Chlorella across latitudes:investigating biochemical composition and antioxidant activities for biotechnological applications
Chloe Zi-En WONG, Ming-Li TEOH,2*, Sook Wah CHAN,3, Nallammai SINGARAM,2, Wendy Ming-Yen TEOH & John BEARDALL
Article
across latitudes:investigating biochemical composition and antioxidant activities for biotechnological applications
Chloe Zi-En WONG1, Ming-Li TEOH1,2*, Sook Wah CHAN1,3, Nallammai SINGARAM1,2, Wendy Ming-Yen TEOH4& John BEARDALL5
1School of Biosciences, Faculty of Health and Medical Sciences, Taylor’s University, Subang Jaya 47500, Malaysia;2Clean Technology Impact Lab, Taylor’s University, Subang Jaya 47500, Malaysia;3Food Security & Nutrition Impact Lab, Taylor’s University, Subang Jaya 47500, Malaysia;4Institute of Technology Management and Entrepreneurship, Universiti Teknikal Malaysia Melaka (UTeM), Durian Tunggal 76100, Malaysia;5School of Biological Sciences, Monash University, Clayton VIC 3800, Australia
With the present day rise of interest in acquiring sustainability in the pharmaceutical industry, there has been an emphasis on finding natural resources to replace the use of synthetic compounds used in products. Microalgae have garnered significant attention owing to their natural and sustainable capability to produce a diverse array of bioactive compounds. Therefore, this study aims to evaluate the biochemical composition and antioxidant properties ofstrains from a tropical region (UMACC 051 andUMACC 038) and a polar region (UMACC 250 andUMACC 234). The cultures were grown for 10 d. At the end of the experiment, the specific growth rate, chlorophyll-contentcarotenoid content, biomass, and biochemical composition such as carbohydrate, protein and lipid content were determined. In addition, the phytochemical properties were determined using a total phenolic assay while the antioxidant activities were determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH). Of allstrains tested, the tropicalUMACC 051 showed the fastest growth rate and biomass while the polarUMACC 234 contained the highest pigment content and tropicalUMACC 038 has the highest total phenolic content. The biochemical composition analysis showed all strains have a high lipid content ranging from 45.36% to 60.30% dry weight. Allstrains exhibited a small amount of antioxidant activity (15.42% to 30.15%) and total phenolic content ranging from 1.91 ± 0.04 to 4.43 ± 0.10 mg GAE·g–1dry weight. The results indicated that polarUMACC 234 has the most potential in containing significant amounts of bioactive compounds.
, polar, tropical, biochemical composition, total phenolic content, DPPH
1 Introduction
In the current era, many companies utilize synthetic materials in their product and the pharmaceutical industry is not immune to this as many compounds used in the commercially available products are a mix of synthetic and non-synthetic chemicals (Newman et al., 2003). Nowadays, with the rise of interest in acquiring sustainability in the pharmaceutical industry, there has been an emphasis on finding natural resources to replace the use of synthetic compounds used in the products (Milanesi et al., 2020). Some of the many synthetic compounds are antioxidants and despite being useful in preventing oxidation reactions in several products such as food, it has been reported that synthetic antioxidants can be carcinogenic and can bring about toxic effects (Milanesi, et al., 2020; Coulombier et al., 2021). Other synthetic medications for different symptoms such as inflammation have also been the subject of speculation regarding their potential to cause adverse side effects. Such concerns have increased the popularity of herbal medicines (Beg et al., 2011). Hence, there has been a need to find natural bioactive compounds as alternatives to these synthetic compounds to combat the risk of serious side effects as well as to provide a more sustainable resource, and microalgae can potentially be one of these sources.
Microalgae are unicellular photosynthetic microorganisms that are diverse and capable of thriving in many different environments around the globe. These microorganisms mainly live in aquatic environments whether it be marine or freshwaters (García et al., 2017). Microalgae can efficiently produce biomass and a wide range of organic compounds through the utilization of solar energy and carbon dioxide which allows them to play a crucial role as the main primary producers of aquatic biological ecosystem (Kiuru et al., 2014). Due to their rapid growth rate and turnover rate, it has been said that microalgae contribute approximately half of the global primary productivity (Gao et al., 2012). With its many properties, microalgae have gathered much attention in the research field.
Microalgae can thrive in many different environments and are even found in extreme parts of the world such as polar or tropical climates. Polar microalgae are one of the many organisms that are found in harsh Arctic environments. These microalgae have evolved several strategies used to cope with the harsh environment and in many cases, this has resulted in the production of an array of bioactive compounds that can be harvested for their potential benefits (Montuori et al., 2023). Polar microalgae have been speculated to be capable of handling cold climates by producing more polyunsaturated fatty acids (PUFAs) (Teoh et al., 2013). PUFAs are not only important for microalgal function but different types, such as linolenic acid, can be used as an essential nutrient in the human diet (Whelan and Fritsche, 2013).
Tropical microalgae are one of the many microorganisms that make up the vast biodiversity of tropical regions. One of the research interests on tropical microalgae is its potential to cultivate microalgal fatty acids (FAs) that can be used as a basis for renewable biodiesel (Aratboni et al., 2019; Correa et al., 2020). For instance, in a study done on tropical microalgae, it was identified thatisolated from Kota Kinabalu, Sabah had a moderately high lipid productivity (42.90 mg·L–1·d–1), highest lipid content (39% dry weight (DW)), high level of monounsaturated fatty acids (MUFAs) and C14–C18 FAs (81.47%), and highest oleic acid proportion (28.38%) which was concluded to be a favorable source for biodiesel production (Andrew et al., 2022).
sp. is one of the most well-studied microalgal genera in the world (Bock et al., 2011).sp. can be found in many regions of the world and are a very adaptive type of microalgae that can withstand an array of different stresses. For instance, in a study onthe microalgae were subjected to a temperature of 40 ℃ during heterotrophic cultivation and were seen to be capable of reaching a maximum specific growth rate of 1.45 d–1(Dai et al., 2022). In another study,sp. was exposed to high levels of free ammonia in wastewater to evaluate its response to a sudden change in its environment. Initially, the free ammonia caused damage to the microalgae’s DNA which stunted the cell growth, and inhibited processes like photosynthesis and nutrient uptake but over time, the microalgae were able to acclimate to the situation by repairing the damaged DNA (Chen et al., 2022). These examples highlight the profound ability ofsp. to adapt to certain stresses and may be useful in different industries.
Microalgae are often used as a food source in their ecosystems for many different organisms and have been used by humans for hundreds of years as a form of food source or nutritional supplement. Currently,andare commonly found in health foods and have been marketed as one of the most nutritious foods in the market (García et al., 2017). In most cases, the biochemical composition of microalgae consists of 40%–60% proteins, 20%–30% carbohydrates, and 10%–20% lipids (Singh et al., 2011). The study of Niccolai et al. (2019) examined the different biochemical composition of 12 microalgae, and it was highlighted that cyanobacteria and thespecies had the most protein content but the least lipid content. The study also noted a difference between the fresh and marine microalgae in terms of their fatty acids compositions with marine microalgae having a higher concentration of PUFAs (Niccolai et al., 2019). The diverse biochemical compositions of microalgae create value as it showcases the potential usages of microalgae in different industries.
The potential health benefits of microalgae have been evaluated in several past studies and antioxidant activity is one of the most well-known characteristics of microalgae in this respect (Yu et al., 2022). The type of antioxidant molecule that can be found in microalgae are ascorbic acid, glutathione, tocopherols, phenolic compounds carotenoids, mycosporine-like amino acids, polysaccharides and phycobiliproteins (Coulombier et al., 2021). With the vast diversity of microalgae around the world, research is still ongoing to identify the antioxidant molecules and some of the past research results for antioxidant activities and total phenolic content (TPC) are presented in Table 1. For instance, studies on 19 Nordic microalgae and five indigenous Malaysian microalgae concluded that all the microalgae were able to exhibit antioxidant properties by containing significant amounts of carotenoids, phenolic compounds, and flavonoids (Tiong et al., 2020; León-Vaz et al., 2023).

Table 1 The antioxidant activity and total phenolic content of microalgae found in different studies
One of the many physiological benefits of microalgae antioxidant compounds is their ability to act as an anti-inflammatory agent (Teoh et al., 2023). Studies have shown that there are correlations between oxidative stress and inflammatory disease (Choo et al., 2020). In a study in Japan, a novelsp. from a Beppu hot spring was concluded to have anti-inflammatory affects due to its antioxidant properties and can be a potential treatment candidate for skin and joint inflammatory disorders (Miyata et al., 2021). With a growing amount of oxidative stress related diseases, finding new sources for antioxidant compounds should still be relevant and conducted.
Therefore, the aim of this study is to evaluate the biochemical composition, phytochemical content, and antioxidant activities of two tropicalstrains (UMACC 051, andUMACC 038) and two polarstrains (UMACC 250 andUMACC 234). The carbohydrate, protein, lipid, antioxidant contents and TPC were determined for each strain. By investigating the intricate biochemical and physiological attributes of microalgae, this study endeavors to uncover novel insights that could have significant implications for their application in various biotechnological sectors.
2 Materials and methods
2.1 Algal culture
In this study, four microalgae consisting of two tropicalstrains and two polarstrains were used for the experiments. The origin of each microalgal strain is listed in Table 2.UMACC 051 andUMACC 038 are tropical strains whileUMACC 250 andUMACC 234 are polar strains. All strains were obtained from University of Malaya Algae Culture Collection (UMACC).

Table 2 Origin of the microalgae strains that were used in this study. All strains are available from the University of Malaya Algae Culture Collection
The standard growth parameters used for growing and maintaining the microalgae were according to Teoh et al. (2004). The microalgal cultures were grown under controlled environmental conditions in growth chambers (Protech Growth Chamber, Tech Lab Scientific Sdn. Bhd.) set to simulate the environment of the origin of each microalgal strain. The tropical strains were kept under 28 ℃ while the polar strains were kept under 4 ℃. All the microalgal cultures were grown under cool white, fluorescent lamps (42mmol·m–2·s–1) that followed a 12︰12 h light-dark cycle. The cultures were grown in Bold’s Basal Medium (Nichols and Bold, 1965).
2.2 Experimental design
strains (750 mL) were grown in 1 L conical flasks with Bold’s Basal Medium for 10 d with a starting OD750reading of 0.2. The tropical strains (UMACC 051 andUMACC 038) were grown at 28 ℃ while the polar strains (UMACC 250 andUMACC 234) were grown at 4 ℃. Throughout the experiment, the growth, chlorophyll-(chl-)and carotenoids were measured every 2 d. On day 10 during the stationary phase, the samples required for the biochemical composition determination were collected and kept in the freezer for future analysis. The remaining cultures were harvested by centrifugation, washed twice with phosphate-buffered saline and dried by freeze drying. Freeze-dried biomass samples were stored at ?20 ℃ before being used for DPPH and TPC assays.
2.3 Determination of specific growth rate, chl-a and carotenoid content
Every 2 d, OD750was measured and the specific growth rate () of each strain was calculated using the following formula (Guillard, 1973):
(d–1) = (ln2– ln1)/(2–1), (1)
where,
2= OD750at2;
1= OD750at1
2and1are the times within the exponential phase,is the specific growth rate in unit d–1.
Using the spectrophotometric method of Strickland and Parsons (1968), chl-and carotenoid content were determined after extraction in acetone from samples filtered using 47 mm diameter glass-fibre filters. The concentration of chl-and carotenoid content were calculated using the equations below (Anuwar et al., 2020). The results were presented as mg·g–1dried weight.
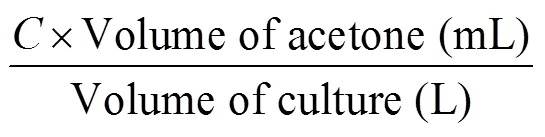

where,
= 11.6 × (OD665) – 1.13 × (OD645) – 0.14 × (OD630).
2.4 Dry weight determination
Before day 10, blank microfiber filters (Whatman CF/C, 47 mm) were dried at 100 ℃ overnight using a drying oven. On day 10, a known volume of microalgae culture was collected and filtered onto the pre-weighed filter paper. The filtered sample was then placed into the drying oven overnight at 100 ℃. After weighing the dried samples, their dry weight (DW) can be calculated using the following formula (Teoh et al., 2013):
DW (mg·L–1) =

2.5 Biochemical analysis
The carbohydrate contents were determined from samples extracted in 2 mol·L–1HCl using the phenol- sulphuric acid method (Kochert et al., 1978). The protein contents were determined using the dye-binding method after extraction in 0.5 mol·L–1NaOH (Bradford, 1976). The lipid content was extracted in methanol-chloroform (volume ratio is 2︰1) and quantified using gravimetry (Bligh and Dyer, 1959).
2.6 Microalgae extraction
Freeze dried biomass was mixed with methanol followed by cell lysis using a probe sonicator (5 s on, 10 s pulse, 40% amplitude, for 2 min). The samples were then centrifuged at 4000 rpm for 10 minutes at a temperature of 4 ℃ and the supernatant was collected as the microalgae extracts (Andriopoulos et al., 2022). Subsequently, the extract was used for the DPPH and TPC assays.
2.7 DPPH scavenging activity
Antioxidant activity of microalgae was tested using the DPPH radical scavenging assay of Venkatesan et al. (2016). A sample (0.1 mL) of microalgae extracts along with 3.9 mL of DPPH solution were mixed together and then were incubated in the dark for 30 min. The absorbance was read at 517 nm using an Epoch microplate reader. Ascorbic acid was used as the standard and DPPH scavenging activity (%) was then calculated using the formula stated below.
DPPH scavenging activity (%) =

where DPPH mixed with methanol is used as blank.
2.8 Total phenolic content
TPC of the microalgae was performed using methods described by Chan et al. (2009) with slight alterations. A total of 1 mL of microalgae extracts along with 1 mL of Folin-Ciocalteu (FC) solution were placed in aluminum- wrapped tubes and mixed thoroughly for 10 s using a vortex mixer before incubating in the dark for 3 min. Once the incubation was done, 800mL of Na2CO3(7.5%) was added, vortexed for 10 s and incubated in the dark for 2 h. The absorbance was read at 765 nm using an Epoch microplate reader (Biotek, USA). Gallic acid was used as the standard and the results were then expressed as mg gallic acid equivalent (GAE) per g of biomass DW (mg GAE·g–1DW).
3 Results
3.1 Growth rate, pigment content and biomass of Chlorella strains
All fourstrains were harvested on day 10 and the specific growth rate (Figure 1), pigment composition (Figures 2, 3) and biomass (Figure 4) were determined. For tropical strains, the highest specific growth rate was obtained by tropicalUMACC 051 (= 0.62 d–1) and for polar strains, the highest specific growth rate was obtained by polarUMACC 234 (= 0.21 d–1). For the chl-content, polarUMACC 234 (19.26 ± 5.38 mg·g–1DW) had significantly the highest value (<0.05) while tropicalUMACC 051 had the lowest chl-content at 8.93 ± 1.96 mg·g–1DW. In terms of carotenoid content, polarUMACC 234 showed the highest reading at 7.93 ± 2.24 mg·g–1DW while tropicalUMACC 051 had the lowest reading at 4.10 ± 0.88 mg·g–1DW. However, between all strains there was no significant difference (>0.05) The ratio of carotenoids to chl-was also calculated (Figure 5) and showed that tropicalUMACC 038 had the highest value while polarUMACC 250 had the lowest value.
Overall, the specific growth rates of the polar strains were shown to be lower in comparison to the tropical strains. TropicalUMACC 051 had a significantly higher specific growth rate. However, polarUMACC 234 showed the highest chl-and carotenoids contents among the strains.

Figure 1 Specific growth rate of tropical and polarstrains. Data are presented as the mean ± SD,=3. Different letters above the columns indicate significant differences between strains (<0.05).
Figure 2 Chlorophyll-content of tropical and polarstrains at the end of the 10 day experiment. Data are presented as the mean ± SD,=3. Different letters above the columns indicate significant differences between strains (<0.05).

Figure 3 Carotenoid content of tropical and polarstrains at the end of the 10 day experiment. Data are presented as the mean ± SD,=3. Different letters above the columns indicate significant differences between strains (<0.05).
Figure 4 Biomass of tropical and polarstrains. Data are presented as the mean ± SD,=3. Different letters above the columns indicate significant differences between strains (<0.05).
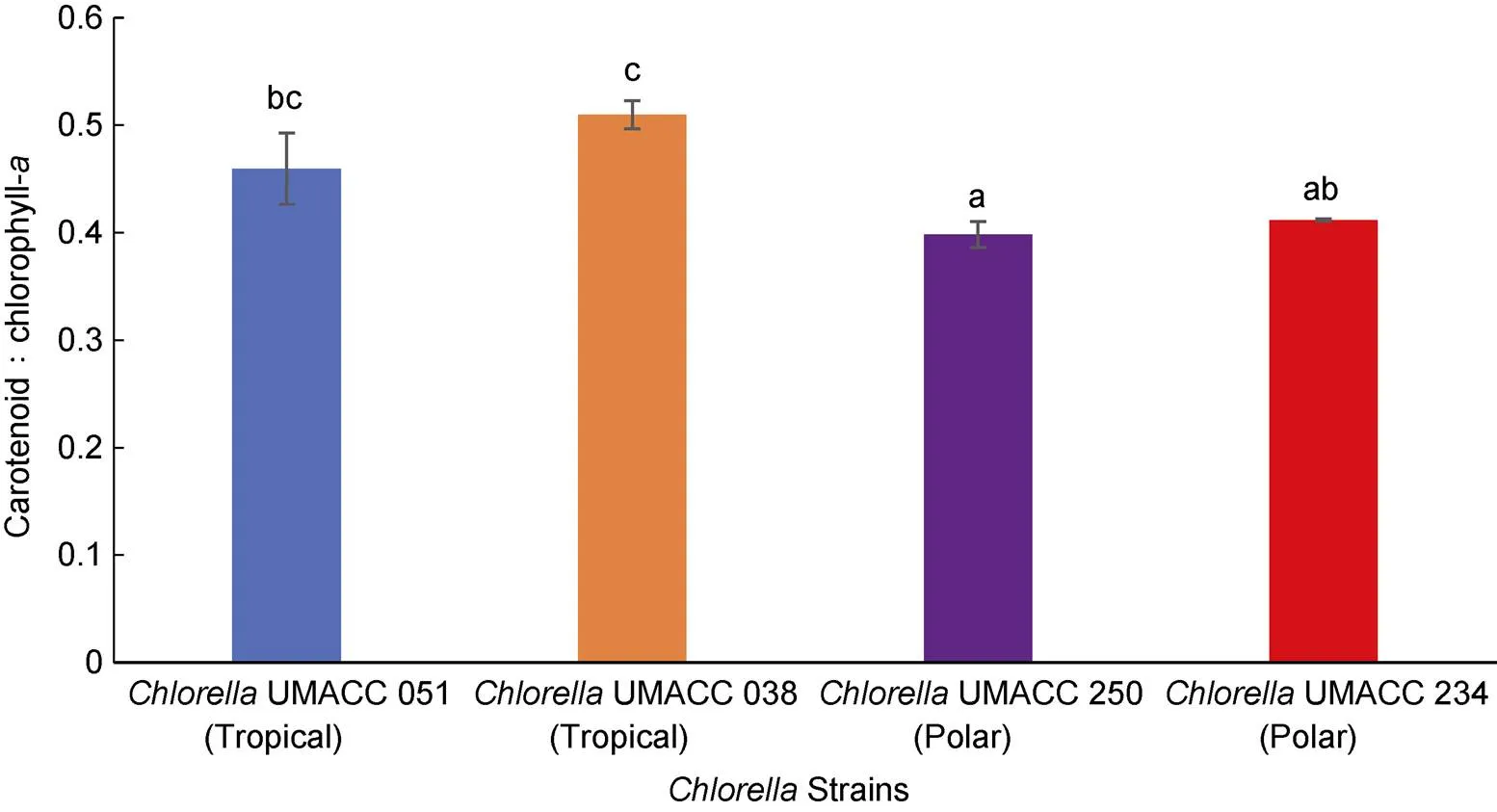
Figure 5 Carotenoid to chlorophyll-ratio of tropical and polarstrains at the end of the 10 day experiment. Data are presented as the mean ± SD,=3. Different letters above the columns indicate significant differences between strains (<0.05).
For the overall biomass (Figure 4), tropicalUMACC 051 was shown to have attained the highest biomass after 10 d incubation (47.77 ± 12.64 mg·L–1) followed by tropicalUMACC 038 (37.78 ± 7.70 mg·L–1). However, the tropicalUMACC 038 and polarUMACC 250 showed no significant difference between strains (>0.05).
3.2 Biochemical composition of Chlorella strains
The biochemical composition, namely protein, carbohydrate, and lipid, of the fourstrains are shown in Figure 6. The polarUMACC 234 has the highest carbohydrate content at 23.47% ± 2.45% DW compared to the other testedstrains (<0.05) and in terms of protein content, it was observed that the tropicalUMACC 051 has the highest protein content at 39.48% ± 8.35% DW but has no significant difference when compared with the polarUMACC 250 and tropicalUMACC 038 (>0.05). The lowest protein content was obtained by the polarUMACC 234 at 24.18% ± 3.24% DW. The protein content of tropicalUMACC 038 was shown to have no significant difference between all strains (>0.05). The lipid content was seen to be the highest in tropicalUMACC 038 and was significantly different compared to tropicalUMACC 051 and polarUMACC 250 (<0.05). It is followed by polarUMACC 234 which had the second highest lipid content but showed no significant difference between the strains (>0.05). TropicalUMACC 051 and polarUMACC 250 showed the lowest lipid content and were not significant different between each other (>0.05). Overall, the main component of thestrains from both tropical and polar strains was shown to be lipid (45.36%–60.30% DW). Carbohydrate showed the lowest percentage in the biochemical composition for all testedstrains.
3.3 Antioxidant activities and TPC of Chlorella strains
As shown in Table 3, polarUMACC 250 had the highest antioxidant activity (30.15% ± 3.22%) followed by tropicalUMACC 051 (23.52% ± 1.18%). TropicalUMACC 038 had the lowest antioxidant activity (15.42% ± 0.30%) and had no significant difference to polarUMACC 234 (>0.05). For TPC, tropicalUMACC 038 was seen to have a significantly highest phenolic content at 4.43 ± 0.10 mg GAE·g–1DW. TropicalUMACC 051 was seen to have the lowest phenolic content at 1.91 ± 0.04 mg GAE·g–1DW. The TPCs of polarUMACC 234 and polarUMACC 250 were seen to have no significant difference (>0.05).

Figure 6 Carbohydrate, protein and lipid contents of tropical and polarstrains. Data are presented as the mean ± SD,=3. Different letters above the columns indicate significant differences between strains (<0.05).

Table 3 DPPH and TPC of methanolic tropical and polar Chlorella extracts. Data are presented as the mean ± SD, n=3. Different superscript letters indicate significant differences among strains (p<0.05)
4 Discussion
With the emphasis on finding new resources of bioactive compounds that are sustainable, microalgae have become one of the frequent subjects of interest. Microalgae are known to contain diverse bioactive compounds such as bioactive peptides, PUFAs, vitamins, phenolics, carotenoids, sterols and more (Zhou J et al., 2022).speciesin recent years, have started to be commercialized as dietary supplements due to their ability to produce bioactive compounds that are absent in plant-derived resources. A range of studies have shown the presence of diverse nutrients and bioactive compounds incells that can promote good health as well as prevent diseases (Rani et al., 2018; Ru et al, 2020).
Among the fourstrains, tropicalUMACC 051 had the highest specific growth rate at= 0.62 d–1while polarUMACC 250 has the lowest specific growth rate at= 0.21 d–1.sp. has a variety of specific growth rates as reported in different studies. The eurythermal nature ofspecies has been shown in a study investigating the effect of culture temperature (ranging from 4 to 30℃) on six different Antarctic microalgae (Teoh et al., 2004). Notably, the two polarsp. strains (UMACC 234 and UMACC 237) investigated in that study were able to grow even at 30 ℃ and both were able to reach their highest specific growth rate at 20 ℃ (Teoh et al., 2004).
In another study, a newly isolated Arcticsp. was subjected to temperatures ranging from 3 to 27 ℃ and was concluded to have high specific growth rates in these temperatures which further portrays the ability forsp. to adapt and thrive in harsh environments (Cao et al., 2016).sp.’s ability to tolerate and continue to grow in a wide range of temperatures can be attributed to changes in gene expression such as differentially expressed genes expressed genes that are known to be related to photosynthesis, carbohydrate metabolism, electron transfer, and cell maintenance (Chong et al., 2011).
Overall,sp. have a high adaptability to their surroundings and can even exceed their ‘normal’ specific growth rates in different temperatures. Although the present study shows tropical strains to have the higher specific growth rates, it should also be acknowledged that there is a possibility for polar strains to surpass tropical strains when placed in different environments. By studyingsp. in their ideal and not ideal environments can help find the most convenient way to cultivate it for future industrial purposes.
In this study, polarUMACC 234 had the highest chl-content while tropicalUMACC 051 had the lowest chl-content. For carotenoids, polarUMACC 234 was observed to have the highest reading compared to the other strains. In terms of the carotenoids to chl-ratio, it was observed that tropicalUMACC 038 has the highest value. The chl-and carotenoid contents from these strains were seen to be similar to otherstrains from different studies such as,sp. FNUB001,andBIN that had chl-that ranged from 11 to 37 mg·g–1DW and carotenoid content that ranged from 4 to 11 mg·g–1DW (Zhong et al., 2018; Guo and Fang, 2020; Fakhri et al., 2021; Bazarnova et al., 2022).
It was observed that the tropical strainUMACC 038 used in this study had the highest carotenoid to chl-ratio (0.51) followed by tropicalUMACC 051 (0.45) which can be contributed to the fact that these strains have been adapted to survive in higher temperatures. Higher temperatures can result in photo-oxidative stress which in turns induces the biosynthesis of carotenoids (Tripathi et al., 2002). Chlorophylls have many health benefits such as antioxidant, antimutagenic, antigenotoxic, anti-cancer, and anti-obesogenic properties (Martins et al., 2023). In terms of carotenoids, these pigments usually have protective roles in microalgae which functions similarly on humans as well and has since led to many discoveries of its potential health benefit. They are best known for their antioxidant properties (Gong and Bassi, 2016). With polarUMACC 234 having the highest chl-and carotenoid content, it shows that it has the potential to be used in the food, cosmetic and pharmaceutical industries.
The final biomass of tropicalUMACC 051 was highest among all strains (<0.05). PolarUMACC 234 had the lowest final biomass, and the other strains showed no significant difference between each other (>0.05). The biomass of microalgae contains various bioactive compounds and often contains carbohydrates, proteins as well as lipids that can be utilized in the food and biofuel industries (Khan et al., 2018). Due to the tropicalUMACC 051 high specific growth rate, the biomass is also seen to be higher compared to the other strains. Therefore, cultivating tropicalUMACC 051 could be seen as more advantageous as it can quickly accumulate biomass to be used.
The biochemical composition analysis of all four strains interestingly showed the main component was lipids, followed by proteins and then carbohydrates. The lipid portion was around 45.36%–60.30% DW, the protein was around 24.18%–39.48% DW and the carbohydrate was around 5.93%–23.47% DW. Carbohydrate being the lowest proportion in the composition is generally in line with most microalgae in different studies that are around 5% to 20%. However, in those studies, the most common trend is that the protein content usually holds the highest proportion of the microalgae biomass at around 40% to 70% (Brown and Jeffrey, 1992; Niccolai et al., 2019; Canelli et al., 2020; Grubi?i? et al., 2022). Microalgae having a high lipid content has been reported elsewhere, as seen in a previous study showing, andspecies to have high lipid content that range from 10% to 67% (Islam et al., 2013; Nascimento et al., 2013). It has also been stated before thathas the potential to reach 5% to 40% lipids per dry weight of biomass when grown in their optimum environment (Becker, 1994). Furthermore, it is to be noted thatsp.’s biochemical composition can vary due to the environment as a study onshowed that varying temperatures can yield differences in the biochemical compositions (Dai et al., 2022).
From a genetic viewpoint, since these strains were not subjected to any environmental stress, it could be possible that these strains naturally have a higher expression of genes that responsible for lipid synthesis such as glycerol-3-phosphate dehydrogenase and phospholipid- diacylglycerol acyltransferase that have been identified inandsp.ABC-001. These genes have been reported to be related to the lipid accumulation and synthesis (Fan et al., 2014; Koh et al., 2023). Therefore, it can be speculated that the strains presented in this study may have high expressions of similar genes but further research on gene expression on these strains would have to be done to confirm such claims.
Based on this current study’s results, the high lipid content of the four strains ofthat ranges between 45.36%–60.30% DWshows a possibility of containing valuable lipid compounds such as unsaturated fatty acid (UFA), PUFA, docosahexaenoic (DHA), eicosapentaenoic acids (EPA) and more. These fatty acids have many physiological functions as they can be anti-microbial, anti-inflammatory, anti-diabetic and even help in cardiovascular diseases. Thus, these bioactive lipids have potential usages in the pharmaceutical, food, cosmetics, and biofuel industries (Zhou L et al., 2022). Seeing that tropicalUMACC 038 and polarUMACC 234 has the highest lipid contents, these two strains may have the potential for microalgae lipid cultivation that utilized in different industries.
In this study, the antioxidant activities of the fourstrains were studied using DPPH and the phytochemical content were studied using TPC test. From the two tests, polarUMACC 250 had the highest antioxidant activity while tropicalUMACC 038 had the lowest antioxidant activity. In terms of the TPC, tropicalUMACC 038 was seen to have the highest TPC while tropicalUMACC 051 has the lowest total TPC.
The antioxidant activities shown in this study were lower in comparison to other studies, but the TPC was similar (Safafar et al., 2015; Haoujar et al., 2019; Andriopoulos et al., 2022). The results of low antioxidant activity from the DPPH measurements perhaps suggest that it is not as suitable a test for examining microalgae’s antioxidant activity. It can also be due to the incompatibility of methanol extracts and DPPH assay. As seen in a study comparing DPPH and 2,2-Azino-bis (3-etilbenzotiazolin- 6-sulfonic acid) (ABTS) methods to determine the antioxidant activity ofit showed that ABTS had a higher antioxidant activity with methanolic extracts ofwhile DPPH has a higher antioxidant activity when water was used instead of methanol (Shalaby and Shanab, 2013). Studies have shown that the solvent used for extraction can impact the sensitivity of DPPH and have highlighted those other solvents such as 80% acetone can perhaps yield better results in DPPH (La et al., 2021; El Mannoubi, 2023).
Apart from that, as stated before thestrains in this study were not subjected to any stress, including oxidative stress, which can perhaps explain the appearance of low antioxidation activity. This can be evident in a study on the effects of magnetic fields (MF) on. MF is speculated to induce oxidative stress on biological systems and to counteract it, the production of antioxidants will increase which was observed inThe DPPH was able to reach a maximum of 185.7% when the culture was exposed to 60 mT for 1 h·d–1(Bauer et al., 2017). Although results presented in this study were low, they still show the possibility of thesestrains to exhibit antioxidant activities, hence TPC test was employed as microalgae has been seen to have high phenolic content which can contribute to their antioxidant activity (Agregán et al., 2018).
However, in this study, despite tropicalUMACC 038 having the highest TPC, it had the lowest antioxidant activity. Apart from the potential lack of compatibility of methanolic extracts and DPPH, there is a possibility that tropicalUMACC 038 has phenolic compounds that are low in antioxidant activity. There are many different types of phenolic compounds and based on their structures, the antioxidant activity can differ. For example, the number of hydroxyl groups present in the phenolic compounds can lead to different antioxidant capacities as it is one of the groups responsible for donating H-atoms to allow an antioxidant effect (Kumar and Goel, 2019).
Moreover, phenolic compounds that are free, esterified, glycosylated, and non-glycosylated can also impact the antioxidant activities (Rice-Evans et al., 1996; Chalas et al., 2001). Therefore, it can be speculated that the tropicalUMACC 038 may have a high TPC but consist of phenolic compounds that are low in antioxidant activity. Overall, both polar and tropical strains showed varying antioxidant capabilities and TPCs, hence there is a potential for further studies to perhaps reconfirm the antioxidant activities of these strains as well as identify the specific compounds that can provide health benefits to human.
This study was able to highlight the differences in terms of growth, pigment, biomass dry weight, biochemical composition, antioxidant activities and phytochemical properties of tropical and polarstrains. However, further studies should be done to identify the specific bioactive compounds that contribute to its antioxidant activities. Furthermore, studies to identify as well as quantify the specific bioactive compounds can also show their possible potential in the food, cosmetic, biofuel and pharmaceutical industries.
5 Conclusion
In the current era, different industries are keen in finding naturally derived bioactive compounds that can potentially replace the synthetic materials as well as finding renewable sources of bioactive compounds. Microalgae have gained much attention due to these goals as they are known to house a plethora of bioactive compounds with varying functions such as acting as an antioxidant and can regenerate these bioactive compounds which can bring a sense of sustainability. With a vast majority of microalgae from different regions that has yet to be studied, this study aimed to determine the specific growth rate, biomass content, biochemical composition, antioxidant activities and TPC of foursp. from tropical and polar regions.
In terms of the growth rate and biomass, the tropical strains were observed to have much higher values than the polar strains and tropicalUMACC 051 is seen to be able to grow fast and create a bigger biomass, making it a potential candidate for cultivation as it is more efficient. Between the four strains, the lipid content was observed to be the highest in the biochemical compositions in all strains, which can be a trait of these species that can be useful in the development of biofuels. The antioxidant activities were seen to be low in all strains but in all strains, there were positive results for the phenolic content that can be further analyzed. Based on the findings, although polarUMACC 234 has one of the slowest growth rates and the smallest biomass, it still can be considered as the strain that has the most potential. Factors such as the growth rate, biomass, and the need to maintain the cultures at 4 ℃ may increase the cost of production but it has significantly the highest chl-, carotenoid, carbohydrate and second highest lipid content despite having the smallest biomass.
This study provided a general screening on tropical and polarstrains and showed that there is a potential that these strains hold bioactive compounds that can be utilized in various industries. With the research findings in this study, thestrains perhaps have a future in biofuels or nutritional foods industries. However further studies to dissect the specific bioactive compounds present in thestrains are needed to determine the exact compounds on a molecular level. Future work should aim to extract these specific bioactive compounds and test it for potential health benefits or toxic effects as well as study the genetic make-up of these strains. Investigations focusing on manipulating various growth factors to explore their influence on the synthesis of specific bioactive compounds in thesestrains are also warranted.
This research was supported by Ministry of Higher Education (MOHE) Malaysia through Fundamental Research Grant Scheme (FRGS/1/2023/STG01/TAYLOR/02/1). The authors would also like to give special thanks to Taylor’s University for the support for this project and University of Malaya for supplying the microalgae cultures. We appreciate Dr. Su Chern Foo as reviewer, one anonymous reviewer and Associate Editor Dr. Cinzia Verde for constructive comments that helped us improve the manuscript.
Agregán R, Munekata P E S, Franco D, et al. 2018. Antioxidant potential of extracts obtained from macro- (,and) and micro-algae (and) assisted by ultrasound. Medicines, 5(2): 33, doi:10.3390/medicines5020033.
Andrew A R, Yong W T L, Misson M, et al. 2022. Selection of tropical microalgae species for mass production based on lipid and fatty acid profiles. Front Energy Res, 10: 912904, doi:10.3389/fenrg.2022. 912904.
Andriopoulos V, Gkioni M D, Koutra E, et al. 2022. Total phenolic content, biomass composition, and antioxidant activity of selected marine microalgal species with potential as aquaculture feed. Antioxidants, 11(7): 1320, doi:10.3390/antiox11071320.
Anuwar S, Teoh M L, Yap W H, et al. 2020. Effects of elevated temperatures on growth and photosynthetic performance of polar. Adv Polar Sci, 31(2): 124-131, doi:10.13679/j.advps.2019. 0040.
Aratboni H A, Rafiei N, Garcia-Granados R, et al. 2019. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Factories, 18(1): 178, doi:10.1186/s12934- 019-1228-4.
Bauer L, Alberto J, Priscila A, et al. 2017. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields incultivations. Bioresour Technol, 244: 1425-1432, doi:10.1016/j.biortech.2017.06.036.
Bazarnova J, Smyatskaya Y A, Shlykova A, et al. 2022. Obtaining fat-soluble pigments—carotenoids from the biomass ofmicroalgae. Appl Sci, 12(7): 3246, doi:10.3390/app12073246.
?Becker E W. 1994. Microalgae: biotechnology and microbiology. Cambridge: University Press.
Beg S, Swain S, Hasan H, et al. 2011. Systematic review of herbals as potential anti-inflammatory agents: recent advances, current clinical status and future perspectives. Pharmacogn Rev, 5(10): 120-137, doi:10.4103/0973-7847.91102.
Bligh E G, Dyer W J. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol, 37(8): 911-917, doi:10.1139/ o59-099.
Bock C, Krienitz L, Pr?schold, T. 2011. Taxonomic reassessment of the genus() using molecular signatures (barcodes), including description of seven new species. 11(2): 293-312, doi:10.5507/fot.2011.028.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72(1/2): 248-254, doi:10.1016/0003-2697(76) 90527-3.
Brown M R, Jeffrey S W. 1992. Biochemical composition of microalgae from the green algal classesand. 1. Amino acids, sugars and pigments. J Exp Mar Biol Ecol, 161(1): 91-113, doi:10.1016/0022-0981(92)90192-D.
Canelli G, Tarnutzer C, Carpine R, et al. 2020. Biochemical and nutritional evaluation ofandbiomasses relevant for food application. Front Nutr, 7: 565996, doi:10.3389/fnut.2020. 565996.
Cao K W, He M, Yang W, et al. 2016. The eurythermal adaptivity and temperature tolerance of a newly isolated psychrotolerant Arcticsp. J Appl Phycol, 28(2): 877-888, doi:10.1007/s10811- 015-0627-0.
Chalas J, Claise C, Edeas M, et al. 2001. Effect of ethyl esterification of phenolic acids on low-density lipoprotein oxidation. Biomed Pharmacother, 55(1): 54-60, doi:10.1016/S0753-3322(00)00011-1.
Chan S W, Lee C Y, Yap C F, et al. 2009. Optimisation of extraction conditions for phenolic compounds from limau purut () peels. Int Food Res J,16(2): 203-213.
Chen Z P, Qiu S, Li M T, et al. 2022. Instant inhibition and subsequent self-adaptation ofsp. toward free ammonia shock in wastewater: physiological and genetic responses. Environ Sci Technol, 56(13): 9641-9650, doi:10.1021/acs.est.1c08001.
Chong G L, Chu W L, Othman R Y, et al. 2011. Differential gene expression of an Antarcticin response to temperature stress. Polar Biol, 34(5): 637-645, doi:10.1007/s00300-010-0918-5.
Choo W T, Teoh M L, Phang S M, et al. 2020. Microalgae as potential anti-inflammatory natural product against human inflammatory skin diseases. Front Pharmacol, 11: 1086, doi:10.3389/fphar.2020.01086.
Choochote W, Suklampoo L, Ochaikul D. 2014. Evaluation of antioxidant capacities of green microalgae. J Appl Phycol, 26(1): 43-48, doi:10.1007/s10811-013-0084-6.
Correa D F, Beyer H L, Possingham H P, et al. 2020. Freeing land from biofuel production through microalgal cultivation in the Neotropical region. Environ Res Lett, 15(9): 094094, doi:10.1088/1748-9326/ ab8d7f.
Coulombier N, Jauffrais T, Lebouvier N. 2021. Antioxidant compounds from microalgae: a review. Mar Drugs, 19(10): 549, doi:10.3390/ md19100549.
Dai Y R, Wang D, Zhu Y R, et al. 2022. Thermal-tolerant potential of ordinaryand the promotion of cell harvesting by heterotrophic cultivation at high temperature. Front Bioeng Biotechnol, 10: 1072942, doi:10.3389/fbioe.2022.1072942.
El Mannoubi I. 2023. Impact of different solvents on extraction yield, phenolic composition,antioxidant and antibacterial activities of deseededfruit. J Umm Al Qura Univ Appl Sci, 9(2): 176-184, doi:10.1007/s43994-023-00031-y.
Fakhri M, Riyani E, Ekawati A, et al. 2021. Biomass, pigment production, and nutrient uptake ofsp. under different photoperiods. Biodiversitas, 22(12): d221215, doi:10.13057/biodiv/ d221215.
?Fan J, Cui Y, Wan M, et al. 2014. Lipid accumulation and biosynthesis genes response of the oleaginousthree nutrition stressors. Biotechnol Biofuels,7(1): 17, doi: 10.1186/1754- 6834-7-17.
Gao K, Helbling E, H?der D, et al. 2012. Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming. Mar Ecol Prog Ser, 470: 167-189, doi:10.3354/ meps10043.
García J L, de Vicente M, Galán B. 2017. Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol, 10(5): 1017-1024, doi:10.1111/1751-7915.12800.
Gong M, Bassi A. 2016. Carotenoids from microalgae: a review of recent developments. Biotechnol Adv, 34(8): 1396-1412, doi:10.1016/j. biotechadv.2016.10.005.
Grubi?i? M, ?antek B, Zori? Z, et al. 2022. Bioprospecting of microalgae isolated from the Adriatic Sea: characterization of biomass, pigment, lipid and fatty acid composition, and antioxidant and antimicrobial activity. Molecules, 27(4): 1248, doi:10.3390/molecules27041248.
Guillard R R L. 1973. Division rates//Stein J R. Handbook of phycological methods: Volume 1. Cambridge: University Press, 289-312.
Guo H, Fang Z. 2020. Effect of light quality on the cultivation of. E3S Web Conf, 143: 02033-02033, doi:10.1051/e3sconf/ 202014302033.
Haoujar I, Cacciola F, Abrini J, et al. 2019. The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules, 24(22): 4037, doi:10.3390/molecules24224037.
Islam M, Magnusson M, Brown R, et al. 2013. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies, 6(11): 5676-5702, doi:10.3390/ en6115676.
Khan M I, Shin J H, Kim J D. 2018. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories, 17(1):36, doi: 10.1186/s12934-018-0879-x.
Kiuru P, D?Auria M, Muller C, et al. 2014. Exploring marine resources for bioactive compounds. Planta Med, 80(14): 1234-1246, doi:10. 1055/s-0034-1383001.
Kochert G, Hellebust J A, Craigie J S. 1978. Carbohydrate determination by the phenol sulfuric acid method//Hellebust J A, Craigie J S. Handbook of phycological methods: Physiological and biochemical methods, Cambridge: Cambridge University Press, 95-97.
Koh H G, Cho J M, Jeon S, et al. 2023. Transcriptional insights intosp. ABC-001: a comparative study of carbon fixation and lipid synthesis under different CO2conditions.Biotechnol Biofuels Bioprod, 16(1): 1-16, doi:10.1186/s13068-023-02358-4.
Kumar N, Goel N. 2019. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep, 24: e00370, doi:10.1016/j.btre.2019.e00370.
La J, Kim M J, Lee J. 2021. Evaluation of solvent effects on the DPPH reactivity for determining the antioxidant activity in oil matrix. Food Sci Biotechnol, 30(3): 367375, doi:10.1007/s10068-020-00874-9.
León-Vaz A, León R, Vigara J, et al. 2023. Exploring Nordic microalgae as a potential novel source of antioxidant and bioactive compounds. New Biotechnol, 73: 1-8, doi:10.1016/j.nbt.2022.12.001.
Martins T, Barros A N, Rosa E, et al. 2023. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: a comprehensive review. Molecules, 28(14): 5344, doi:10.3390/molecules28145344.
Milanesi M, Runfola A, Guercini S. 2020. Pharmaceutical industry riding the wave of sustainability: review and opportunities for future research. J Clean Prod, 261: 121204, doi:10.1016/j.jclepro.2020.121204.
Miyata M, Iwata S, Mifude C K, et al. 2021. A novelsp. downregulates inflammatory gene expression in skin and articular cells. Altern Ther Health Med, 27(1): 28-34.
Montuori E, Saggiomo M, Lauritano C. 2023. Microalgae from cold environments and their possible biotechnological applications. Mar Drugs, 21(5): 292, doi:10.3390/md21050292.
Nascimento I A, Marques S S I, Cabanelas I T D, et al. 2013. Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res, 6(1): 1-13, doi:10.1007/s12155-012-9222-2.
Newman D J, Cragg G M, Snader K M. 2003. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod, 66(7): 1022-1037, doi:10.1021/np030096l.
Niccolai A, Chini Zittelli G, Rodolfi L, et al. 2019. Microalgae of interest as food source: biochemical composition and digestibility. Algal Res, 42: 101617, doi:10.1016/j.algal.2019.101617.
Nichols H W, Bold H C. 1965.Gen. et sp. nov. J Phycol, 1(1): 34-38, doi:10.1111/j.1529-8817.1965.tb04552.x.
Rani K, Sandal N, Sahoo P K. 2018. A comprehensive review on-its composition, health benefits, market and regulatory scenario. Pharma Innov J,(7): 584-589.
Rice-Evans C A, Miller N J, Paganga G. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 20(7): 933-956, doi:10.1016/0891-5849(95)02227-9.
Ru I T K, Sung Y Y, Jusoh M, et al. 2020.: a perspective on its potential for combining high biomass with high value bioproducts. Appl Phycol, 1(1): 2-11, doi:10.1080/26388081. 2020.1715256.
Safafar H, van Wagenen J, M?ller P, et al. 2015. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar Drugs, 13(12):7339-7356, doi:10.3390/md13127069
Shalaby E A, Shanab S M M. 2013. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of. Indian J Mar Sci, 42(5): 556-564.
Singh A, Nigam P S, Murphy J D. 2011. Mechanism and challenges in commercialisation of algal biofuels. Bioresour Technol, 102(1): 26-34, doi:10.1016/j.biortech.2010.06.057.
Strickland J D H, Parsons T R. 1968. A practical handbook of seawater analysis. Fish Res Board Can Bull, 167: 311.
Teoh M L, Choo W L, Marchant H, et al. 2004. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J Appl Phycol, 16(6): 421-430, doi:10.1007/s10811-004-5502-3.
Teoh M L, Choo W T, Anuwar S, et al. 2023. Chapter 23: Microalgae- based products and their immunomodulatory activities// Jacob-Lopes E, Queiroz M I, Maroneze M M, et al. (eds.) Handbook of Food and Feed from Microalgae, Amsterdam: Academic Press, 279-290, doi:10. 1016/b978-0-323-99196-4.00023-1.
Teoh M L, Phang S M, Chu W L. 2013. Response of Antarctic, temperate, and tropical microalgae to temperature stress. J Appl Phycol, 25(1): 285-297, doi:10.1007/s10811-012-9863-8.
Tiong I K R, Nagappan T, Abdul Wahid M E, et al. 2020. Antioxidant capacity of five microalgae species and their effect on heat shock protein 70 expression in the brine shrimp Artemia. Aquac Rep, 18: 100433, doi:10.1016/j.aqrep.2020.100433.
Tripathi U, Sarada R, Ravishankar G A. 2002. Effect of culture conditions on growth of green alga—and astaxanthin production. Acta Physiol Plant, 24(3): 323-329, doi:10.1007/s11738- 002-0058-9.
Venkatesan J, Kim S K, Shim M S. 2016. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae. Nanomaterials, 6(12): 235, doi:10.3390/ nano6120235.
Whelan J, Fritsche K. 2013. Linoleic acid. Adv Nutr, 4(3): 311-312, doi:10.3945/an.113.003772.
Widowati I, Zainuri M, Kusumaningrum H P, et al. 2017. Antioxidant activity of three microalgae,andclone Tahiti. IOP Conf Ser: Earth Environ Sci, 55: 012067, doi:10.1088/1755-1315/55/1/012067.
?Yu Z, Yan H, Xie K, et al. 2022. Research progresses on the physiological and pharmacological benefits of microalgae-derived biomolecules. Foods, 11(18): 2806, doi:10.3390/foods11182806.
?Zhong Y, Jin P, Ji C. 2018. A comprehensive comparable study of the physiological properties of four microalgal species under different light wavelength conditions. Planta, 248(2): 489-498, doi: 10.1007/ s00425-018-2899-5.
Zhou J, Wang M, Saraiva J A, et al. 2022. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem, 384: 132236, doi:10.1016/j.foodchem.2022.132236.
Zhou L, Li K, Duan X. et al. 2022. Bioactive compounds in microalgae and their potential health benefits. Food Biosci, 49: 101932, doi:10.1016/j.fbio.2022.101932.
: Wong C Z-E, Teoh M-L, Chan S W, et al.across latitudes: investigating biochemical composition and antioxidant activities for biotechnological applications. Adv Polar Sci, 2023, 34(4): 340-351,doi: 10.12429/j.advps.2023.0028
10.12429/j.advps.2023.0028
4 November 2023;
22 December 2023;
30 December 2023
, ORCID: 0000-0001-7979-6045, E-mail:MingLi.Teoh@taylors.edu.my
 Advances in Polar Science2023年4期
Advances in Polar Science2023年4期
- Advances in Polar Science的其它文章
- Space physics and astronomy research from Chinese polar stations:current and future directions
- Variations and relations between chlorophyll concentrations and physical-ecological processes near the West Antarctic Peninsula
- Chemical composition of natural waters at Broknes Peninsula,Larsemann Hills,Antarctica
- Evaluation of meteorological predictions by the WRF model at Barrow,Alaska and Summit,Greenland in the Arctic in April 2019
- Current state of research on microplastics in the marine-atmosphere environment of the Arctic region
- Carbon isotope ratios of n-alkanoic acids:new organic proxies for paleo-productivity in Antarctic ponds
