Acute flare of systemic lupus erythematosus with extensive gastrointestinal involvement: A case report and review of literature
Hua Huang, Ping Li, Dan Zhang, Ming-Xuan Zhang, Kai Yu
Abstract
Key Words: Systemic lupus erythematosus; Gastrointestinal involvement; Lupus enteritis; Lupus mesenteric vasculitis; Ultrasonography; Computer tomography; Case report
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic multisystemic autoimmune disease of unknown etiology. Under the influence of genetic predisposition and environmental risk factors, alterations in the immune response include hyperactivation of T and B lymphocytes, loss of self-tolerance and formation of circulating pathogenic immune complexes and subsequent deposition in several organs, thereby causing damage. The incidence of SLE is approximately 30–70 cases per 100000 individuals in China[1]. Females of reproductive age are mostly affected.
Gastrointestinal (GI) symptoms are present in > 50% of SLE patients throughout the course of the disease[2]. Among the wide spectrum of SLE-associated GI complications, including abdominal serositis/peritonitis, protein-losing enteropathy, intestinal pseudo-obstruction, hepatic involvement, and pancreatitis, lupus mesenteric vasculitis (LMV) is rare, but one of the most serious complications of SLE with high mortality[3]. Early diagnosis of LMV is crucial for prompt treatment.
LMV or lupus enteritis (LE), was originally proposed by Hoffman and Katz[4] in 1980. The early clinical presentation of LE comprises abdominal pain, nausea, vomiting, or severe GI bleeding, which is unremarkable and non-specific and is easily mistaken for infectious gastroenteritis and medication-related adverse effects. The rarity of LE in SLE, which makes it unincluded in any SLE classification criteria or weighted in the SLE Disease Activity Index (SLEDAI), makes early clinical suspicion difficult to elicit. In the British Isles Lupus Assessment Group disease activity index, LE is defined as either vasculitis or inflammation of the small or large bowel with supportive imaging and/or biopsy findings[5]. Since histological evidence is difficult to obtain, it is important to find a rapid, reliable, and safe diagnostic tool. This case review aimed to identify possible strategies for early diagnosis of LE among women of reproductive age.
CASE PRESENTATION
Chief complaints
A 27-year-old woman was referred to our department on account of abdominal pain that had persisted for six days.
History of present illness
The patient was initially transferred to our department after an enhanced abdominal computed tomography (CT) examination and GI decompression.
History of past illness
The patient had no photosensitivity, alopecia, oral ulcers, arthritis, or Raynaud’s phenomenon, and was previously healthy.
Personal and family history
The patient had no family history of SLE.
Physical examination
Upon examination, the abdomen was distended with no tenderness or rebound pain. No palpable masses or active bowel sounds were detected.
Laboratory examinations
Antinuclear antibody titer was 1:320, and anti-U1 ribonucleoprotein and anti-Sjogren's syndrome A antibodies were positive, whereas anti-double-stranded DNA (anti-dsDNA) antibody and anti-Smith antibody were negative. The levels of C3 and C4 compliments decreased to 0.245 g/L (0.7–1.4 g/L) and 0.024 g/L (0.1–0.4 g/L), respectively. Immunoglobulin G (IgG) level was 10.45 g/L (7–16 g/L), and direct antiglobulin "Coombs" test results were positive. Routine blood tests showed a white blood cell count of 1.40 × 1010/L (3.5× 109–9.5 × 109/L), platelet count of 2.01 × 1011/L (1.25 × 1011–3.50 × 1011/L), and hemoglobin level of 114 g/L (115–150 g/L). Urine microscopy revealed a pathological tube-type 1 per low power field. Liver enzyme levels and renal function were normal. Moreover, serum albumin level was 33.2 g/L (40–55 g/L); erythrocyte sedimentation rate (ESR) was 3 mm/h (0-22 mm/h); C-reactive protein (CRP) level was 1.28 mg/L (< 10 mg/L); procalcitonin level was 0.293 ng/mL (0–0.046 ng/mL); blood potassium level was 2.9 mmol/L (3.5–5.3 mmol/L); cardiac enzyme levels were normal; and 24 h total urine protein level was 0.51 g/d (0.01–0.15 g/d).
Imaging examinations
Lung CT showed bilateral pleural effusion (Figure 1). Enhanced abdominal CT showed the typical "target and comb signs" performance (Figure 2). Further investigation revealed LMV and incomplete intestinal obstruction. Some segmental bowel walls of the transverse colon were thickened by approximately 5 mm, and the main color flow of the superior mesenteric artery was well filled without embolism (Figures 3 and 4).
FINAL DIAGNOSIS
According to the 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for SLE, autoimmune hemolysis (4 points) + pleural or pericardial effusion (5 points) + low C3 and C4 (4 points) + proteinuria (4 points) = 17 points, which was > 10 points, and the patient met the diagnostic criteria; therefore, LMV and lupus nephritis were diagnosed. The SLEDAI-2K score was eight.
TREATMENT
After fasting, GI decompression, intravenous nutrition, methylprednisolone 80 mg per day intravenously, and immunoglobulin 20 g per day intravenously for five days, the symptoms were relieved, and the patient was able to tolerate semiliquid food. Furthermore, the methylprednisolone dose was gradually decreased to 40 mg per day intravenously. Fourteen days later, the methylprednisolone dose was adjusted to 36 mg per day orally, and this dosage was maintained for approximately one month, even after the patient was discharged. Moreover, she was treated with cyclophosphamide 0.2 g qod intravenously twice with a cumulative dose of 0.4 g, which was switched to mycophenolate mofetil (MMF) 0.75 g bid orally. Owing to the GI inflammation, intravenous antibiotics were administered for 15 d. Somatostatin reduced the intestinal inflammation, exudation, and gastric acid secretion. Nadroparin calcium was administered to prevent micro- or small mesenteric thrombosis.
OUTCOME AND FOLLOW-UP
Twenty days after the treatment, the patient was able to eat semi-liquid food without stomach discomfort. Bowel sounds returned to normal. Laboratory investigations showed complement levels had increased. The IgG level was 15.6 g/L (7–16 g/L), and renal function, hepatic enzyme levels, and electrolyte levels were normal. Routine urine and stool test results were normal. Ten days after the treatment, ultrasonography was used to assess the patient’s condition, which demonstrated that the local intestinal wall was approximately 0.35 cm at its thickest part (Figure 5), and that there was no effusion in the pleural, abdominal, or pelvic cavities. Most of the intestinal wall thickness decreased to a normal size. Finally, the intestinal lumen did not expand, and the multiserous cavity effusion disappeared. The abdominal signs and symptoms reduced, and the patient tolerated full feeding and was discharged.
The patient was follow-up at two-weekly intervals for the first three months and monthly thereafter. One year later, the patient was treated with methylprednisolone, which was tapered to 4 mg per day, MMF 0.75 g bid, and hydroxychloroquine 0.2 g bid. The C4 complement level was normalized, but the C3 complement level was 0.663 g/L (0.7–1.4 g/L), still slightly below the normal level. Table 1 shows the timeline from symptom onset to the last follow-up visit.
DISCUSSION
LE, also known LMV[6], is rare and one of the most devastating complications of SLE. The incidence of LMV is estimated to range between 0.2%–9.7% among patients already diagnosed with SLE and between 13.0%–62.5% in patients with isolated LE as the initial presentation of SLE[7].
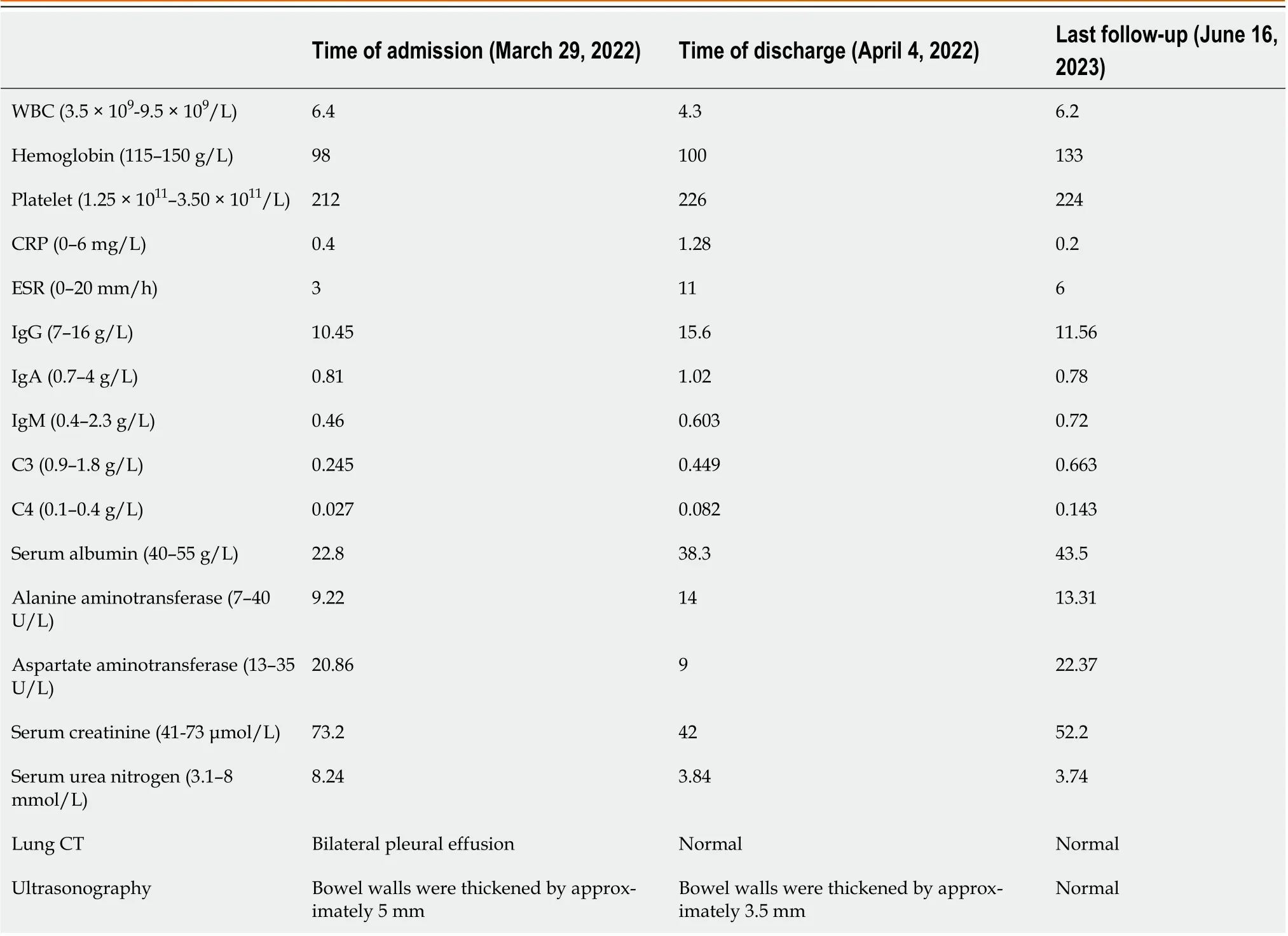
Table 1 Timeline from symptom onset to the last follow-up visit
The pathophysiology of LMV is leukocytoclastic vasculitis secondary to the deposition of immune complexes in vascular walls, and antiphospholipid antibody-associated vasculopathy leading to thrombosis[8]. Ischemia causes the greatest damage to the mucosal mesenteric vessels, followed by the vasculature of the muscularis propria, submucosa, and plasma membrane[6]. Certain triggering factors (bacterial or viral infections, chemicals,etc.) can activate endothelial cells and expose them to cryptic antigens, resulting in the production of anti-endothelial cell antibodies, activation of complement, and deposition of immune complexes on inflamed blood vessel walls, which can lead to vascular damage and thrombosis[6]. The literature reported that vasculitis was histologically confirmed by biopsy samples of the affected intestinal segments[9]. The submucosa, filled with a mild diffuse inflammatory infiltrate of mononuclear cells, becomes edematous, and hemorrhage can be observed mostly in the muscular and subserosal layers; fibrinoid necrosis, leukocytoclasis on the vascular wall, and fibrin thrombus formation can be observed in the subserosal vessels.
The initial presenting signs and symptoms of LE are heterogeneous and commonplace and are usually mistaken for GI infections, peptic ulcers, lithiasis cholecystitis, or side effects of medications. However, the most common symptom in more than 90% of patients is abdominal pain of varying intensity caused by intestinal ischemia secondary to vasculitis[10]. Signs and symptoms of impaired GI motility and peritonitis are also common in patients with SLE. Diarrhea was frequent, with a frequency of 43.5%–64.7% in different studies[11]. Vomiting was found among 39.5%–72.2% of Asian patients[12]. Ascites was also frequently reported in the literature (27.9%–94.1%). This was also observed in our patient. GI bleeding is a rare but serious presentation that may be due to intestinal ischemia and intestinal necrosis. If not recognized in time, it may lead to perforation with a mortality rate of up to 50%[13]. The ileum and jejunum were the most frequently involved intestinal sites (> 80%) reported in the literature[14].
Timely and accurate diagnosis of LMV is a great challenge due to the lack of knowledge about LMV. Therefore, it is necessary to explore the factors associated with LMV.
Several studies have found that patients accompanied by oral ulcers or lupus urinary tract involvement have a greater risk of encountering LMV[15-17]. Therefore, clinicians should suspect LMV when clinical laboratory results suggest lupus involvement of oral ulcers and the urinary system.
Previous studies have shown that there is no significant difference in SLEDAI scores in patients with SLE presenting with abdominal pain, regardless of the presence or absence of LE[14]. In patients with LMV, there was no difference in SLEDAI scores for serious adverse events, such as intestinal hemorrhage, infarction, perforation, or death due to serious complications, suggesting that SLE activity does not correlate with the severity of LMV[9]. Although SLEDAI was higher at baseline in LE patients than in non-LE patients, it did not predict the development of LE[18].
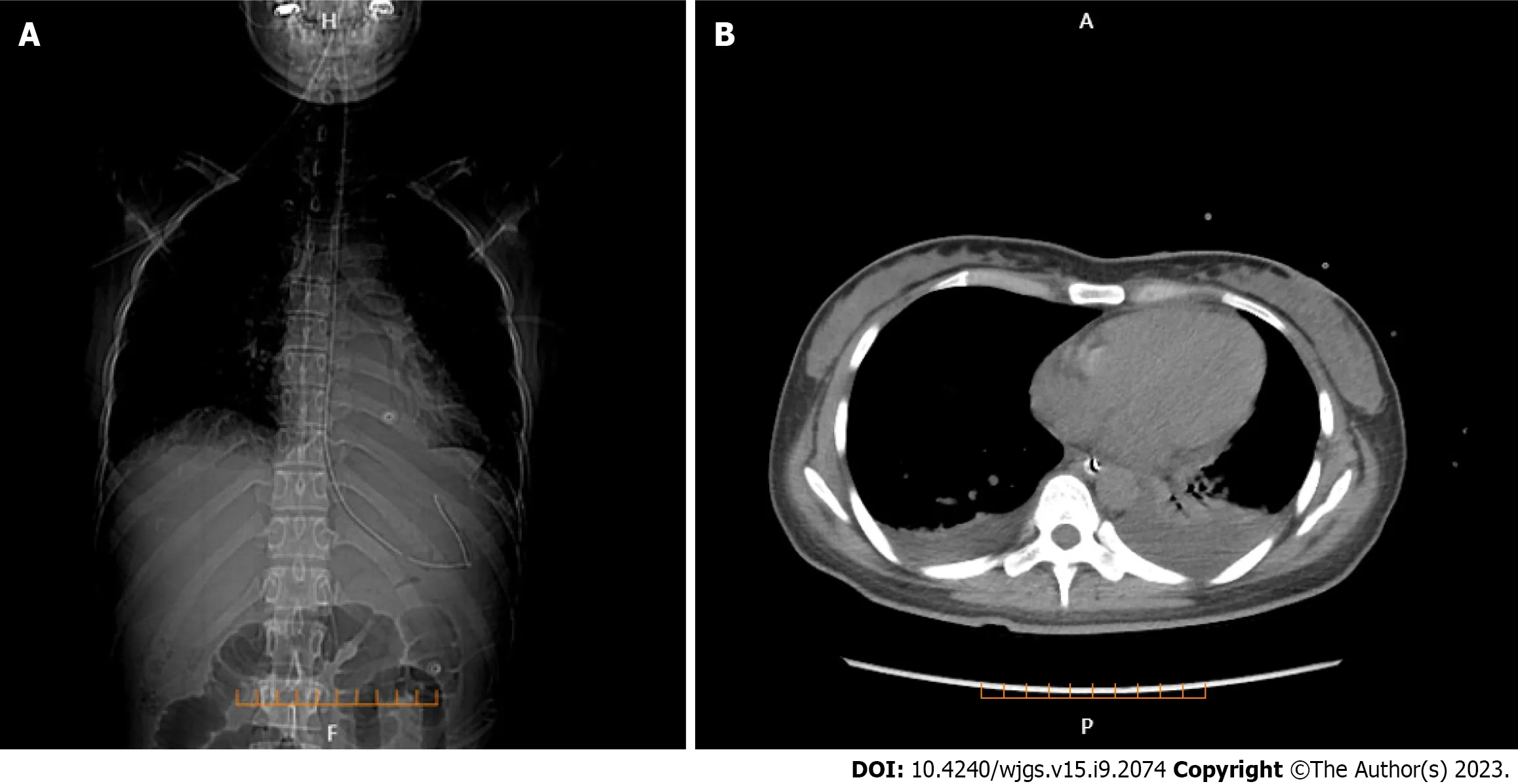
Figure 1 Lung computed tomography. A: Bilateral pleural effusion and the position of gastrointestinal decompression tube (coronal plane); B: Bilateral pleural effusion (axial plane).
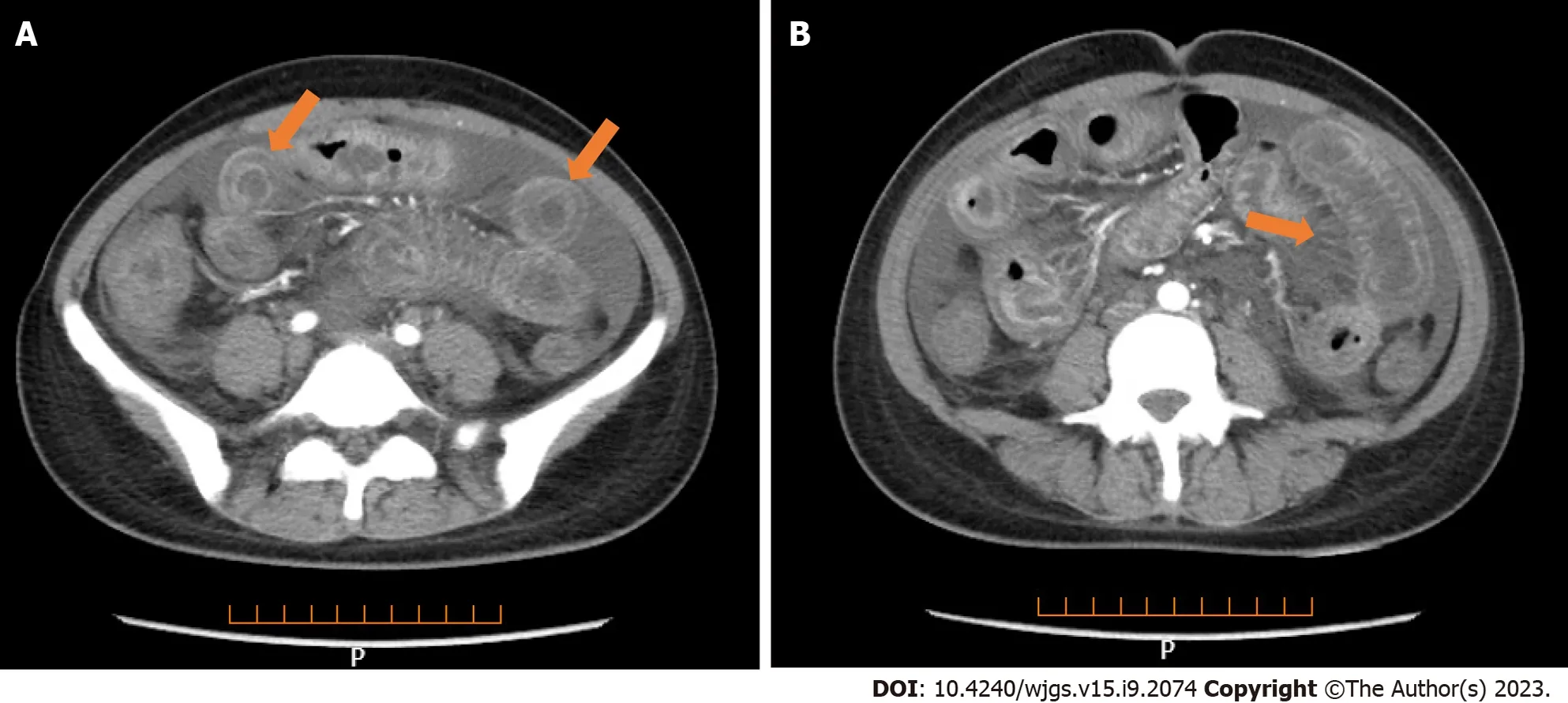
Figure 2 Full enhanced abdominal computed tomography. A: Thickened bowel loops (target sign); B: Engorgement and increased visibility of the mesenteric vessels (comb sign).
Laboratory tests are of value in the diagnosis of LE. However, CRP is usually normal and complement levels are decreased[7]. In seropositive cases, antibodies were more reliable, with increased titers of antinuclear, anti-dsDNA, and anti-Smith antibodies, which were approximately 92%, 80%, and 20%, respectively[19]. These autoimmune and inflammatory markers, while informative in diagnosis, are not essential. LE can occur without clinically active lupus[7]. Other researchers did not find laboratory parameters, including complement, ESR, CRP, lupus-related antibodies, and antiphospholipid antibodies to be correlated with the risk of LE[8,14].
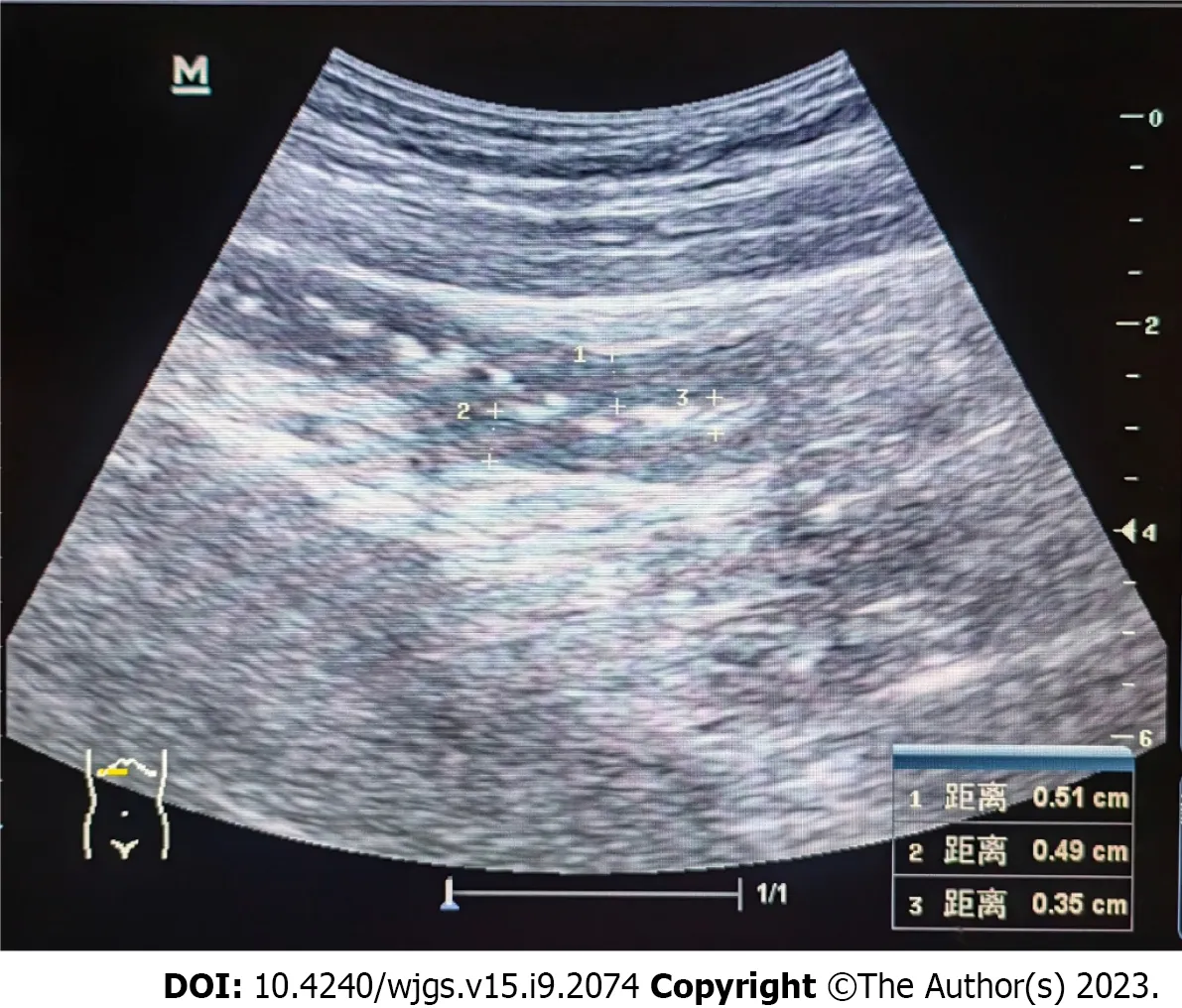
Figure 3 Ultrasonography of the transverse colon showing thickened segmental bowel walls of approximately 5 mm.

Figure 4 Ultrasonography of the superior mesenteric artery showing the main color flow of the superior mesenteric artery was well-filled without embolism.
Multiple methods, including ultrasonography, CT, magnetic resonance imaging, and GI endoscopy, have been used to evaluate LMV. However, CT is fast and non-invasive and can be used to perform image reconstruction and postprocessing, to observe the LMV in multiple directions, and to observe lesions of the mesentery and blood vessels. Enhanced abdominal CT is more sensitive in detecting intestinal abnormalities, as it can reveal thickened and swollen bowel walls that contain enhanced mucosal, edematous submucosal, and enhanced serosal layers. Normal bowel wall thickness visualized by CT was < 3 mm; 3–5 mm thick bowel walls were considered mildly thickened, 6–7 mm moderately thickened, and > 8 mm markedly thickened[20]. This categorization is important in judging the occurrence of LE[9,21]. There are three typical abdominal CT findings in patients with LE, and these can appear alone or concurrently: Bowel wall thickening (> 3.0 mm), which leads to separation of the mucosa and muscle layers and “target sign” appearance; mesenteric vasodilation with “comb sign” appearance; and increased attenuation of mesenteric fat[15,19,22-25]. The co-occurrence of “target sign” and “comb sign” is particularly specific to LE, and can be used to establish a diagnosis. Unfortunately, these imaging manifestations are not uncommon in inflammatory bowel disease, intestinal ischemia, and mesenteric vein thrombosis.
Magnetic resonance enterography can also be used to diagnose LE. The main advantages of magnetic resonance enterography are that it allows for a holistic assessment of the bowel, both intestinal and extra-intestinal, as well as a high degree of safety as the examination can be repeated in a short period of time[26]. However, the slightly longer scan time, the radiologist's manipulation, and the patient's tolerance of the contrast agent may limit magnetic resonance enterography.
Ultrasonography is likely to be the most readily available imaging method, and a valuable alternative to CT when the latter is not available or is contraindicated. Being a safe and more accessible technique, ultrasonography was the first imaging examination when our patient presented to the emergency room. The study[27] have described patients with LE who had characteristic intestinal wall edema and ascites based on ultrasonography. Ultrasonography revealed thickening of the intestinal wall, in which the submucosal edema of the Kerckring fold resembled an accordion[28], dilation of intestinal segments, increased reflectivity of mesenteric fat, and mild ascites. CT confirmed these features, suggesting that both methods have similar sensitivities for LE diagnosis. Regarding specificity, ultrasonography has similar limitations with CT. If the patient is a young woman of childbearing age, ultrasonography is likely to be the best screening examination for suspected LMV diagnosis and follow-up[14]. Therefore, ultrasonography is a reliable and accessible tool.

Figure 5 Ultrasonography showing the local intestinal wall with approximately 0.35 cm at its thickest part.
Although the gold standard for diagnosing LE is pathology, the positive rate is low. Therefore, endoscopy is not used as a routine diagnostic tool. Only a few cases present intestinal edema, congestion and ischemia with or without ulceration or necrosis[7].
Due to the lack of prospective randomized controlled clinical trials, there are currently no available guidelines or recommendations for LE treatment.
The prognosis is good when the diagnosis is made early, and high-dose steroids are started in a timely manner; however, patients may occasionally experience recurrence. Glucocorticoids are the primary medications used to treat LE. Cyclophosphamide is one of the most commonly used immunosuppressants used for SLE treatment in China, and its combination with corticosteroids results in a good response[29]. Cyclophosphamide, whether intravenous pulse or not, has been useful in preventing relapses of lupus flares[2]. However, considering its lower reproductive toxicity, we switched to MMF treatment, which is preferred in young men and women due to the high risk of testicular and ovarian failure following cyclophosphamide[30]. Although rituximab, an anti-CD20 antibody, showed promising potential in observational studies, the LUNAR randomized controlled trials did not show that rituximab combined with MMF had additional benefits during remission compared with MMF alone[31].
LMV causes inflammation in the intestinal wall, which enhances its permeability. Dysregulation of the intestinal microbiota can lead to intestinal infection. Antibiotics were administered to control the risk of infection. Octreotide treatment may be effective, owing to its immunomodulatory effects, regulation of intestinal microvasculature blood flow, and amelioration of lymphatic dilatation[32]. Considering the aforementioned effects of somatostatin, we administered it to the patient and good outcomes were achieved.
CONCLUSION
Despite being rare, LMV must always be considered in any SLE patient presenting with GI symptoms. The diagnosis of LMV requires a combination of history and clinical and immunological indicators. Moreover, enhanced CT examination can reveal the extent and scope of GI lesions involved in SLE and provide evidence for timely clinical diagnosis and treatment. For pregnant women, or physicians in primary hospitals, ultrasonography can be an alternative method for preliminary screening and follow-up.
ACKNOWLEDGEMENTS
We are grateful to the patient for permitting us to publish this study.
FOOTNOTES
Author contributions:Huang H and Li P contributed to manuscript drafting; Zhang MX was the physician responsible for the patient's diagnosis and treatment in the Rheumatology and Immunology Department; Zhang D was the patient’s nutritionist and provided nutritional guidance; Yu K was the patient’s admitting physician in the Gastroenterology Department; All authors were responsible for the revision of the manuscript and final approval for submission.
Informed consent statement:Consent was obtained from the patient, both verbally and in written form and has been attached to this submission.
Conflict-of-interest statement:The authors declare no conflicts of interest.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Hua Huang 0009-0006-8989-7193; Ping Li 0000-0001-9008-0937; Dan Zhang 0009-0004-4056-7340; Ming-Xuan Zhang 0009-0008-0821-9775; Kai Yu 0009-0001-8623-8639.
S-Editor:Lin C
L-Editor:A
P-Editor:Guo X
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
