Puccinia triticina effector protein Pt_21 interacts with wheat thaumatinlike protein TaTLP1 to inhibit its antifungal activity and suppress wheat apoplast immunity
Fei Wng,Songsong Shen,Zhonghi Cui,Shito Yun,Ping Qu,Hui Ji,Linshuo Meng,Xioyu Ho,Dqun Liu,Lisong M,,,Hiyn Wng,
a College of Plant Protection,Hebei Agricultural University/Technological Innovation Center for Biological Control of Crop Diseases and Insect Pests of Hebei Province,Baoding 071001,Hebei,China
b Center of Agriculture Through Science and Education,Hebei Agricultural University,Baoding 071001,Hebei,China
c The State Key Laboratory of North China Crop Improvement and Regulation,Hebei Agricultural University,Baoding 071001,Hebei,China
Keywords: Wheat Puccinia triticina Effector Thaumatin-like protein Anitifungal activity
ABSTRACT Puccinia triticina (Pt),as the causal agent of wheat leaf rust,employs a plethora of effector proteins to modulate wheat immunity for successful colonization.Understanding the molecular mechanisms underlying Pt effector-mediated wheat susceptibility remains largely unexplored.In this study,an effector Pt_21 was identified to interact with the apoplast-localized wheat thaumatin-like protein TaTLP1 using a yeast two-hybrid assay and the Pt_21-TaTLP1 interaction was characterized.The interaction between Pt_21 and TaTLP1 was validated by in vivo co-immunoprecipitation assay.A TaTLP1 variant,TaTLP1C71A,that was identified by the site-directed mutagenesis failed to interact with Pt_21.Pt_21 was able to suppress Bax-mediated cell death in leaves of Nicotiana benthamiana and inhibit TaTLP1-mediated antifungal activity.Furthermore,infiltration of recombinant protein Pt_21 into leaves of transgenic wheat line overexpressing TaTLP1 enhanced the disease development of leaf rust compared to that in wild-type leaves.These findings demonstrate that Pt_21 suppresses host defense response by directly targeting wheat TaTLP1 and inhibiting its antifungal activity,which broadens our understanding of the molecular mechanisms underlying Pt effector-mediated susceptibility in wheat.
1.Introduction
Wheat (Triticum aestivumL.) leaf rust caused byPuccinia triticinaEriks.(Pt) is one of the most widely occurring and severe diseases in wheat-growing regions worldwide [1].It causes wheat yield loss ranging from 5% to 15%,but can result in a minimum of 50% yield loss when the rust infection occurs at the seedling stage of wheat [2].Compared to stripe and stem rusts caused byPuccinia striiformisf.sp.tritici(Pst)andPuccinia graminisf.sp.tritici(Pgt),respectively,the annual yield loss incited by leaf rust maintains at a similar level due to its high frequency and wide range of occurrence[3].Given the impacts of global climate change,temperature and humidity conditions may favor the proliferation and epidemic ofPt,which will pose a serious threat to wheat production.Deployment of resistant wheat cultivars is one of the practical methods to control this disease.Currently,more than 80 leaf rust(Lr)resistance genes conferring a gene-for-gene race-specific resistance againstPthave been reported in wheat[4].However,during host-pathogen coevolution,widely deployed wheat resistance genes are often easily overcome by highly adaptable leaf rust pathogens [5],which stresses the urgent need to develop new and alternative means for controlling this devastating disease.To develop such strategies,a solid understanding of the molecular mechanisms ofPtinfecting wheat plants is needed.
As an obligate biotrophic fungus,Ptforms specialized infection structure haustoria during infection of the wheat plant.Haustoria functions to acquire nutrients from the host plant and secrets effectors into the host cell [6].Effector proteins from haustoriaforming wheat rust pathogens,such asPstandPgt,have been reported to be involved in virulence by targeting diverse host proteins [7,8].For example,Psteffector Hasp98 contributes to virulence by suppressing H2O2accumulation and inhibiting the kinase activity of wheat mitogen-activated protein kinase 4(TaMPK4)via direct interaction with TaMPK4[9].Effector proteins PEC6 fromPstcan suppress pattern-triggered immunity and interact with adenosine kinases to facilitate pathogen infection and growth [10].Interestingly,the chloroplast-targeting effector Pst_12806 targets a chloroplast protein TaISP that is a putative component of the cytochrome b6-f complex to impair host photosynthesis and production of chloroplast-derived reactive oxygen species (ROS),thereby promotingPstinfection [11].Recently,thePstarginine-rich effector Pst_A23 with host nuclear localization regulates host pre-mRNA splicing by direct binding to the alternative splicing sites of host pre-mRNA to suppress host immune response [12].Previous studies showed that host pathogenesisrelated proteins (PRs) are targeted by multiple fungal virulence factors,including effectors.For example,Parastagonospora nodorumeffector SnTox3 interacts with wheat TaPR1 to inhibit the release of the C-terminal region carrying the CAPE1 peptide from TaPR1,thereby suppressing wheat immune response [13].A cerato-platanin protein SsCP1 fromSclerotinia sclerotiorumwas found to interact withArabidopsis thalianaPR1 in the apoplast of the plant cells,contributing to the virulence ofS.sclerotiorum[14].PstmicroRNA-like RNA 1 (Pst-milR1) binds to the wheat pathogenesis-related 2 (PR2) gene to suppress its expression,thereby repressing plant immune response [15].A conserved fungal effector PNPi fromPstinteracts with wheat TaPR1a protein to suppress host defense response [16].These studies indicate that fungal pathogens utilize a common strategy by targeting PRs to promote pathogenicity [17,18].
Plant thaumatin-like proteins (TLPs) that are also named PR5 belong to one of the PR protein families that have been evidenced to be involved in plant resistance in response to diverse biotic stresses [19].An increasing number of studies demonstrate that PR5 confers antifungal activity in various plant species [20,21].TLPs display other enzymatic activities,including glucanase activity [22],xylanase inhibitor [23],and anti-pest activities [24].In addition,TLPs can be targeted by different plant pathogens to promote their infection.For example,Botrytis cinereaelicitor protein BcIEB1 protects the fungus by targeting tobacco PR5 to inhibit its antifungal activity [25].Verticillium dahliaPevD1 interacts with cotton PR5-like protein GhPR5 to protectV.dahliaagainst the antifungal activity of GhPR5,thereby facilitating fungus infection[26].Kumar et al.[27]found that the secreted cysteine-rich Alt a 1 protein fromTrichoderma virenspromotes root colonization likely via direct interaction with maize PR5 to inactivate its function usingde novomodeling and protein-protein docking analysis.Alt a 1 protein related to virulence and pathogenicity ofAlternaria alternatainteracts with a kiwi PR5 and partially inhibits the βglucanase activity of PR5[28].Although many plant pathogens target TLP to suppress host defense,howPtmanipulates TLP to enhance its infection remains largely unknown.
We identified aPteffector protein (Pt_21) that interacts with TaTLP1 in the apoplastic space,and the cysteine residue located at position 71 in the N-terminal region of TaTLP1 is responsible for the interaction.In addition,we found that Pt_21 can suppress Bax-induced cell death inNicotiana benthamianaleaves and inhibit the antifungal activity of TaTLP1in vitroandin vivoto facilitatePtinfection.Our findings confirmed thatPtalso targets TLP protein to suppress host defenses,which broadens our understanding ofPtvirulence mechanisms.
2.Materials and methods
2.1.Plant materials,Pt race,and sample collection
Wheat near-isogenic line Thatcher,TaTLP1-overexpressing transgenic line (TaTLP1-OE),wild-type (WT) Jinan 1 (JW1) wheat andN.benthamianaare preserved in the Laboratory of Wheat Leaf Rust,Hebei Agricultural University.Ptrace PHNT (isolate 07-10-426-1) was used in all leaf rust inoculation that was performed as previously described by Roelfs [29].The second leaves of seedlings inoculated with PHNT or distilled water (control) for RNA extraction were harvested at 0,24,48,72,96,120,144,and 168 h post inoculation(hpi).All samples were immediately frozen in liquid nitrogen and stored at -80 °C.Each treatment included three independent biological replicates.
2.2.Screening of yeast two-hybrid (Y2H) library
Y2H library was previously constructed from PHNT-infected susceptible Chinese Spring wheat[30].The titer of the Y2H library was 2.4×106cfu mL-1,which meets the criterion for Y2H screening (Fig.S1).For Y2H assays,the bait vector pGBKT7-ΔspTaTLP1 was transformed into Y2HGold yeast strain using LiAc and PEG/LiAc (Clontech,Kyoto,Japan).Screening of interacting targets of ΔspTaTLP1 was conducted using the Matchmaker Gold Y2H System (Clontech).Positive colonies were selected for colony PCR using Matchmaker Insert Check PCR Mix 2 (Clontech) and sequenced at Sangon Biotech Co.,Ltd.(Shanghai,China).Sequence analyses were performed using the Ensembl Fungi(http://fungi.ensembl.org/index.html) and NCBI (https://www.ncbi.nlm.nih.gov/)databases.The protein molecular mass was predicted by Prot-Param (https://web.expasy.org/protparam/).Localization of the secreted protein was predicted by Cell-PLoc (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc/).ThePteffector protein was predicted by EffectorP-fungi 3.0 (https://effectorp.csiro.au/).The signal peptide of protein was identified using SignalP 5.0 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0).
2.3.Y2H validation and Co-immunoprecipitation (Co-IP) assay
Primers were designed to amplify the coding regions ofPt_21without signal peptides (Table S1).The recombinant vector pGBKT7-ΔspPt_21 was constructed.Cysteines (Cys29,Cys71,Cys80,Cys85,Cys91,Cys129,Cys137,Cys142,Cys159,and Cys170)were mutated to alanine in TaTLP1 by polypeptide synthesis,and ten pGADT7 recombinant vectors were constructed.The Matchmaker Gold Y2H System was used to verify the interaction between ΔspTaTLP1(bait)and ΔspPt_21(prey)or ΔspPt_21(bait)and ten TaTLP1 mutants (prey).The bait and prey plasmids were co-transformed into yeast strain Y2HGold according to the manufacturer’s instructions.Transformed yeast cells were assayed for growth on synthetic dropout SD/-Trp-Leu plates and SD/-Trp-Leu-His-Ade plates containing X-α-Galactosidase (X-α-Gal) and Aureobasidin A (AbA).
The recombinant protein pCamA-ΔspPt_21 was transformed into 4-week-oldN.benthamianabyAgrobacterium tumefaciens.At 48 h post agroinfiltration,total protein was extracted with extraction buffer(50 mmol L-1Tris pH 7.5,150 mmol L-1NaCl,1%NP-40,5 mmol L-1DTT,100× Protease Inhibitor Cocktail,For Bacterial Cell,with EDTA).ΔspTaTLP1,ΔspPt_21,and TaTLP1 mutant protein TaTLP1C71Awere induced by isopropyl β-d-1-thiogalactopyranoside (IPTG) with a final concentration of 0.5 mmol [31].For Co-IP assays,a mixture of 2 mL ΔspPt_21 and ΔspTaTLP1 or ΔspPt_21 and TaTLP1C71Aproteins containing 100 μL GST agarose beads were incubated at 4°C for 4 h with gentle shaking.The precipitated proteins were washed five times with washing buffer (50 mmol L-1Tris-HCl pH 8.0,1.25 mol L-1NaCl,1.5 mmol L-1EDTA,and 2 mmol L-1DTT).The precipitated proteins and crude proteins(input)were detected by immunoblotting with an anti-GST antibody and anti-GFP antibody (Solarbio,Beijing,China).
2.4.Co-localization assays
Red fluorescent fusion constructs were produced by cloning the coding sequence ofTaTLP1into the vector pGWB454.GFP fluorescent fusion constructs were produced by cloning the coding sequence ofPt_21into the vector pCamA (Table S1).ResuspendedAgrobacteriumstrains (GV3101) carrying pGWB454-TaTLP1 and pCamA-Pt_21 at a final OD600=0.8 were mixed in equal proportions immediately prior to infiltrations.AllN.benthamianainfiltrations were performed on 4-5-week-old plants.Fluorescence in leaves ofN.benthamianawas monitored at 48 h after agroinfiltration and after a plasmolysis assay by adding 1 mol L-1NaCl to the infiltrated leaves for 5 min,and then imaged directly using a confocal laser scanning microscope (Olympus FluoView FV1000,Tokyo,Japan).
2.5.RNA extraction and qPCR analysis
Total RNA of Thatcher wheat was extracted using an M5 Plant RNeasy Complex Mini kit(Mei5bio,Beijing,China).cDNA was synthesized using an M5 Super Plus qPCR RT kit with gDNA remover(Mei5bio,Beijing,China).qPCR was conducted using 2× M5 HiPer Realtime PCR Super Mix (Mei5bio,Beijing) with an ABI QuantStudio 5 instrument (ABI,Waltham,MA,USA).The expression ofPt_21was investigated,and the β-actingene (GenBank accession OAV91054) was used to calibrate expression levels of queried genes.Data were analyzed by the 2-ΔΔCT method [32].Statistical significances of differences were calculated using one-way analysis of variance(ANOVA)and least-significant difference(LSD)analysis in SPSS 26.0 (IBM SPSS Statistics,IBM,Armonk,NY,USA).Data are presented as means±SEM of three biological replicates from single experiments.
2.6.Yeast secretion trap assay
The yeast signal sequence trap system was used to validate the signal peptide function of Pt_21 as described previously [33].The signal peptide sequence ofPt_21was cloned into vector pSUC2T7-M13ORI (pSUC2) using specific primers (Table S1) and then transformed into an invertase mutant yeast strain YTK12.CMD-W medium was used to screen the positive colonies.The secretory function was confirmed by growth on YPRAA plates containing raffinose and lacking glucose.Invertase activity was detected by the reduction of 2,3,5-triphenyltetrazolium chloride(TTC)to insoluble red-colored 1,3,5-triphenylformazan(TPF)according to procedures and conditions described previously [34].
2.7.Agrobacterium infiltration assays
Bax,a cell death-promoting protein of the Bcl-2 family in mice[35],causes programmed cell death (PCD) inN.benthamiana,representing a similar defense-related cell death [36].Inhibition of Bax-induced cell death is often regarded as a criterion for suppressing plant immunity [37].Suppression of Bax-triggered cell death by effector ΔspPt_21 and four mutant ΔspPt_21 was determined by theAgrobacterium-mediated transient expression method [38].The sequences of ΔspPt_21and mutant ΔspPt_21were introduced into pCamA vector.ResuspendedAgrobacteriumcultures carrying pCamA,ΔspPt_21,and ΔspPt_21 mutants at a final OD600of 0.5 and MMAi buffer (5 g L-1MS salts,20 g L-1sucrose,and 10 mmol L-1MES,200 mmol L-1Acetosyringone)were infiltrated intoN.benthamianaleaves.The infiltration sites were challenged 24 h later with recombinant anAgrobacteriumculture carrying Bax at a final OD600of 1.Symptoms were monitored and recorded from 2 to 4 d after infiltration.
2.8.Antifungal activity assays in vitro
TaTLP1,TaTLP1C71A,andPt_21were individually cloned into pGEX-6P-3 (with GST tag) and expressed inEscherichia coli.Crude proteins were induced by IPTG with a final concentration of 0.5 mmol [31].A GST-Agarose Label Kit (TRAN,Beijing,China)was used to bind GST-tagged protein.The purified protein products were separated by 15% SDS-PAGE and visualized by Coomassie Blue staining.Ptrace PHNT was tested for growth response in the presence of TaTLP1+Pt_21 and TaTLP1C71A+Pt_21 complex,and TaTLP1,TaTLP1C71A,and Pt_21 alone.Petri dishes containing 20 mL agar medium containing TaTLP1,TaTLP1C71A,and Pt_21 protein(0.1 mg mL-1)or TaTLP1 plus Pt_21 and TaTLP1C71Aplus Pt_21(0.1 mg mL-1) were incubated in darkness at 25 °C and germination of spores and hyphal length was determined with a Nikon Ti2-LAPP Ti2 Laser Application System (Nikon Corporation,Minato-ku,Tokyo,Japan).The same experiment was also carried out with sterile water,1×PBS buffer,and GST as negative controls.
2.9.Antifungal activity assays in planta
In our previous study,TaTLP1was transformed into vector pLGY-02 under the control of the maize ubiquitin promoter (ubi)and transferred into cultivar JW1 by theAgrobacterium-mediated method to create a transgenic cultivarTaTLP1-OE [39].Therefore,TaTLP1-OE and JW1 (WT) lines were used for infiltration assays.GST,Buffer,and Pt_21 proteins were diluted to 0.1 mg mL-1in 1× PBS buffer (137 mmol L-1NaCl,2.7 mmol L-1KCl,10 mmol L-1Na2HPO4,and 2 mmol L-1KH2PO4) and were infiltrated into the first and second leaves ofTaTLP1-OE and JW1 lines.TaTLP1,Pt_21,TaTLP1C71A,TaTLP1+Pt_21,and TaTLP1C71A+Pt_21 proteins were infiltrated into the leaves of JW1 lines using a 0.5-mL syringe,GST and buffer as the negative control.Inoculations withPtrace PHNT were carried out 24 h after protein infiltration,and disease responses were observed after 14 d post inoculation (dpi).In all these experiments,each treatment included 3-5 plants and was repeated at least two times.The numbers of urediniospores were quantified using ImageJ software [7,40].The 3,3-diaminobenzidine(DAB,Solarbio,Beijing,China)staining was conducted following the protocols described previously [41] and was then viewed by differential interference contrast in an optical microscopy.
3.Results
3.1.Pt effector Pt_21 interacts with wheat TaTLP1
To assess whether TaTLP1 is targeted byPteffector proteins in thePt-wheat compatible interaction,screening a yeast Y2H prey library derived from PHNT-infected susceptible Chinese Spring wheat was conducted using ΔspTaTLP1(lacking the signal peptide)as a bait.Twenty-three positive candidate targets,including 13Ptgenes and 10 wheat genes,were identified(Tables S2,S3),in which Pt_21,with the highest clone number and predicted extracellular localization,was selected for further analysis.Pt_21has an open reading frame (ORF) of 459 bp encoding a 17 kDa protein of 153 amino acids (Protein ID OAV91388) without a known conserved domain and containing a cysteine residue (Fig.S2).
To validate the interaction between Pt_21 and TaTLP1,a Y2H assay was conducted using pGBKT7-ΔspTaTLP1 as bait and pGADT7-ΔspPt_21 as prey.Fig.1A showed that the yeast transformants co-expressing ΔspTaTLP1 and ΔspPt_21 were able to grow and produce blue coloration on selection plates consistent with the positive control ΔspTaPR1 and ΔspTaTLP1.However,the yeast transformants co-expressing either the ΔspPt_21 and empty pGBKT7 vector or ΔspTaTLP1 bait and empty pGADT7 vector were not able to grow on the selective plates(Fig.1A).To further confirm this association,the Co-IP was performed using transiently produced ΔspPt_21-GFP protein inN.benthamianaand ΔspTaTLP1-GST protein produced inE.coli.ΔspPt_21-GFP co-immunoprecipitated with ΔspTaTLP1-GST (Fig.1B).Furthermore,we transiently expressed RFP-tagged TaTLP1 (with signal peptide) and GFP-tagged Pt_21 (with signal peptide) inN.benthamianaleaves using agroinfiltration and microscopy observation showed that GFP-tagged Pt_21 and RFP-tagged TaTLP1 colocalized in the apoplast ofN.benthamianaleave,as evidenced by the plasmolysis assay (Fig.1C).However,the GFP control was localized in the cytosol and nucleus ofN.benthamianaleaves after the plasmolysis assay(Fig.S3).Taken together,these findings confirm that Pt_21 interacts with TaTLP1 and the interaction can occur in the apoplast.

Fig.1.Pt_21 directly interacts with TaTLP1 protein and both colocalize to plant apoplastic space.(A)Interaction of ΔspTaTLP1 and ΔspPt_21 was detected by Y2H.Growth of yeast strains coexpressing ΔspTaTLP1 fused to the GAL4 DNA binding domain(BD)with ΔspPt_21 fused to the GAL4 activation domain(AD)on control media lacking leucine and tryptophan (SD-WL) or selective media additionally lacking histidine (SD-HAWL-X-α-Gal).ΔspTaPR1 (BD) and ΔspTaTLP1 (AD) were the positive controls.A 10-fold dilution series is shown.(B)The Co-IP assay confirmed the interaction between ΔspPt_21 and ΔspTaTLP1.Western blots of total proteins transiently expressing the marked constructs and proteins eluted from GST beads were detected with the anti-GFP or anti-GST antibody.The protein marker was labeled on the left.(C)Transiently expressed Pt_21-GFP and TaTLP1-RFP proteins in the N.benthamiana leaf epidermal cells by agroinfiltration.The yellow arrow indicates the apoplastic space (AP).Scale bars,50 μm.
3.2.Pt_21 is highly induced during Pt infection and encodes a protein containing a functional signal peptide
To investigate the expression profiles ofPt_21during thePtwheat compatible interaction,we quantified the expression ofPt_21at different hours after PHNT inoculation in Thatcher wheat.Fig.2A shows that the expression levels ofPt_21are significantly induced at 24,48,72,96,and 120 hpi,respectively,compared to that at 0 hpi.At 24 hpi,the expression ofPt_21reached the highest level,suggesting thatPt_21is involved in the early stage ofPtinfection.

Fig.2.Expression profiles of Pt_21 in compatible interaction and functional validation of the putative N-terminal signal peptide.(A) The transcriptional level of Pt_21 gene.The relative expression is expressed as fold change relative to mock inoculated plants at 0 hpi.The y-axis indicates the amounts of Pt_21 genes transcript normalized to the β-Actin gene.The x-axis indicates sampling times.Values are means ± SEM of three independent experiments.Significant differences were assessed using one-way analysis of variance (ANOVA) and least-significant difference (LSD) analysis (P <0.01).(B) Functional validation of the putative Nterminal signal peptide of Pt_21 using the yeast invertase secretion assay.Yeast strain YTK12 carrying pSUC2-SP-Pt_21,which expresses two different signal peptides fused in frame to the mature invertase gene SUC2,were able to grow in YPRAA (Yeast-Peptone-Raffinose-Antimycin A) medium with raffinose as sole carbon source.YTK12 and Mg87 were used as the negative control and pSUC2-SP-TaTLP1 was used as the positive control.Invertase activity was detected with 2,3,5-triphenyltetrazolium chloride (TTC).The red color indicates invertase activity.
To examine whether Pt_21 contains a functional signal peptide,its predicted signal peptide coding sequence was cloned into a yeast secretion trap vector pSUC2.The yeast strain YTK12 transformed with SP-Pt_21 or positive control the signal peptide of TaTLP1 (SP-TaTLP1) construct was able to grow on CMD-W and YPRAA media (Fig.2B).The 2,3,5-triphenyltetrazolium chloride(TTC)-treated SP-TaTLP1 and SP-Pt_21 culture filtrates turned red,whereas the negative control culture filtrates treated with TTC remained colorless (Fig.2B).These results indicate that Pt_21 contains a functional signal peptide responsible for the secretion of Pt_21 into the plant cell.
3.3.The N-terminal region of Pt_21 is required for the suppression of Bax-induced cell death
To examine the ability of Pt_21 to inhibit Bax-induced cell death,the vector pCamA-ΔspPt_21 was introduced intoAgrobacteriumand the agroinfiltration ofN.benthamianaleaves was conducted.After 24 h,pEarlyGate100-Bax was infiltrated into the regions transiently expressingPt_21.Fig.3 showed that ΔspPt_21 completely suppressed Bax-induced cell death inN.benthamiana.However,the negative control containing the empty pCamA vector did not inhibit Bax-induced cell death.
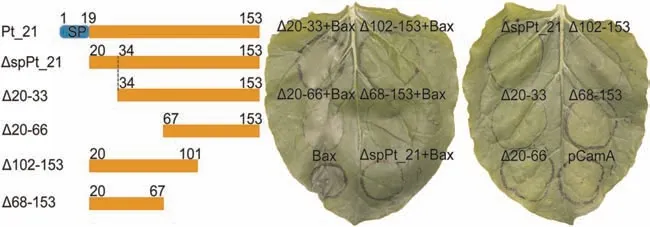
Fig.3.Pt_21 suppresses Bax-induced cell death and the N-terminal region of Pt_21 is required for the suppression.Agrobacterium-mediated transient expression of ΔspPt_21(a signal peptide deletion mutant) and Pt_21 (two N-terminal truncations:Δ20-33 and Δ20-66;two C-terminal truncations:Δ102-153 and Δ68-153) in N.benthamiana.ΔspPt_21 and mutants were transiently expressed in leaves 24 h prior to infiltration with cells expressing Bax.
To determine the minimal region of Pt_21 that is required to suppress Bax-induced cell death,four truncated versions of Pt_21 were generated,including two N-terminally truncated and two C-terminally truncated versions.As shown in Fig.3,the expressions of two C-terminally truncated Pt_21: Δ102-153 and Δ68-153 were able to suppress Bax-induced cell death,whereas the two N-terminal truncations,Δ20-33 and Δ20-66,failed to suppress Bax-induced cell death.In addition,green fluorescent signals were observed in the transiently expressed GFP-tagged truncated Pt_21 and the empty vector pCamA with GFP tag(Fig.S4),indicating the accumulation of truncated Pt_21 proteins after transient expression inN.benthamianaleaves.Taken together,these results demonstrate that Pt_21 can inhibit Bax-induced cell death and the N-terminal region of Pt_21 is indispensable for this suppression.
3.4.Amino acid Cys71 at the N-terminus of TaTLP1 is essential for the interaction with ΔspPt_21
To evaluate whether cysteine residues of TaTLP1 affect the interaction between TaTLP1 and ΔspPt_21,site-directed mutagenesis of the five cysteine residues at the N-terminal region (Cys29,Cys71,Cys80,Cys85,and Cys91) and five cysteine residues at the C-terminal region (Cys129,Cys137,Cys142,Cys159,and Cys170)was conducted,respectively (Fig.S5).All these cysteine residues were mutated to alanine.The Y2H results showed that yeast Y2HGold transformants co-expressing ΔspPt_21 and nine individual ΔspTaTLP1 mutated proteins(C29A,C80A,C85A,C91A,C129A,C137A,Cys142,C159A,and C170A)were able to grow and produce blue coloration on the selection medium,which was in accordance with the positive control ΔspTaPR1 and ΔspTaTLP1(Fig.4A).However,TaTLP1C71Afailed to interact with ΔspPt_21,indicating that Cys71 at the N-terminal region of ΔspTaTLP1 is indispensable for interaction with ΔspPt_21.The Co-IP assay was conducted to validate that Cys71 at the N-terminal region of TaTLP1 is indispensable for interaction with ΔspPt_21 using recombinant TaTLP1C71A-GST protein produced inE.coli(Fig.S6)and transiently expressed ΔspPt_21-GFP protein inN.benthamiana.Fig.4B showed that TaTLP1C71A-GST failed to co-immunoprecipitate with green fluorescence protein Pt_21-GFP,whereas the WT ΔspTaTLP1-GST was able to co-immunoprecipitate with ΔspPt_21-GFP.Collectively,these findings indicate that Cys71 at the N-terminal region of TaTLP1 is necessary for its interaction with ΔspPt_21.

Fig.4.Cys71 at the N-terminus of TaTLP1 is essential for the interaction with ΔspPt_21.(A) All transformants were able to grow on synthetic dropout medium without leucine and tryptophan (SD-WL) medium.Yeast colonies that were able to grow on a selective medium [SD medium without leucine,tryptophan,histidine,and adenine supplemented with X-α-Gal and AureobasidinA (SD-HAWL)] and displayed blue coloration confirmed the protein-protein interaction.The positive control was ΔspTaPR1-ΔspTaTLP1.pGADT7 and pGBKT7 were used as the negative controls.(B)Validation of the interaction between ΔspTaTLP1 and ΔspPt_21,TaTLP1C71A and ΔspPt_21 by the Co-IP assay.Total proteins from N. benthamiana leaves transiently-expressing ΔspPt_21-GFP,ΔspTaTLP1-GST,and TaTLP1C71A-GST were expressed in E.coli.ΔspPt_21 protein mixed with TaTLP1C71A or ΔspTaTLP1 proteins containing GST agarose beads were detected with the anti-GST and anti-GFP polyclonal antibodies.
3.5.Pt_21 suppresses the antifungal activity of TaTLP1 via targeting Cys71
We examined the effect of Pt_21 on the antifungal activity of TaTLP1 using recombinant protein Pt_21 produced inE.coli(Fig.S7).Consistent with the previous study,urediniospores germination and hyphal growth were significantly inhibited in the presence of TaTLP1 protein(Fig.5D,I,J).In contrast,in the presence of TaTLP1+Pt_21 protein,urediniospore germination and hyphal growth were significantly increased compared to the treatment with TaTLP1(P<0.05)(Fig.5F,I,J).Interestingly,we observed that urediniospores germination and hyphal growth were significantly restricted in the treatment with TaTLP1+Pt_21 compared to the negative controls treated with water,buffer,and GST protein,suggesting that TaTLP1+Pt_21 is not able to completely inhibit the antifungal activity (Fig.5A-C,E).Pt_21 protein did not affect the germination of urediniospores and hyphal growth as the urediniospores in the treatment with Pt_21 displayed similar germination rates and hyphal length with negative controls(Fig.5A-C,E,I,J).In conclusion,Pt_21 can inhibit the antifungal activity of TaTLP1.

Fig.5.Pt_21 inhibits the antifungal activity of TaTLP1 in vitro.Urediospore germination and hyphal development of Pt in the presence of protein on the 2%agar medium in darkness for 12 h at 22-25 °C.(A) Sterile water.(B) 1× PBS Buffer (C) Glutathione S-Transferase (GST).(D) TaTLP1.(E) Pt_21.(F) TaTLP1+Pt_21.(G) TaTLP1C71A.(H)TaTLP1C71A+Pt_21.(I,J) The hyphal length and urediniospore germination rate were visualized using Nikon Ti2-LAPP Ti2 Laser Application System,were measured using Image J,and were counted using Excel,respectively.Values are means±SEM of three independent biological replicates.Significant differences were assessed using one-way analysis of variance(ANOVA)and the Duncan’s multiple range test in SPSS 26.0(P <0.05).Protein was used at the same concentration.Sterile water,the 1×PBS buffer and the GST protein were used as control,and photographs were taken at 10× magnification.Scale bar,100 μm.
To further test whether Cys71 affects the antifungal activity of TaTLP1,the TaTLP1 mutant protein TaTLP1C71Awas expressed inE.coli(Fig.S6).After incubating urediniospore with mutant TaTLP1C71A,the hyphae lengths and germination rates were similar to TaTLP1 but lower than that treated with TaTLP1+Pt_21(Fig.5G,I,J).Moreover,TaTLP1C71A+Pt_21 significantly restricted the growth of hyphae compared to TaTLP1+Pt_21 protein (Fig.5H,I,J).These results indicate that C71A is not required for the antifungal activity of TaTLP1,but is required for the Pt_21-mediated suppression of TaTLP1 antifungal activity.
3.6.Pt_21 inhibits TaTLP1-mediated wheat defense responses to Pt in planta
To assess whether Pt_21 affects TaTLP1-mediated wheat defense in response toPtinfection,a disease assay was conducted by inoculatingPtrace PHNT on the leaves ofTaTLP1-overexpressing (TaTLP1-OE) lines at 24 h post infiltration of purified recombinant Pt_21.The susceptible and recipient wheat JW1 was included as a negative control.At 14 dpi,many necrotic spots and limited urediniospores were observed on the leaves ofTaTLP1-OE lines infiltrated with GST protein and buffer.However,the enhanced number ofPturediniospores were observed around the necrotic spots on the leaves ofTaTLP1-OE lines infiltrated with Pt_21 protein,but less than the negative controls (Fig.6A).These results suggest thatTaTLP1-OE lines infiltrated with Pt_21 showed significantly enhanced wheat susceptibility toPt.To further demonstrate that Pt_21 suppresses TaTLP1-mediated defense response via direct interaction,the purified TaTLP1,TaTLP1C71A,Pt_21,TaTLP1+Pt_21,and TaTLP1C71A+Pt_21 proteins were infiltrated into leaves of the JW1 lines,respectively.Infiltration of buffer and purified GST protein was included as negative controls.At 14 dpi,enhanced disease development was observed on the leaves co-infiltrated with TaTLP1 and Pt_21 compared to the leaves infiltrated with TaTLP1 or TaTLP1C71A,as evidenced by increased numbers of urediniospores on the leaves infiltrated with TaTLP1 and Pt_21 (Fig.6B).The number of urediniospores on the leaves infiltrated with TaTLP1C71Aprotein was similar to those on the leaves infiltrated with TaTLP1,but less than that infiltrated with TaTLP1+Pt_21,and water or GST protein.Moreover,less amount ofPturediniospores appeared on JW1 lines infiltrated with TaTLP1C71A+Pt_21 protein compared to that infiltrated with TaTLP1+Pt_21 (Fig.6B).To further confirm this observation,we measured the accumulation of H2O2using the DAB staining method.Pronounced staining of H2O2in the infected leaves ofTaTLP1-OE lines was observed.By contrast,the infected leaves of JW1 plant showed limited and weak staining (Fig.6C).These results indicate that Pt_21 can inhibit the antifungal activity of TaTLP1in plantaby interacting with TaTLP1,and the Cys71 of TaTLP1 at the N-terminus is required for the inhibition.

Fig.6.Pt_21 inhibits the antifungal activity of TaTLP1 in planta.(A,B)GST,buffer,and Pt_21(0.1 mg mL-1)were infiltrated into the first and second leaves of TaTLP1-OE and JW1 lines.Black lines indicate the infiltration zones.TaTLP1,TaTLP1C71A,TaTLP1+Pt_21,and TaTLP1C71A+Pt_21(0.1 mg mL-1)were infiltrated into the leaves of JW1 lines.Buffer and GST are negative controls.(C)The production of H2O2 and necrosis were observed in these leaves at 24,48,and 120 hpi.The accumulation of H2O2 at infection sites was detected by the DAB staining method and viewed under an optical microscopy with differential interference contrast setups.Scale bars,20 mm.
4.Discussion
Pathogenesis-related (PR) proteins induced by the infection of diverse plant pathogens are the key components of plant immunity[42].TLPs belong to the PR5 protein family,which are known to play an important role in plant defense against different pathogens[43,44].Our previous study showed that stable transgenic wheat line overexpressingTaTLP1displayed increased β-1,3-glucanase activity and enhanced resistance toPtand TaTLP1 interacts with TaPR1 in the plant apoplast to potentiate the resistance toPt[45,46].In this study,we identified that wheat leaf rust pathogen employs an effector protein Pt_21 to inhibit the antifungal activity of TaTLP1in vitroandin plantaand thereby promotesPtinfection.This inhibition is caused by the direct interaction between Pt_21 and TaTLP1 and the Cys71 at the N-terminus of TaTLP1 is required for this interaction but not required for the antifungal activity of TaTLP1.The increasing number of studies showed that plant fungal pathogens target PR proteins and thereby suppress plant immune responses to facilitate their infection.Our observations are consistent with previous findings that plant pathogens utilize virulence factors to target PR proteins to suppress host resistance.Therefore,we need further broaden our understanding of the molecular mechanisms underlying PR proteins manipulated by pathogen effectors in the future,which will aid us in developing CRISPRCas9-edited PR crops to reduce pathogen infection.
Our results showed that Pt_21 contains a functional signal peptide using yeast secretion trap assay and Pt_21 and TaTLP1 colocalize in the apoplast ofN.benthamianaplants where Pt_21 interacts with TaTLP1 (Figs.1C,2),which is similar to previous reports[16,47].It has been documented that the yeast secretion trap assay has been extensively used to validate the function of the signal peptide of plant fungal pathogen effector proteins.Sun et al.[48]reported that all the examined effector candidates ofFusarium oxysporumf.sp.lycopersicihave functional signal peptides using the yeast secretion trap assay.Wheat apoplast as the frontier line of resistance in response to leaf rust has been reported [49].An apoplast-localized wheat PR14 lipid transfer protein TaLTP3 is physically associated with wheat TaPR1a protein in the apoplast to improve wheat resistance to leaf rust pathogen [50].Interestingly,the wheat TaTLP1 also interacts with TaPR1 in the apoplast and this TaTLP1-TaPR1 complex exhibits stronger antifungal activity to leaf rust pathogen[46].These findings indicate that wheat PR proteins form the complex in the apoplast to defend against the infection of leaf rust.
Previous studies showed that the mature proteins of plant fungal effectors often feature a high number of cysteine residues,have no known conserved domains,and are highly expressed upon pathogen infection [51].Our studies found that Pt_21 is unique toPtafter blast searching in the Ensembl Fungi,but there are three homologs of Pt_21 present in thePtgenome,in which PTTG_05699 and Pt_21 share 90% similarity,and the similarity between PTTG_05706 and PTTG_05707 with Pt_21 is 46%(Fig.S8).Although the formation of disulfide bonds can stabilize the protein,there are no cysteine residues in the mature Pt_21 protein,suggesting that Pt_21 is likely unstable in the apoplast and might form a homodimer or a protein complex in the apoplast to function.The expression ofPt_21is significantly induced at 24 hpi (Fig.2A),which is the critical stage of haustoria formation and suppression of plant defense[52].The percentage of infection sites showing H2O2accumulated area was 40.0% at 12 hpi in the incompatible interaction between wheat andPst,and at 24 hpi the percentage of infection sites showing H2O2accumulation further increased [53].Based on these findings,we can concede thatPt_21is involved in the initial infection of wheat byPt,to inhibit the host defense response.
Truncated Pt_21 variants display different abilities to inhibit Bax-mediated cell death inN.benthamianaleaves (Fig.3).Surprisingly,deletion of the C-terminal 86 amino acids of Pt_21 did not impair its ability to suppress Bax-induced cell death,while the N-terminal amino acids from 34 to 66 are dispensable for inhibiting Bax-induced cell death (Fig.3).However,we did not test whether this minimal region of Pt_21 still can interact with TaTLP1.Using deletion mutants of Avr1b,Dou et al.[54]found that the K,W,and Y domains ofPhytophthora sojaeeffector Avr1b are responsible for the avirulence function.It has been documented that most TLPs contain 10 or 16 conserved cysteine residues forming disulfide bonds that can stabilize proteins and provide resistance to unfavorable pH,proteases,and heat-induced denaturation [55,56].In our study,cysteine residues of TaTLP1 were mutated to alanine to minimize the disruption of the folding structure of the WT TaTLP1 protein based on previous findings[57].Our findings showed that the Cys71 at the N-terminal region of TaTLP1 is necessary for its interaction with Pt_21(Fig.4A).However,Cys71 is not required for the antifungal activity of TaTLP1(Fig.6),indicating that Cys71 is not the key functional residue of TaTLP1,which is similar to our previous study that mutations of all N-terminal cysteine residues are not able to affect the interaction between TaTLP1 and TaPR1 [46].For a future study,we need to determine whether the minimal region of Pt_21 including 33 amino acids that are indispensable for suppressing Bax-induced cell death is required for the interaction with TaTLP1.
Using protein-mediated growth-inhibition assays ofPtpathogensin vitro,we found that the recombinant effector protein Pt_21 can promote urediospore germination and hyphal growth(Fig.5).In addition,the protein-mediated disease assay in different wheat lines showed that the infiltration of Pt_21 protein into wheat leaves ofTaTLP1-OE lines can suppress TaTLP1-mediated immune response compared to the controls(Fig.6).These findings support that Pt_21 contributes toPtvirulence by directly targeting wheat TaTLP1 proteins.
In summary,our results reveal thatPtexploits a secreted effector Pt_21 to suppress wheat defense by interacting with TaTLP1,thereby inhibiting the antifungal activity of TaTLP1in vivoandin vitro.Pt_21 also can suppress Bax-induced cell death.In addition,the TaTLP1 variant,TaTLP1C71A,is not able to interact with Pt_21.Infiltration of recombinant protein Pt_21 into leaves ofTaTLP1-OE enhanced the disease development of leaf rust compared to that in JW1 leaves.These findings broaden our understanding of the virulence mechanisms exploited byPteffector proteins.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Fei Wang:Conceptualization,Data curation,Methodology,Investigation,Validation,Writing-original draft,Writing-review&editing.Songsong Shen:Methodology,Data curation,Investigation,Validation.Zhongchi Cui:Methodology,Data curation,Investigation,Validation.Shitao Yuan:Methodology,Investigation.Ping Qu:Methodology,Investigation.Hui Jia:Methodology,Investigation.Linshuo Meng:Methodology,Data curation,Investigation,Validation.Xiaoyu Hao:Investigation.Daqun Liu:Supervision.Lisong Ma:Conceptualization,Writing -review &editing.Haiyan Wang:Conceptualization,Writing-review&editing,Project administration,Resources,Supervision.
Acknowledgments
We thank Prof.R.A.McIntosh(Plant Breeding Institute,University of Sydney) for review and English editing of the manuscript;Prof.M.Hossein Borhan (Agriculture and Agri-Food Canada) for providing the gateway vectors;Prof.Zhimin Hao (Hebei Agricultural University) for providing the pGEX-6P-3 plasmids.This work was supported by the National Natural Science Foundation of China (32172384 and 31501623),the Natural Science Foundation of Hebei (C2020204028),the Key Research and Development Project of Hebei Province(20326505D),and the‘‘Hundred Talents Program”for the Introduction of High-level Overseas Talents in Hebei Province (E2020100004).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.04.006.
- The Crop Journal的其它文章
- Reversible protein phosphorylation,a central signaling hub to regulate carbohydrate metabolic networks
- Genetic and environmental control of rice tillering
- High-throughput phenotyping of plant leaf morphological,physiological,and biochemical traits on multiple scales using optical sensing
- The R2R3-MYB transcription factor GaPC controls petal coloration in cotton
- The photosensory function of Zmphot1 differs from that of Atphot1 due to the C-terminus of Zmphot1 during phototropic response
- Disruption of LEAF LESION MIMIC 4 affects ABA synthesis and ROS accumulation in rice

