A new prognostic model for drug-induced liver injury especially suitable for Chinese population
Yan-Fei Chen,Lan-Juan Li
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases,National Clinical Research Center for Infectious Diseases,Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases,The First Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou 310 0 03,China
Drug-induced liver injury (DILI) is a rare side effect of drugs caused by all kinds of prescription or over-the-counter chemicals,biological agents,traditional Chinese medicine (TCM),natural medicine (NM),health products,dietary supplements and their metabolites,and even excipients,which can lead to jaundice,liver failure,or even death.Although it is rare in term of single drug,the occurrence of DILI in all liver injuries is not low due to the wide range of drugs and foods involved.Moreover,there was an increasing trend of incidence of DILI since 2010 worldwide,with Asian regions showing the highest incidence [1].
In this issue ofHepatobiliary&PancreaticDiseasesInternational,Wang et al.developed a new model,DILI mortality predictive(DMP) score,for prediction of the risk of liver-related death or liver transplantation (LT) in DILI patients [2].The DMP score was based on international normalized ratio (INR),total bilirubin (TB),albumin (ALB),aspartate aminotransferase (AST)/alanine aminotransferase (ALT) and platelet (PLT) count at DILI onset.Although these variables had been used in previous models to predict serious outcomes (acute liver failure and death) in DILI patients,this was the first time to develop and validate a model with a large cohort of Chinese DILI patients,which showed better performance than other models developed in Western populations.
At present,the mortality of DILI varies greatly across different countries and populations [3–16](Table 1).The highest mortality reported was in India;Devarbhavi et al.demonstrated that the overall 90-day mortality was 17.3%.However,since 58% of the DILI was caused by a combination of four anti-tuberculous drugs in that study,the high mortality might be influenced by comorbidity of tuberculosis [13].In a retrospective study on DILI in China,the incidence of death/LT was only 0.4% [17].The major problems of previous epidemiology studies with mortality data including the population included in various study protocols (inpatient or outpatient),the observed clinical outcomes (acute liver failure or mortality),and the duration of follow-up (28 days,2 months,6 months,or 12 months) are not uniform,which makes it impossible to compare the results.Thus,a comparative study of mortality in DILI was warranted with stratification of regions,implicated drugs,and commodity diseases in further studies.
There have been several prognostic models in use to predict acute liver failure or death in DILI patients,including Hy’s law,new Hy’s law,Robles-Diaz model,drug-induced liver toxicity acute liver failure (DrILTox ALF) score,Ghabril model,and the DMP score [2,18–21](Table 2).Besides,model for end-stage liver disease (MELD) score was often used to predict 30-day mortality in patients with end-stage liver diseases,but not restricted to DILI.Among these prediction models,only the Ghabril model and the DMP score were designed for prediction of death/LT in DILI.Different clinical characteristics were implicated in these models,thus they might have strength and weakness in regarding to different circumstances.Wang et al.previously compared the accuracy of six models in a cohort of DILI patients hospitalized at two tertiary hospitals in Beijing,and confirmed that MELD score and Ghabril model have the best predictive performance in the prediction of mortality within 12 months after DILI onset [3].In the article of this issue by Wang et al.[2],the DMP score was superior in comparison with previously proposed predictive scores.The area under the receiver operating characteristic curves (AUCs) of the DMP score were significantly higher than those of Hy’s law,new Hy’s law,Robles-Diaz model,DrILTox ALF score,and Ghabril model for the prediction of 28-day,90-day and 6-month mortality,which implies that this model is superior to others for Chinese population with DILI.

Table.1Epidemiology studies with mortality data in drug-induced liver injury.
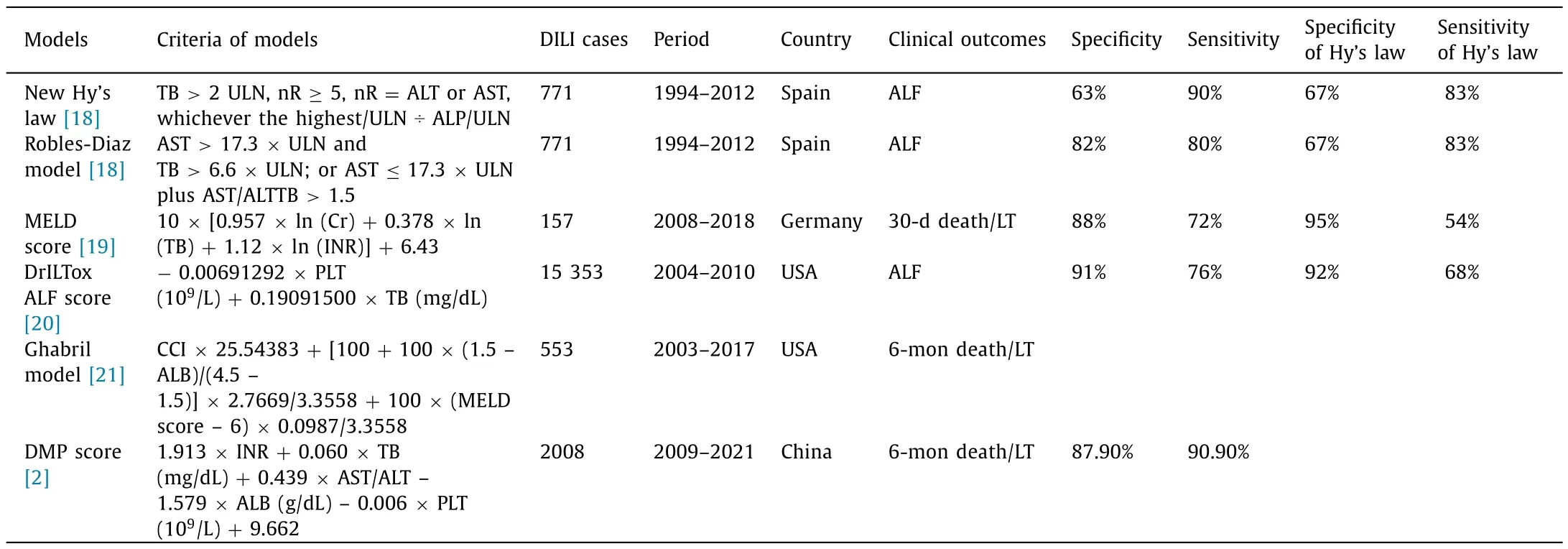
Table 2Prognostic model studies in DILI.
Since Asia harbor the highest incidence of DILI worldwide,China was supposed to be the country with the largest population of patients with DILI.Moreover,drugs implicated in DILI were quite different between Western and Eastern countries,with antibiotics being the leading causes of DILI in the West,whereas TCM and NM are major causes of DILI in Eastern countries.Also,genetic risk factors are associated with the incidence of DILI,which might explain the different characteristics of DILI between different regions.Traditional models used for DILI,like Rucam and Hy’s law might not be sensitive enough for DILI patients caused by TCM and NM [17].Understanding the specific risk factors of DILI in China could help make therapeutic strategies to deal with the emerging health problems.
Wang et al.proposed a prognostic model to predict death within 6 months with a large Chinese DILI cohort.Better performance of this model was demonstrated in comparison with previously developed models using two other independent validation cohorts.Thus,this model is supposed to be especially suitable for Chinese DILI patients.However,there might be some bias because of the retrospective research design.Also,patients with underlying liver diseases were excluded in this study.Whether this model is suitable for patients with liver diseases background needs to be further validated.Taken all together,a prospective analysis with a wider range of patients,including outpatients as well as those with underlying liver diseases,is warranted in future research.
Acknowledgments
None.
CRediTauthorshipcontributionstatement
Yan-FeiChen:Funding acquisition,Writing– original draft.Lan-JuanLi:Conceptualization,Writing– review &editing.
Funding
This study is supported by grants from National Key R&D Program of China (2022YFC36020 0 0) and Zhejiang Provincial Natural Science Foundation of China (LZ22H030 0 01).
Ethicalapproval
Not needed.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2023年6期
Hepatobiliary & Pancreatic Diseases International2023年6期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Post-hepatectomy liver failure: A timeline centered review
- INSTRUCTIONS FOR AUTHORS
- Value and prognostic factors of repeat hepatectomy for recurrent colorectal liver metastasis
- Older liver grafts from donation after circulatory death are associated with impaired survival and higher incidence of biliarynon-anastomotic structure
- Development and validation of a novel model to predict liver-related mortality in patients with idiosyncratic drug-induced liver injury
- Clinical-radiomics predictors to identify the suitability of transarterial chemoembolization treatment in intermediate-stage hepatocellular carcinoma: A multicenter study
