Molecular Dynamics Simulation of Shock Response of CL-20 Cocrystals Containing Void Defects
Chnglin Li , Wei Yng , Qing Gn ,*, Yjun Wng , Lin Ling , Weno Zhng ,Shungfei Zhu , Chnggen Feng
a State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China
b School of Materials Science & Engineering, Beijing Institute of Technology, Beijing 100081, China
Keywords: CL-20 co-crystals Molecular dynamics simulation Reactive force field Impact response Hot spot Void defect
ABSTRACT To investigate the effect of void defects on the shock response of hexanitrohexaazaisowurtzitane(CL-20)co-crystals, shock responses of CL-20 co-crystals with energetic materials ligands trinitrotoluene (TNT),1,3-dinitrobenzene (DNB), solvents ligands dimethyl carbonate (DMC) and gamma-butyrolactone (GBL)with void were simulated,using molecular dynamics method and reactive force field.It is found that the CL-20 co-crystals with void defects will form hot spots when impacted, significantly affecting the decomposition of molecules around the void.The degree of molecular fragmentation is relatively low under the reflection velocity of 2 km/s, and the main reactions are the formation of dimer and the shedding of nitro groups.The existence of voids reduces the safety of CL-20 co-crystals, which induced the sensitivity of energetic co-crystals CL-20/TNT and CL-20/DNB to increase more significantly.Detonation has occurred under the reflection velocity of 4 km/s,energetic co-crystals are easier to polymerize than solvent co-crystals,and are not obviously affected by voids.The results show that the energy of the wave decreases after sweeping over the void, which reduces the chemical reaction frequency downstream of the void and affects the detonation performance, especially the solvent co-crystals.
1.Introduction
CL-20,as one of the most important energetic materials applied,was limited application due to its disadvantages of high sensitivity[1] and low mechanical properties [2].The co-crystals formed by CL-20 and other compounds can greatly reduce the sensitivity [3],improve the safety of materials and effectively expand the scope of application on the basis of retaining high energy,which provides a new idea for the preparation of high-energy and low-sensitivity energetic materials.The design of co-crystals is an effective method to improve its properties from the molecular level [4],which could also balance its high energy and insensitive properties[5],better than the mixed explosive[6].At present,a variety of CL-20 co-crystals with energetic materials(TNT,DNB,benzotrifuroxan(BTF) [7-10], etc.) have been prepared, showing excellent performance in high energy and insensitive.In addition,the co-crystals of CL-20 prepared with solvents(DMC[11],GBL[5],etc.)also benefit from reducing its sensitivity.To evaluate the property of CL-20 cocrystals, its shock response characteristics gained much attention recently,of which molecular dynamics simulation[12]method and reactive force field [13] with low gradients correction (ReaxFF-lg)[14]show an advantage.The initial parameters of ReaxFF lg reactive force field are obtained by training the physical and chemical properties of nitromethane,TATB,RDX,and PETN,and are generally applicable to them.However, as a nitro compound, CL-20 has similar physical and chemical properties to the above compounds,so ReaxFF-lg reactive force field has also been used by many researchers to explore the microscopic properties of CL-20 [15-17],and has drawn a conclusion consistent with the experimental results.
Recent shock simulations focus on the relationship between shock velocity and the response of CL-20 co-crystals[18].Liu et al.[15] studied the influence of shock velocity on the response of CL-20/TNT co-crystal, CL-20 would be polymerized to form a dimer and no decomposition occurred under impact at low velocity(0.8-1.0 km/s) while under shock velocity of 2-4 km/s, the cocrystals structure decomposition caused by the impact is dominant,and the amount of dimer decreases gradually.Zhang et al.[16]simulated the shock decomposition process of CL-20/BTF, the results showed that the shock wave sensitivity of CL-20 at different directions has slight differences.According to Liu et al.[17],CL-20/HMX co-crystals undergo periods of induction, fast compression,slow compression,and expansion under shock velocity of 4-10 km/s,and different wavelet front velocities were observed in CL-20 and HMX, respectively.However, During the preparation of energetic materials, defects of different kinds, shapes, and sizes often occur.The above studies consider little about the effect of defects on the shock response of CL-20 co-crystal, which would come from the preparation and crystallization processes, such as voids, doping,and dislocations [19-21].Although Hang et al.[22-24] revealed the adverse effects of defects on the sensitivity of CL-20/NQ,CL-20/TNT, and CL-20/DNB co-crystals by static equilibrium model, the shock response of CL-20 co-crystals with defects have not been reported.

Fig.1.(a) Molecular structures of CL-20/TNT; (b) CL-20/DNB; (c) CL-20/DMC; (d) CL-20/GBL co-crystals molecular structures and perfect/defective co-crystals supercells.

Table 1Cell parameters of built CL-20 perfect and void defect co-crystals.
In this paper,in order to study the impact of defects on the shock response of CL-20 co-crystals,and the effect of the ligands,the void collapse,initial chemical reaction,and hot spot formation of CL-20/TNT, CL-20/DNB, CL-20/DMC and CL-20/GBL co-crystals were studied by molecular dynamics simulation and ReaxFF-lg reactive force field,the influence of defects on shock sensitivity of CL-20 cocrystals was discussed from the molecular level, aims to provide a reference for the shock response mechanism of CL-20 co-crystals.
2.Methods
2.1.Model establishment and calculation
The supercells of perfect and void-containing energetic cocrystals CL-20/TNT [7] and CL-20/DNB [9], solvent co-crystals CL-20/DMC [11] and CL-20/GBL [5] are established based on crystal data of Cambridge crystal database, shown in Fig.1.The specific sizes and atomic numbers of each supercell are shown in Table 1.All simulations were performed using the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) [25].Periodic boundary conditions are applied to these supercells in thea,b, andcdirections.The conjugate gradient algorithm [26] is used to minimize lattice energy, Maxwell Boltzmann random distribution [27]is used to assign the initial velocity of the atom,and the Berendsen thermostat[28]is used to relax the supercell for 15 ps at 298 K.The relaxation structure is obtained by 15 ps relaxation at 298 K and 0 GPa with 0.1 fs timestep using the NPT ensemble and Nos'e-Hoover thermostat and barostat [29,30], and the atomic nearest neighbor table is generated every ten timesteps.The comparison of the size and density of the perfect supercell after relaxation with the experimental value is shown in Table 2.It can be seen that the error between the simulation value of the lattice constant and the experimental value is small, which shows that the ReaxFF-lg force field can accurately describe the physical properties of CL-20 cocrystals.
Set directioncas a nonperiodic boundary condition and set up a reflective wall [31] to realize the shock induced simulation.Supercells whose void radii are 0 and 20 ? are respectively reflected along directioncat a velocity of 2 km/s and 4 km/s.The total simulation time lasts 5 ps,and 0.1 fs timestep were used during the simulation.The spatial position of the atom, molecular type information and position are output every 50 fs, the spatial position of the atom is output every 0.04 ps,the bond level is calculated every 1 fs, and the average bond level is calculated every 5 fs.Derive bond-level data,and determine the breakage and formation cut-off of interatomic bonds based on the bond-level table between C,H,O,and N[32].Then observe the phenomenon of void collapse and hotspot generation during shock process, count the number of molecules generated and the frequency of chemical reactions, and compare the results of perfect and void-containing co-crystals.

Table 2Cell parameters and density values (ρ/(g·cm-3)) of CL-20 Perfect co-crystals cells after relaxation.
2.2.Data processing method
(1) Wavefront analysis method of shock model
In order to study the wavefront moving state after the shock,the shock data at different times are analyzed to investigate the propagation process of the shock wave in the model.The wavefront velocity under each impacted state is obtained by linear fitting.The position determined by the average value of the two velocities in front of and behind the wavefront is taken as the wavefront position.The shock wave position-time curve is drawn, and the wave velocity is approved by the shock Hugoniot formula.
According to the average velocity of each particle along the impact direction after the shock wave sweep, determine the particle velocity behind the wave of the relatively static reference system,and then combine it with the initial velocity of the impact model to obtain the particle velocity behind the wave relative to the impact model.
μpis the particle velocity behind the wave, μp0is the particle velocity behind the wave of the relatively static reference system,andVs0is the initial shock velocity of the system, in km/s.In this paper,the reflective wall impact method is used to give the model a velocityVs0in the opposite direction to the impact direction,so the wave velocityDof the wavefront relative to the impact model is as follows:
whereD0is the wavefront velocity of the stationary reference system, in km/s.The above equation and the impact Hugoniot equationPn=ρ0upD were combined as
wherePnis the wavefront pressure,unit GPa;ρ0is the density of the system before impact, g/cm3; D0is the wavefront velocity of the relative static reference system,and Vs0is the initial shock velocity of the system, in km/s.Since the average velocity of each impact model atom along the impact direction after wavefront scanning is close to 0 [34],μp0is regarded as 0.
(2) Analysis of two-dimensional temperature distribution
In order to investigate the morphology of high-temperature hot spots caused by void collapse,a two-dimensional temperature field diagram was calculated.The regional temperature analysis is based on the energy equipartition theorem[33],which connects the total translational kinetic energy and total energy of atoms in the region,and converts them to obtain the regional temperature.The average velocity of the centroid was subtracted from the velocity of the atom to obtain the relative velocity of the atom, the relative translational kinetic energy was calculated, then the energy equalization theorem was applied to calculate the temperature in the region.The average value of the velocity of the atom in the region swept by the shock model wavefront is close to 0 [34], the atomic translational kinetic energy in this region is about the relative translational kinetic energy, and the temperature of the area is not affected by the wavefront is regarded as 298 K.The influence region of the wavefront determines the temperature based on the average translational kinetic energy, which is determined by the energy equalization theorem.
whereCkeis the translational kinetic energy in kJ/mol;NAis the Avogadro constant with the value of 6.02×1023;kis the Boltzmann constant with the value of 1.38×10-23J/K;Tis the temperature of the region, unit K.According to the formula, the conversion relationship between the translational kinetic energy of atoms and the temperature in the region can be obtained as follows:
Each model region is divided into 100 × 40, calculate the average temperature of each region, thus drawing the twodimensional temperature field diagram of void collapse.
(3) Analysis of atomic trajectories and molecular species
In the process of molecular dynamics simulation, the positions of all atoms in the system follow the equations of motion determined by the forcefield.The physical quantity of each atom is output at a preset timestep of atomic information.The Open Visualization Tool (OVITO) [35] is used to process the trajectory data, and the atomic number, atomic type, charge, atomic coordinates, atomic velocity, kinetic energy, potential energy, and other physical quantities are output every 0.1 ps.In addition, in order to avoid accidental chemical bonds, the cutoff of average bond order [32] is created to determine whether there is a bond between atoms, thus identify the species of molecular fragments under the condition of maintaining the integrity of molecules.
In order to investigate the chemical reaction rules during each shock, the main reaction products and their distribution were analyzed.The products include undecomposed CL-20 molecules,co-crystal ligand molecules, and chemical species such as N2,NO2,NO3, HCN, H2, CO2,and H2O.In the simulation process, the types and positions of molecular fragments are derived every 0.1 ps,and the relative number and distribution of the main groups are analyzed over time, so as to obtain the curve of the number of molecules over time and the distribution of each molecule in the supercell at a specific time.The relative contentCmof particles is defined as follows:
wherenis the number of molecules in the system andn0is the number of molecules in the unreacted system.In the initial unreacted state,Cmof CL-20 molecule equals to 1.
(4) Chemical elementary reaction analysis
In order to investigate the chemical reaction path in the two cases of shock compression and wavefront reverse stretching,here uses the chemical reaction frequency statistical method to analyze.This method obtains the chemical reaction through the change of the bonding relationship between adjacent timesteps (that is, it is regarded as one step completed within the step interval),and does not discuss the reaction process within the step interval.An example of elementary reaction discrimination is shown in Fig.2.

Fig.2.Examples of organic substitution reaction.
As shown in Fig.2, each group is numbered at past and next timesteps, obtain the affiliation of each atomic group to the molecule.Analyze the changes of molecular groups at adjacent timesteps,tracing the new affiliated molecules of atoms after reactions,and traverse the molecular structure containing the newly assigned atoms,finally integrate the results obtained from the traversal into the elementary reaction formula.In addition, this article uses CL-20/DNB as the benchmark to normalize the reaction frequency obtained by this method,which is convenient for the comparison of different crystal orientation supercells.
3.Results and discussion
3.1.Analysis of shock wavefront displacement
The wavefront position of different perfect impact models with time is shown in Fig.3.Determine the wavefront moving speed of each impact speed according to Eq.(2),and calculate the wavefront pressure using the impact Hugoniot formula, which is compared with the simulation results in this paper, as shown in Table 3.The corresponding column analysis diagram is shown in Fig.4.
It can be seen from Fig.3 that the wavefront velocity of CL-20/DMC and CL-20/GBL solvent co-crystals under 2 km/s impact is significantly lower than that of CL-20/TNT and CL-20/DNB energetic co-crystals,while the difference is small under 4 km/s impact,so it can be inferred that energetic co-crystals is more prone to detonation.
The error between the simulation result of pressure and the calculation result of impact Hugoniot formula is less than 5%,which proves that ReaxFF-lg reactive force field can accurately express the impact response of CL-20 co-crystals.The wavefront pressure at 2 km/s shock is about 30 GPa, which is less than the CL-20 cocrystals detonation pressure, indicating that these two velocity shocks are not enough to cause CL-20 detonation.However,under the impact of 4 km/s velocity, the pressure of each co-crystal’s wavefront has been greater than the detonation pressure.It is believed that CL-20 has been detonated at this time, and the detonation pressure of energetic co-crystals is the highest.

Fig.3.Wavefront velocity of different models.

Table 3Comparison between simulated value and calculateda value of shock wave velocity pressure.

Fig.4.Wavefront velocity histogram of different models.
3.2.Hotspot generation process
Taking CL-20/GBL co-crystals as an example, the twodimensional temperature field diagram of shock induced supercells with void defect is drawn based on the energy equipartition theorem, and analyzed the void collapse and hot spot generation process at different timesteps, as shown in Fig.5.

Fig.5.Hot spot evolution of CL-20/GBL co-crystals with defects shocked by(a)2 km/s and (b) 4 km/s.
It can be seen from Fig.5 that in the CL-20/GBL co-crystals containing voids,impact of the shock wave intensifies the thermal motion of the atoms upstream of the cavity, accelerates the collision with the downstream atoms in the cavity to generate hot spots,and no mass accumulation occurs when the cavity is collapsed.Under low velocity of 2 km/s, the hot spot is slightly flat, and the recovery speed of the depression is slow after the wavefront sweeps through the cavity.Under high velocity of 4 km/s, the hot spot range became large and tapered,indicating that the increase in shock velocity can extend the size of the hot spot in the impact direction, which is consistent with the high-speed impact simulation results of RDX with voids[36].The central temperatures of hot spots formed by void collapse of four kinds of CL-20 cocrystals are slightly different, under the impact of 2 km/s, the maximum temperatures and pressures of energetic co-crystals and solvent co-crystals are both about 4050 K and 45.0 GPa under the impact of 4 km/s, the maximum temperatures of energetic cocrystals and solvent co-crystals are about 12,000 K, 95.8 GPa and 10,500 K, 92.2 GPa, respectively.In energetic co-crystals, the hot spot temperature of CL-20/TNT is slightly higher than 12,000 K,which is supposed to be related to the different chemical energy release of different ligand molecules.Under the same impact conditions, the void in energetic co-crystals delays the shockwave longer,and there is more energy exchange between the wavefront and the hot spot, which corresponds to the higher hot spot temperature of the energetic co-crystals above.In addition,the residual energy is less after the wavefront sweeps through the void, which makes it more difficult for downstream molecules to react than upstream.
3.3.Quantity and distribution of main species
This chapter investigates the relative content, number, and molecules fragmentation degree of species in the perfect and voidcontaining CL-20 co-crystals under the reflect velocity of 2 km/s and 4 km/s, as shown in Figs.6 and 7.The dotted line represents perfect supercells, and the solid line represents void-containing supercells.

Fig.6.(a)Changes of the numbers of CL-20;(b)Ligand;(c)Molecules fragmentation degree;(d)NO2 in co-crystals under impact of 2 km/s(solid line is void containing co-crystals,dotted line is perfect co-crystals).
Under the reflect velocity of 2 km/s, the number of CL-20 molecules and co-crystals ligands in the co-crystals containing defects decreases continuously.Molecule’s fragmentation degree almost remains unchanged before the cavity collapsed (0-1.76 ps), and then increases step by step.The corresponding curve in the perfect model changes smoothly, as shown in Fig.6.Nitrogen containing small species such as N2and NO2are generated by shock, and the number of small species such as H2O, CO2and HCN is very small,similar to the results of CL-20/TNT co-crystals simulation [37].Before the void collapsed in each supercell, the reaction speed of CL-20 and ligand is the same.However, after the void-containing supercell undergoes the void collapse stage, the reaction speed around the hot spot accelerates.In the void-containing supercells,the number of molecules involved in the reaction is significantly greater than that of the perfect supercells.Due to the compound effect of void collapse and reverse stretching, molecular fragmentation degree also shows an increasing trend, while the perfect model remains relatively flat in the above parameters.After the wavefront was swept,no CL-20 molecules in the supercell had been recovered to complete molecules.For perfect CL-20/DMC and voidcontaining CL-20/DMC, the reaction intensity and the molecular fragmentation degree change relatively smoothly, which is presumed to be caused by the stronger C-H…O hydrogen bond between molecules [11].
The molecular reaction amount of energetic co-crystals components is much higher than that of solvent co-crystals.It can be seen that the shock response properties between energetic cocrystals and solvent co-crystals are quite different.The introduction of voids has a greater impact on the number of CL-20 and its ligand molecules,molecular fragmentation degree,and the number of products in the energetic co-crystals than solvent co-crystals.The molecular reaction of each component in the void-containing supercells is more intense than that of the perfect supercells,indicating that the hot spots caused by voids will significantly increase the sensitivity of the CL-20 co-crystals, especially the energetic co-crystals.The molecular fragmentation and the number of small species generated in CL-20 co-crystals are lower than those of pure CL-20 [38], indicating that co-crystals modification can significantly reduce sensitivity [5].At this shock velocity, CL-20 molecules and co-crystal ligands react significantly only when hot spots are generated,and there is no significant change in molecular fragmentation degree, with no detonation reaction occurring.
Under the reflect velocity of 4 km/s, the molecular reaction amount, the number of product species and the molecular fragmentation of these supercells are significantly higher than those under 2 km/s impact, as shown in Fig.7.Before the void collapse(0-1.04 ps), the number of molecules in perfect and defective cocrystals is similar, but the reaction rate of atoms near the hot spot is much higher than that of other atoms after void collapse,resulting in a more dramatic change in the number of components of the void-containing co-crystals than in the perfect co-crystals.When the wavefront sweeps through the whole co-crystals, most of the CL-20 molecules react completely,on the contrary,the ligand molecules react incompletely, which is consistent with the simulation results of defect-containing CL-20/DNB [39].Due to the difference in the density of nitro functional groups, the reaction volume of solvent ligands is significantly lower than that of energetic ligands, and the amount of N2and NO2generated in the solvent co-crystals is also lower, which proves that the detonation performance and sensitivity of solvent co-crystals are lower than that of energetic co-crystals.The molecular number of CL-20/DMC supercell is the most smoothly under the shock of this velocity,preliminarily judged that the sensitivity reduction effect of CL-20/DMC co-crystals is the best among the four types of co-crystals,but the detonation performance is weak.In energetic co-crystals,CL-20/DNB’s fragmentation degree and the amount of N2species are slightly less than those in CL-20/TNT, and the number of residual CL-20 molecules and in CL-20/DNB is larger.It can be seen that the sensitivity of CL-20/DNB of the two co-crystals is lower,which is consistent with the conclusion of CL-20/DNB preparation and test results[39].

Table 4Molecular distribution of co-crystals ligands under the velocity of 4 km/s.

Table 4 (continued)
The results show that the existence of voids has a great influence on the number of co-crystals ligand molecules.It is speculated that the molecular will dimerize first as the wavefront scanning under low-velocity reflects.However,the CL-20 reaction amount and the ligand molecular reaction amount in the void-containing supercells are relatively less than perfect supercells, speculated that the existence of voids causes the wavefront energy to concentrate on the cavity collapse, thus reducing the energy of wavefront and slows down the chemical reaction frequency downstream of the cavity.In order to verify this conjecture, in four kinds of CL-20 co-crystal shock models containing voids, the distribution of co-crystal molecules with wavefront positions upstream of the void,downstream of the void,and swept through the model were plotted,as shown in Table 4, corresponding to the number of co-crystals ligand molecules-time change curve in Fig.5(b).
3.4.Chemical elementary reaction frequency
The chemical elementary reaction analysis method was used to investigate the influence of voids on the chemical reaction of shock induced CL-20 co-crystals, and the shock response process was divided into two stages: cavity collapse and hot spot fading.The high-frequency elementary reactions and frequency of perfect and void-containing CL-20 co-crystals under shock velocity of 2 km/s and 4 km/s were investigated, as shown in Tables 5 and 6.
The main reactions under the shock of 2 km/s include adsorption of CL-20 with ligands, nitro shedding and dimerization reactions,as shown in Table 5.In the cavity collapse stage(0.64-1.76 ps), the dimerization reaction between CL-20 and ligand occurred mainly,and the frequency of CL-20/GBL was the highest,but the net reaction frequency was low,indicating that the wavefront energy is close to the activation energy of the reaction.The solvent cocrystals CL-20/DMC and CL-20/GBL have a higher net frequency of denitration reactions, but the energetic ligands also undergo nitro shedding, which leads to similar NO2production in each supercell during the cavity collapse stage, corresponding to the result of the aforementioned NO2quantity change curve (see Fig.6(d)).In the hot spot fading stage (1.76-5.00 ps), various dimerization reactions were more frequent,CL-20/GBL still has the highest frequency, corresponding to the analysis of the tightest bond between the CL-20 and the ligand in chapter 3.2,and have the better desensitization effect.The number of small carboncontaining molecules generated is very small.It is speculated that CL-20 molecules have a weak ring-opening reaction under the impact of 2 km/s, and the dimerization reaction frequency is greater than the corresponding reverse reaction,meaning that the velocity cannot trigger the detonation of each CL-20 co-crystal[16].
It is found that the chemical reaction of perfect co-crystals is generated during the wavefront scanning and sparse stretching[40], while the hot spot formed in the void-containing co-crystals,which increases the reaction rate around the hot spot,resulting in a significant difference in reaction frequency.Besides the frequency of dimerization reaction between CL-20 and ligand is higher in perfect co-crystals, indicating that there is little correlation with this reaction and the void.The generation reaction frequency of small species in the void-containing supercell is higher.It is speculated that this type of reaction is mainly caused by abnormally high-speed atoms impacting the hot spots downstream of the cavity.In the hot spot fading stage, the net frequency of nitro shedding of CL-20 in void-contained co-crystals is much greater than that in perfect co-crystals, of which CL-20/DNB shows the highest frequency, which is consistent with the above conclusion,the molecular reaction intensity of CL-20 and the production of N2and NO2in the void-contained co-crystals are higher than those in perfect co-crystals (seen in Fig.6).
It can be seen from Table 6 that under the shock of 4 km/s, thepriority of nitro group shed from CL-20 molecules in the cavity collapse stage(0.48-1.04 ps)is much higher than that from ligand TNT and DNB molecules.In CL-20/DMC, the ligand lost its methyl group and self-decomposed, while the other ligands did not undergo high-frequency self-decomposition reaction.At this shock velocity, there is still a dimerization reaction between CL-20 molecules and ligand molecules, but with the exception of CL-20/TNT,the generation frequency of each dimer is lower than 2 km/s,indicating that the priority of the polymerization reaction is reduced,and the molecules tend to decompose.
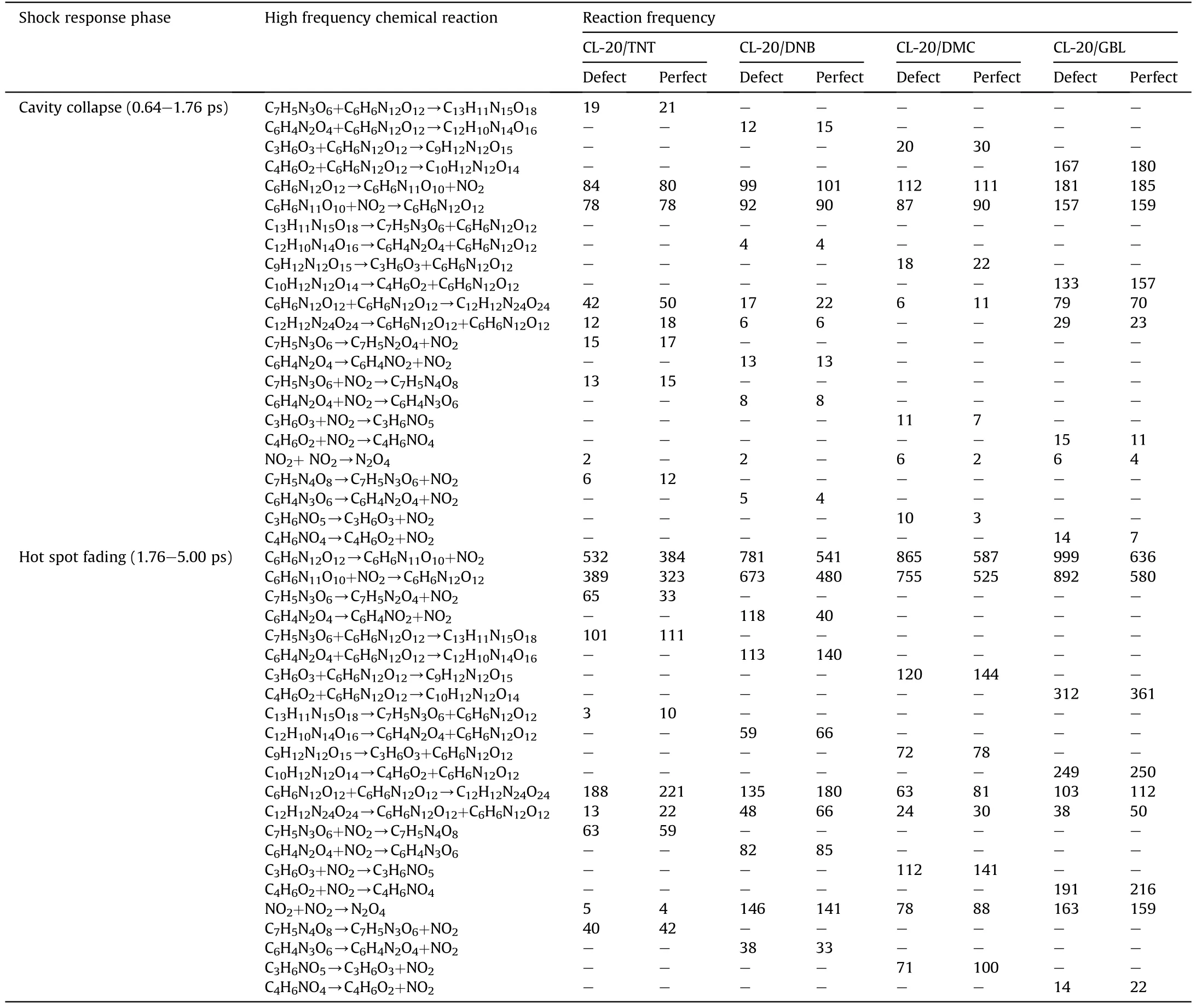
Table 5Reaction frequency of CL-20 co-crystals unit under shock velocity of 2 km/s.
In the hot spot fading stage (1.04-4.00 ps), high-frequency reactions involve small species,generating H2O,HN2O,H2O2et al.It is considered that the detonation of each co-crystal has occurred under the impact of this velocity.The main reaction frequency of the perfect co-crystal is higher than that of the void-containing cocrystal, the gap between the two in the solvent co-crystal is large,and it is consistent with the above conclusion that the effect of void defects on the detonation performance of solvent co-crystal is higher than that of energetic co-crystal.The molecules in the energetic co-crystals CL-20/TNT and CL-20/DNB undergo polymer reactions and stack to form super-large carbon-containing groups.CL-20/TNT is the most prone to polymer reactions, while solvent co-crystals are more difficult for this reaction,and the existence of voids has little effect on the size of the super-large molecular clusters.The results of the two-dimensional temperature field diagram show that the void in CL-20/TNT delays the shockwave longer and has the most energy exchange.The reaction frequency statistics table shows that the small species reaction frequency of CL-20/TNT is greater than that of the other co-crystals.It is speculated that both are factors that affect the hot spot formation temperature.
4.Discussion
Here in two energetic eutectic CL-20/TNT, CL-20/DNB and two solvent co-crystals CL-20/DMC, CL-20/GBL perfect and defectcontaining model impact response are studied, the effect of void defects on the formation of hot spots,the type,and quantity of mainproducts and the reaction frequency of species were analyzed.Nomura et al.[36] simulated the RDX impact with defects and showed that the hot spot spreads in a cone shape when the shock velocity is high.The research in this paper shows that the hot spot of void-containing CL-20 co-crystals would be elongated along the shock direction,which is similar to Nomura’s conclusion.Liu et al.[15]simulated the piston impact of CL-20/TNT energetic co-crystal and showed that there is no detonation at 2 km/s impact, CL-20 polymerization and decomposition reactions exist simultaneously;under 4 km/s impact,the direct decomposition of perfect and void-containing co-crystals dominates, which is consistent with the product analysis and reaction frequency results in this research.It is speculated that CL-20 co-crystals with solvent components cannot produce super-large molecular clusters under the high-speed impact, and defects have little effect on the size of super-large molecular clusters generated.

Table 6Reaction frequency of CL-20 co-crystals unit under shock velocity of 4 km/s.
Here in the statistical results of the shock response frequency of each co-crystals model (see Table 6) show that CL-20 solvent cocrystal is extremely difficult to generate super-large molecular clusters at the initial stage of impact, and it is difficult to cause aggregation.Wang et al.[39] speculated that the CL-20/DNB cocrystal has lower sensitivity than CL-20/TNT in their co-crystal preparation experiments, here compares the number of small species products (see Figs.3 and 4) to verify this conclusion.It is also found that the effect of void defects on CL-20/TNT is greater than CL-20/DNB.When detonation occurs under the high-speed impact (4 km/s), there is little difference in the number of species and reaction frequency among these types of co-crystals, but the introduction of voids would significantly reduce the frequency of species generation of each co-crystal when the hot spot faded,and reduce the detonation performance of each CL-20 co-crystal, and more significant impact on the solvent co-crystals are observed.
5.Conclusions
ReaxFF-lg force field was performed to analyze the formation of shock-induced hot spots, the number of molecules, the main products,and the reaction frequency of CL-20/TNT,CL-20/DNB,CL-20/DMC, and CL-20/GBL co-crystals.The conclusions are listed as follows.
(1) The hot spot evolution process shows that as the shock velocity increases,the size of the hot spot becomes longer along the shock direction,and the shape of the hot spot becomes a cone at 4 km/s.The different types of co-crystal ligands have a significant impact on the hot spot temperature.Under the same conditions, the void in the energetic co-crystal has a greater retardation effect on the wavefront,and there is more energy exchange between the wavefront and the void,resulting in a high hot spot temperature.
(2) Under the reflect velocity of 2 km/s,the main reaction of CL-20 co-crystal is the synthesis of dimers and the shedding of nitro groups, without the formation of a large number of species and no detonation.Compared with perfect crystal,the existence of voids aggravates the chemical reactions,reduces the safety of CL-20 co-crystals,and the sensitivity of void-containing energetic co-crystal increases more obviously.
(3) The sensitivity of CL-20/DNB in energetic co-crystals is lower than that of CL-20/TNT under the impact of 4 km/s, indicating that the sensitivity reduction effect is better.Energetic co-crystal is more prone to polymerization reaction than solvent co-crystals and would unaffected by voids, while solvent co-crystal is less sensitive and harder to agglomerate.Under the shock of this velocity, both the perfect and the hole-containing CL-20 co-crystals detonate, and the wavefront energy is reduced after sweeping through the cavity,reducing the frequency of chemical reactions downstream of the cavity and weakening the detonation performance.In addition, the effect of void defects on the detonation performance of CL-20 solvent co-crystal is higher than that of CL-20 energetic co-crystal.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (22275018), and the Project of State Key Laboratory of Explosion Science and Technology (Beijing Institute of Technology) (Grant No.QNKT20-04).
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels

