye enhances the liver and cyst wall remains unenhanced; C: How indocyanine green (ICG) guidance can avoid transecting the liver parenchyma during cyst wall excision; D: The cyst wall with positive ICG staining is excised using stapling technology to reduce bile leak risk. ICG: Indocyanine green.
Additionally, studies have also evaluated the use of ICG cholangiography for use in robotic cholecystectomies. In a retrospective study of 184 robotic cholecystectomies by Espositoet al[54] demonstrated this with ICG FI allowing visualization of minimally 1 biliary structure in 99% (182/184) cases, with no laparoscopic or open conversions required.
Gallbladder cancer: Gallbladder cancer (GBC) is associated with high mortality, with a 5-year survival rate of less than 5%[55]. The mainstay of treatment for GBC remains radical resection of the gallbladder, including a central hepatectomy and regional lymphadenectomy. Recent advancements in this area include the increasing use of minimally invasive robotic surgery[56]. Ahmad reported the use of ICG FI in robotic radical resections for GBC in 10 patients, for the purposes of identifying the cystic duct junction with the CBD. This was made easy as NIR FI is a standard feature in daVincia surgical robots[56]. In addition, AJCC guidelines recommend removal and evaluation of 6 LNs in GBC resection, however this is rarely achieved[57]. The use of ICG guided regional lymphadenectomies may hence improve our ability to achieve this while reducing the risk of bile duct devascularization, and overcome visualization challenges from scarring and adhesions from previous operations[58,59].
Choledochal cyst excision: The utility of ICG is also explored in identification of pancreatico-biliary junction and distal end of bile duct in a patient with choledochal cyst scheduled for laparoscopic excision[60]. The authors innovated a novel method of exploiting the protein affinity of ICG by mixing ICG with the patient’s own bile juice aspirated from the gallbladder during surgery.
Bilio-enteric anastomosis: In patients undergoing hepaticojejunostomy for a variety of indications, ICG is shown to increase the detection of intra-operative bile leak from the anastomosis, thus allowing surgeons to reinforce the suture line and reducing the risk of post-operative biliary fistulas[61].
Pancreas
Tumour detection: During pancreatic tumour surgery, the extent of the tumour is typically evaluated intraoperatively through visual inspection or, in some cases, with the aid of intraoperative ultrasound. However, accurately delineating tumour boundaries can be difficult due to the presence of inflamed surrounding tissue[62]. Insufficient identification of tumour margins can lead to incomplete tumour resection, a predicament that has been shown to contribute to high recurrence rates ranging from 68% to 72%, as reported in a study by Griffinet al[63].
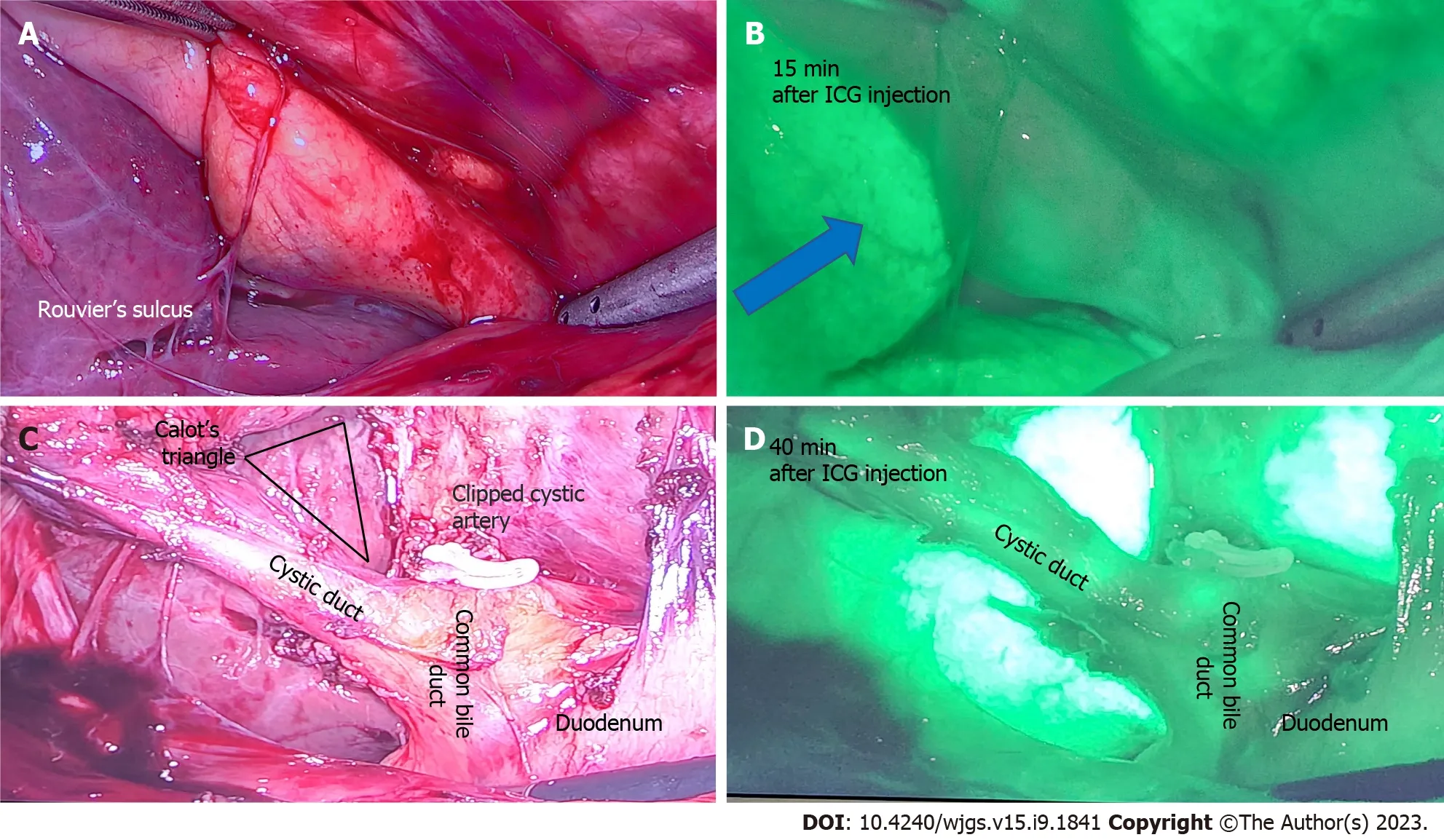
Figure 4 A 50-year-old patient undergoing elective laparoscopic cholecystectomy for previous acute cholecystitis was injected with 4 mL of indocyanine green dye after insertion of camera port. A: Rouvier’s sulcus and corresponding; B: After 15 min of injection shows the dye enhances the liver (blue arrow) and indocyanine green (ICG) is yet to be excreted in biliary tree; C: Calot’s triangle with a critical view of safety and clipped cystic artery; D: At 40 min after ICG injection shows beginning of biliary excretion in cystic duct and common bile duct. ICG: Indocyanine green.
Novel NIR fluorescent agents have been developed that target tumour-specific cell surface markers, enzymatic activity, or increased glucose metabolism[64-66]. However, these tumour-specific agents are not yet available for clinical use. ICG on the other hand, can identify tumours based on the enhanced permeability and retention (EPR) effect. This effect refers to the dye’s ability to accumulate in tumour spaces for prolonged periods due to the highly porous vessels and poorly developed lymphatics, despite not being tumour specific[67]. However, the EPR effect has been found to be less effective in identifying pancreatic tumours compared to other malignancies such as breast cancer. A study conducted by Huttemanet al[62] revealed that only 12.5% (1/8) patients had a clear fluorescence hotspot corresponding to an adenocarcinoma, with no other useful results noted for the remaining patients. This can be attributed to healthy pancreatic cells having almost equal ICG uptake as tumour cells. The COLPAN study concluded that single-bolus intraoperative ICG was effective in delimiting the area of high fluorescence corresponding to functional pancreatic neuroendocrine tumours. Peak tumour fluorescence was obtained 20 min post administration, and ICG also concentrated in peripancreatic LNs[68].Assessment of pancreatic perfusion post-pancreaticoduodenectomy: ICG dye can be utilised to confirm adequate perfusion of the pancreatic remnant during surgery. Traditional methods for assessing perfusion include clinical inspection of normal bleeding from the cut surface of the pancreas or Doppler ultrasonography for real-time arterial flow[69,70]. However, ultrasonography has limited spatial resolution and is not proficient in identifying concealed arteries, venous perfusion, or microperfusion[71]. In contrast, ICG binds to plasma lipoproteins, remaining within the intravascular space. ICG is administered intravenously during surgery, and its fluorescence in the remnant confirms adequate perfusion, as demonstrated in a case study by Iguchiet al[72]. Therefore, it is an effective method for evaluating all vascular supply means of the remnant pancreas.
Adrenals
Use in adrenalectomy: Laparoscopic and robotic techniques are now the gold-standard for adrenalectomies, but it hampers surgeons’ ability to receive tactile feedback, which is important for discerning tumour edges and vascular structures[73]. The use of ICG enables differentiation between the hyperfluorescent adrenocortical tissue and hypofluorescent retroperitoneal tissue, facilitating dissection[74]. The best contrast between the adrenal and retroperitoneal fatty tissues was observed 5 min post-injection of ICG[75].
Moreover, ICG guided cortical-sparing adrenalectomy allows for intraoperative visualisation of the boundaries between the normal adrenal cortex and medullary tumour[74]. Phaeochromocytomas were non-fluorescent while healthy cortical tissue was brightly fluorescent, and hence Kahramangilet al[76] reported how when the phaeochromocytoma was small and did not penetrate the cortex, the whole adrenal appeared heterogeneously fluorescent and hence ICG usage was not helpful. It was only when the tumour was large, was the non-fluorescence appreciable for guiding resection.
Following the intravenous administration of ICG, the sequence of enhancement was the arterial anatomy, followed by the adrenal parenchyma, and lastly the adrenal vein. The identification of the vasculature is important, particularly for cases with distorted anatomy such as large adrenal neoplasms, and potentially allows for decreased blood loss[77]. Of note however, the identification of the adrenal vein was inconsistent in a larger prospective study of 100 patients[76].
Spleen
Laparoscopic splenectomy, as compared to open, has been shown to improve outcomes including blood loss, length of stay and reduction in wound complications[78]. It is unlikely that routine use of ICG would be indicated in straightforward cases. However, it could be useful in the identification and division of the splenic artery and vein in cases where there is anatomic distortion or adhesions from prior inflammation[79]. This is important as bleeding from these vessels can be substantial, and it is more difficult to obtain control in laparoscopic or robotic surgery compared to open surgery. ICG has been shown to be useful in selected cases during splenic surgery as described below.
Splenic aneurysmectomy: ICG has been reported to be helpful in the treatment of splenic artery aneurysms, an extremely rare disorder[80]. Bertolucciet al[81] reported a case where ICG was used in a laparoscopic splenic artery aneurysmectomy to confirm successful clip and resection of aneurysm. The use of ICG FI also enabled assessment of splenic blood supply, allowing for laparoscopic partial splenectomy in 4 patients[79].
Splenic cysts: Dome resection for splenic cysts allows for the preservation of splenic immunological function and has become the primary technique to treat splenic cysts. Masuyaet al[82] reported the successful use of ICG fluorescence to assess for the thinning area of the cyst to be punctured. This is beneficial to allow preservation of normal parenchyma and avoid unnecessary splenectomy.
Small bowel
Perfusion assessment: There has been growing use of minimally invasive surgery for the treatment of small bowel pathology in recent years, but laparoscopy reduces the ability to discern signs of irreversible vascular insufficiency such as absence of peristaltic movements, mesenteric pulsations, and discolouration of the bowel wall. ICG angiography for assessment of bowel perfusion aids in determining need and extent of bowel resection.
Use in small bowel obstruction: In the setting of small bowel obstruction, Guerraet al[83] reported the use of ICG fluorescence in 7 patients for assessment of bowel viability. ICG was administered intravenously and in small 2 mL boluses to assess the intestinal microcirculation. Bowel segments that demonstrated patchy fluorescence or nonfluorescence were then resected. ICG as an adjunct for assessment of bowel perfusion is important, as inability to assess bowel viability is the second most common reason for conversion to open surgery in patients with small bowel obstruction[84]. Likewise, Gangulyet al[85] reported the use of ICG FI in 2 patients with incarcerated inguinal hernias containing small bowel. The involved bowel presented dusky areas but ICG administration revealed sufficient fluorescence and bowel resection was avoided.
Use in small bowel ischemia: In mesenteric ischemia, it can be challenging to macroscopically differentiate between reversible and irreversible ischaemic bowel. Intraoperative ICG FI makes it possible to detect non-viable intestine that is not apparent to the naked eye. This may reduce the need for repeated laparotomies to reassess bowel viability[86]. In occlusive mesenteric ischemia, it is logical to determine the region of bowel to resect based on the vascular supply as evident on CT angiogram[87]. However, in non-occlusive mesenteric ischemia, hypoperfusion is due to mesenteric vasoconstriction which makes identifying the precise segment of non-viable bowel difficult. ICG plays a crucial role in helping surgeons determine intraoperatively which regions of the bowel are adequately perfused, and hence decide on the need or extent of resection[86].
Colorectal
In colorectal surgery, ICG’s applications are varied including fluorescent tumour localisation, LN mapping and intraoperative angiography for anastomosis perfusion assessment[88]. Fluorescence guided visualisation continues to gain popularity amongst colorectal surgeons due to its reliability, safety, and ease of use. A survey of 37 centres in the Italian ColoRectal Anastomotic Leakage study group reported that 78.4% (29/37) of centres used fluorescence in all laparoscopic colorectal resections, and 65.5% of surgeons strongly believed the use of FI will become a minimum requirement in the future[89]. Studies have also demonstrated the use of ICG FI in robotic colorectal surgeries[90].
Assessment of bowel perfusion at site of intended anastomosis: ALs are a known complication of colorectal surgery with incidence between 3%-19%[91]. This is associated with increased morbidity and mortality, prolonged hospital stay, and a potential association with an increased risk of cancer recurrence, translating to worse long-term outcomes[92,93]. Bowel vascularity is a modifiable risk factor for anastomotic healing, hence the utility of ICG fluorescence angiography for intraoperative confirmation of favourable bowel perfusion prior to anastomosis. A retrospective matched-pairs analysis has demonstrated that ICG angiography suggested a change of proximal colonic resection line location in 16.4% and significantly reduced AL rates by 4%[94]. A recent meta-analysis of 4037 patients comparing AL rates between colorectal surgery with and without ICG showed that ICG angiography significantly reduced the AL rate by 4%, which translated to a reduced risk of reoperation and 5.6% reduction in overall complications[95]. This is confirmed by a larger meta-analysis of 25 studies with 7735 patients by Trastulliet al[96], which found that ICG angiography led to a reduction in AL rate compared to standard methods of anastomosis perfusion assessment (OR = 0.39, 95%CI: 0.31-0.49,P< 0.001). The NNT for ICG to prevent 1 additional AL is 23 patients. Figure 5 illustrates the use of ICG to confirm well vascularised bowel at the site of intended bowel transection and subsequent anastomosis.
Some limitations include the qualitative nature of the assessment for ICG fluorescence in the bowel which can be subjective, with no standard on dose of ICG and observation time. Research has hence been conducted on the quantitative analysis of colonic perfusion, with an evaluation of fluorescence intensity and perfusion time factors. A Korean study has determined that factors related to perfusion time, such as time from first fluorescence increase to maximum fluorescence, are significant predictors of anastomotic complications[97]. At present there is no consensus on the routine use of ICG for assessment of anastomotic perfusion in colorectal surgery. In spite of this, a recent cost analysis by Liuet al[98] on routine ICG use for anastomotic perfusion assessment found it cost-effective.
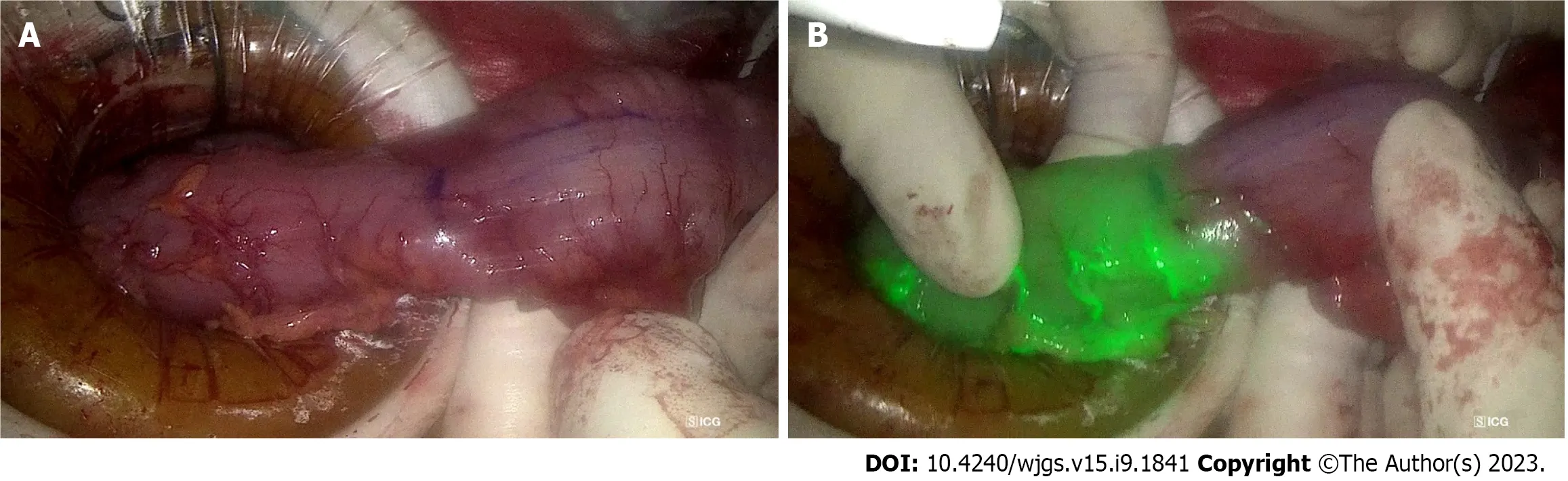
Figure 5 The utility of indocyanine green dye in laparoscopic anterior resection. A: The descending colon prepared for proximal transection during laparoscopic anterior resection, with the purple line indicating intended transection site, 5 cm proximal to tumour; B: The indocyanine green angiography confirms good vascularity at the site of intended transection, prior to creation of colo-rectal anastomosis.
Perfusion assessment of other structures: Perfusion assessment using ICG has also been performed for pedicled omentoplasty in pelvic surgery, gracilis muscle flaps and anal advancement flaps for perianal fistula[99]. In a study assessing the role of ICG dye in pedicled opemtoplasties, 80% (12/15) of patients had a larger resection than intended as ICG was able to identify areas of malperfused omentum that was not visible under standard white light. While this added an extra median of 8 min (range 3-39 min) to the surgical time, it can be argued that this is a worthwhile limitation[100].
Tumour localisation: Preoperative endoscopic tattooing of colonic lesions using India ink was first described in 1975 by Ponsky and King[101] for the purposes of intraoperative localisation. This is necessary in the setting of minimally invasive surgery in view of the inability to palpate the colorectum intraoperatively to allow identification of lesions. ICG tumour marking has been employed to allow precise intraoperative identification of small lesions without affecting the visibility of the surgical field and tissue planes with colour dye while in conventional viewing mode. The preferred interval between endoscopic submucosal injection of ICG and surgery varies. Leeet al[102] endoscopically injected 1-1.5 mL of ICG preoperatively and found that tattoos placed within 2 d of surgery were more often visualised (95%) than if they were placed earlier (40%). In contrast, a Japanese study injecting 0.5 mg of ICG submucosally described 100% intraoperative detection rates within 6 d and significant decrease after 7 d[103]. Furthermore, a prospective case series by Orsiet al[104] on 10 patients who underwent robotic colorectal resections also demonstrated the utility of ICG as a preoperative tumour marking dye for robotic surgeries.
Lymphatic mapping: ICG can further be used for LN mapping in colorectal cancer (CRC) patients, similar to that for other gastrointestinal malignancies. ICG spreads through lymphatic drainage from distal perivascular space with slow interstitial fluid reabsorption when ICG is injected into the colonic wall[88]. Concentration and dosing of ICG utilised in the literature varies, with injections performed either subserosal laparoscopically or submucosal endoscopically[105]. In patients with CRC, ICG is useful for two purposes. Firstly, ICG dye injection guides lymphatic mapping to facilitate harvesting of the draining LNs for oncological resection during colorectal resection. Secondly, ICG dye injection helps identify the SLN and provide information to surgeons for resection and is an area of ongoing research initiatives.
A systematic review of 12 studies found the rate of SLN accuracy in T1 CRC to be between 89%-100% when various dyes are used, including ICG and patent blue[106]. However, there is no consensus on the applicability of SLN identification in colorectal cancer. Current practice of complete mesocolic excision and total mesorectal excision ensures enbloc lymphovascular clearance. The role of lymphatic mapping in colorectal cancer could potentially be in early tumour stages to allow for conservative surgical resections but more research is required in this aspect[107].
Lateral pelvic LN dissection: Lateral pelvic LN dissection (LPLD) is recommended for patients diagnosed with mid-tolow advanced rectal cancer, due to the estimated 11%-22% incidence of lateral pelvic LN metastases (LPNM) in patients with T3/4 rectal cancer[108]. LPNM is an important factor for local recurrence, and is treated as a systemic disease due to common occurrence of distant metastasis[109,110]. Zhouet al[109] evaluated the use of ICG FI for LPLD, and found significantly reduced blood loss and a greater number of LNs harvested, but no difference in operative time nor postoperative complications. In another longer-term propensity score-matched cohort study, Watanabeet al[111] reported decreased 3-year cumulative lateral local recurrence rate in the ICG-FI group. In addition, Yasuiet al[112] and Nouraet al[113] proposed the use of ICG FI to identify SLNs in patients without suspected LPNM. However, further prospective studies are required in this regard.
Ureteral visualization: Ureteral injuries, while rare with an incidence of around 0.28% of colorectal surgeries, are associated with increased mortality, morbidity, length of stay, and healthcare costs[114]. Intraureteral ICG administration has been used for intraoperative ureteral identification to reduce iatrogenic injuries, and also allows for the early identification of any ureteral injury for immediate repair. Administration requires cystoscopy and ureteral catheterisation, and allows for 4 to 12 h of ureteral visualisation[115]. Most studies used 5 mL of 2.5 mg/mL ICG for each ureter. A systematic review of 7 retrospective studies found this safe and effective, although the risks of ureteral catheterisation include ureteral injury itself and infectious complications[116,117].
Urethral identification: Urethral injury is a dreaded complication in transanal total mesorectal excision and abdominoperineal resection, and is increasing in incidence with more minimally invasive transanal surgery being performed[118]. Studies have demonstrated successful visualisation of the urethra with ICG mixed with Instillagel?and ICG-silicon coated Foley catheters, albeit in cadavers[99].
Identification of nerves: The pelvic autonomic nerves are crucial for regulation of anorectal and urogenital function, but may be damaged during colorectal surgery. A pilot study by Jinet al[119] demonstrated that intravenous administration of 5 mg/kg ICG 24 h preoperatively allowed for the visualisation of the splanchnic, inferior mesenteric artery and sacral plexus during laparoscopic colorectal resection. This technique still requires further research, but could potentially aid in identification and protection of the pelvic autonomic nerves during laparoscopic colorectal resections.
Peritoneal
Peritoneal metastases occur in up to 30% of colorectal cancer patients (metachronous more than synchronous), and 75% of ovarian cancer patients present with peritoneal disease on diagnosis[120,121]. Conventional imaging modalities such as CT and magnetic resonance imaging have poor sensitivity in detecting small peritoneal nodules, requiring surgical exploration or cytological examination of peritoneal washings for complete evaluation of the peritoneal cavity[122,123]. However, small nodules may remain undetected during the surgeon’s visual and tactile assessment. In the context of a diagnostic exploration, this can impact staging and management. In the therapeutic setting, this can affect the completeness of cytoreduction and subsequent long term outcomes. ICG offers a potential solution to this diagnostic challenge, with its theoretical ability to detect micro peritoneal implants using the EPR effect[124]. In a systematic review of 71 patients with 322 peritoneal nodules assessed, ICG demonstrated promising sensitivity and specificity in detecting nodules at 88.2% and 77.8%, respectively[125]. However, there are restrictions to its utility in mucinous colorectal carcinomas, which have poor affinity for ICG. There is a possible role for ICG fluorescence as an adjunct to improve detection of peritoneal metastases in colon and ovarian cancer, but more studies are warranted.
Vascular
Wound healing post-amputation in patients with peripheral artery disease or chronic limb threatening ischaemia is often poor due to the poor vascular status and underlying comorbidities including diabetes mellitus or smoking[126]. ICG NIR FI post-amputation or post-revascularization is one proposed method for assessing regional tissue perfusion in predicting wound healing, determining level of amputation and to assess global limb perfusion. Van Den Hovenet al[127] performed a pilot study where ICG NIR FI was performed in 15 patients post-amputation, and noted that impaired wound healing corresponded to regions of low fluorescence in patients, and accurately predicted postoperative skin necrosis in 4 cases.
Bowel ischaemia is a known postoperative complication of abdominal aortic aneurysm (AAA) repair due to malperfusion of the peripheral arteries, with its associated mortality up to 50%[128,129]. ICG angiography provides visualization of peripheral intestinal blood flow, which can be used to determine whether there is sufficient vascular supply to perfuse the bowels. This information can help to guide decisions regarding whether the inferior mesenteric arteries (IMA) and internal iliac arteries (IIA) need to be reconstructed or preserved. In a study conducted by Yamamotoet al[129] involving 10 open AAA repairs, the use of ICG angiography resulted in at least 1 IMA or IIA being reconstructed in 8 cases that would not have been done otherwise. This approach helps to ensure that postoperative bowel ischemia, which would require a second surgery, is minimized.
Other abdominal organs
Beyond the organs discussed above, ICG is also used in other abdominal organs beyond the purview of a gastrointestinal surgeon. For example, ICG has been used to define tumour margins from normal kidney, identify branches of the main renal artery in partial nephrectomies, and assess microperfusion to predict early graft function in kidney transplant patients[130-132]. In gynaecological surgery, similar applications were noted in identifying SLNs in endometrial, cervical and vulvar malignancies[133]. It also is used for ureteral identification and localizing endometriosis nodules[134].
DISCUSSION
Current uses
ICG plays a crucial role in the field of gastrointestinal surgery, specifically in the optimization of oncological resections and comprehension of vascular supply. The key factors that contribute to successful cancer resections with low recurrence rates involve precise identification and localisation of the tumour, adequate resection of the tumour with ample margins, and complete removal of the lymphatics[33]. ICG serves as a useful tool in facilitating these steps, enhancing their efficiency and accuracy.
In oncological resections of various organs such as the oesophagus, stomach, hepatobiliary system, and the bowels, lymphatic mapping through ICG is widely employed. This method ensures the precise identification of SLNs and aids in determining the extent of LN dissection[135]. Literature also confirms that ICG can be used to identify tumours intraoperatively, specificallyviathe EPR effect. This helps to assure surgeons that they have resected sufficient tissue to prevent positive margins that may mandate a second operation. This, in turn, enables surgeons to operate with greater confidence that the cancer has been adequately removed, while simultaneously reducing the need for more extensive surgeries when they are not necessary.
ICG also provides surgeons with a better understanding of vascular supply, thereby preventing intraoperative accidental injuries, especially in cases where vessels are difficult to visualize or have aberrant anatomy[135]. Additionally, it facilitates complete vessel anastomoses to prevent leaks. Furthermore, ICG aids in ensuring sufficient perfusion of organs following resections, thereby decreasing the risk of postoperative ischemia. In summary, ICG provides surgeons with valuable insights into the vessels involved in surgery, which significantly reduces surgical morbidity, leading to shorter postoperative complications and ICU stays.
Given these applications of ICG in gastrointestinal surgery, it is only natural that ICG FI is primarily used in minimally invasive surgery or robotic surgery. ICG enables the mitigation of traditional drawbacks such as the lack of tactile feedback and subjective judgment error for tissue perfusion and viability. It remains used in open surgery still in more oncological contexts, such as SLN mapping.
Future direction
ICG has a myriad of clinical applications and many emerging applications. Despite this, the accessibility, availability, affordability, and adoption remain an unmet need that needs to be met by collaborative initiatives of the medicoindustrial complex. To begin with, standardized evidence base guidelines need to be developed, disseminated, and implemented for safe adoption in routine clinical practice.
Optimising ICG: Patient factors, dye factors, equipment, and method of assessing fluorescence intensity are factors that implicate and affect the utility of ICG. Patient factors include obesity and inflammation. Erikssonet al[136] showed a decreased rate of successful SLN mapping with increased patient body mass index.
Dye factors include the dose and concentration, timing, and route of administration, and increasingly also whether the dye is mixed with any other substances. For the purpose of SLN identification, prolonged accumulation of ICG in the sentinel nodes is crucial. By complexing ICG with HSA in the optimal molar composition, a higher fluorescence can be obtained to aid in this[137].
In addition, other dyes such as ZW-800 and VM678, among many others, have been tested in animal studies, with results showing better pharmacokinetic properties and target-to-background ratio. However, cost remains a barrier for these dyes[138]. ICG coating of the tubes and stents can be made possible with potential future clinical application in surgery. For example, ICG-coated ureteral stents can be useful in colorectal, gynecological, and urological procedures.
Fundamentally, there remains no widely accepted protocols for the use of ICG in most applications, with decisions such as dosing regimens left up to the surgeons’ expertise. Further research and study should focus on this area to optimise protocols to ensure the successful use of ICG.
Targeted contrast agents: It would also be useful to look beyond ICG, and develop new contrast agents that better target unique pathologies. This can be doneviaidentifying antibodies or ligands for proteins and receptors on cancer cell surfaces, and substrates for cancer specific metabolic pathways. A large proportion of these dyes are ICG-based, as they can be incorporated into hardware that are already in operative rooms. Other cyanine based dyes can also be incorporated with minor modifications in these machinery[139].
There are already several tumour specific dyes produced clinically. LUM015 is a cyanine based dye that targets cathepsin, which is a protease secreted by cancer cells at a higher level than healthy cells. LUM015 targets breast cancer and sarcomas specifically and is not affected by breast density, as compared to ICG, making it more accurate[140].
Studies have also shown that ICG-like fluorescent dyes can be tagged to artificially created antibodies of cell-surface tumour markers. Promising antibodies have been developed for carcinoembryonic antigen (CEA) for colorectal, breast, lung, and gastric cancer, prostate-specific antigen for prostate cancer, and cancer antigen 125 for ovarian cancer[139]. For example, XenoLight CF750 is an anti-CEA antibody conjugated to ICG and NIR probe. It was able to detect peritoneal tumour deposits in all 4 gastric cancer cell lines, including micrometastases < 2 mm in mouse models[141].
The ability to target cancers specifically allows for better cancer detection and surgical margins, and hence this is an area of research that shows great promise. Regardless, further research should be conducted for all applications of ICG to confirm the improvement in outcomes.
CONCLUSION
ICG has wide clinical utility to enhance safety and accuracy of gastrointestinal surgery to improve patient outcomes, both in surgical oncology and in general. With the ongoing advancements in technology and research, the future of FI remains promising and will continue to revolutionize surgery. However, ICG should not be considered as a panacea to guide surgical conduct, and surgeons need to exercise own’s informed judgment based on individual skills, experience and training.
FOOTNOTES
Author contributions:Shelat VG contributed to the conceptualization, supervision and project administration of the manuscript; Lim ZY involved in the methodology of this study; Lim ZY and Mohan S curated data; Lim ZY, Mohan S, Balasubramaniam S, Ahmed S, Siew CCH, and Shelat VG wrote the manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Singapore
ORCID number:Zavier Yongxuan Lim 0000-0001-7639-5123; Swetha Mohan 0000-0001-8463-1579; Sunder Balasubramaniam 0000-0002-4983-7157; Saleem Ahmed 0000-0001-6767-4825; Caroline Ching Hsia Siew 0000-0003-0250-6390; Vishal G Shelat 0000-0003-3988-8142.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Zhao S
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
