A new loss-of-function allele 28y reveals a role of ARGONAUTE1 in limiting asymmetric division of stomatal lineage ground cell
Kezhen Yang,Min Jiangand Jie Le
Key Laboratory of Plant Molecular Physiology,Institute of Botany,the Chinese Academy of Sciences,Beijing 100093,China.?These authors contributed equally to this work.*Correspondence:lejie@ibcas.ac.cn
INTRODUCTION
Stomata are specialized epidermal structures that regulate CO2influx used in photosynthesis as well as water vapor loss between plants and their environment(Casson and Hetherington 2010).In Arabidopsis,stomata develop from protodermal cells after at least one asymmetric division and a symmetric division.The meristemoid mother cell(MMC)undergoes asymmetric entry division to produce a small,triangular meristemoid and a larger sister cell,a stomatal lineage ground cell(SLGC).The meristemoid differentiates into a guard mother cell(GMC).Then,the GMC undergoes a single symmetric cell division to generate a pair of guard cells(GCs).The SLGC can directly differentiate into pavement cell or undergoes an asymmetric spacing division to generate a satellite meristemoid,and eventually develops into a new stoma(Bergmann and Sack 2007).
There is at least one pavement cell between two stomata(“one-cell-spacing” rule),suggesting that signals must be transduced between cells during stomatal development.In Arabidopsis,plasma membrane receptors TOO MANY MOUTHS(TMM)(Yang and Sack 1995;Nadeau and Sack 2002),ERECTA(ER)family(Shpak 2013),and putative ligands(Hara et al.2007;Hunt and Gray 2009;Abrash and Bergmann 2010;Sugano et al.2010),are predicted to participate in stomatal signal transduction.For instance,EPF2 and EPF1 secreted from meristemoids or GMCs are perceived by TMM and ER family receptors in neighbor cells and provide the positional information to regulate the frequency and orientation of asymmetric spacing divisions(Lee et al.2012).
In turn,these signals are transduced via a mitogenactivated protein kinase(MAPK)cascade,which includes YODA,MKK4/5,and MPK3/6(Bergmann 2004;Lampard et al.2008,2009).Three basic-helix-loop-helix(bHLH)transcription factors,SPEECHLESS(SPCH),MUTE,and FAMA,are required for successive stages of development including lineage initiation and proliferation,as well as terminal differentiation(Ohashi-Ito and Bergmann 2006;MacAlister et al.2007;Pillitteri et al.2007).SPCH is essential for MMC formation,stomatal entry division,and maintenance of meristemoid stem cell activity(MacAlister et al.2007).MUTE promotes the transition of meristemoids into GMCs and is required for asymmetric amplifying division of meristemoids(Pillitteri et al.2007).FAMA plays a role in GMC differentiation and proliferation(Ohashi-Ito and Bergmann 2006).Additional bHLH proteins INDUCER OF CBF EXPRESSION1(ICE1)/SCREAM(SCRM)and SCRM2,directly interact with SPCH,MUTE,and FAMA during stomatal development(Kanaoka et al.2008).MAPK signaling cascade likely regulates stomatal lineage entry through its control of the phosphorylation status of SPCH.Overexpression of SPCH driven by its own promoter promotes the formation of stomatal meristemoids and leads to extra stomata in clusters(Lampard et al.2008).The phytohormone brassinosteroid(BR)regulates stomatal development through SPCH phosphorylation either directly by the serine/threonine glycogen synthase kinase 3/SHAGGY-like BIN2(BRASSINOSTEROID INSENSITIVE 2),or indirectly via YODA or MKKs in the MAPK signaling pathway(Gudesblat et al.2012;Kim et al.2012;Khan et al.2013).The degradation of SPCH induced by MPK or BIN2 phosphorylation prevents epidermal cells from entering into the stomatal cell lineage(Lampard et al.2008;Gudesblat et al.2012).
MicroRNAs(miRNAs)play important roles in regulating gene expression in multicellular plants and animals(Carrington and Ambros 2003).The miR824 regulates asymmetric division of satellite meristemoids through repressing AGAMOUS-LIKE16(AGL16)in stomatal lineage cells(Kutter et al.2007).A recent study found that the components of the miRNA pathway HYPONASTIC LEAVES1(HYL1),ARGONAUTE1(AGO1),and HUA ENHANCER1(HEN1)genes,participate in the proximal–distal and adaxial-abaxial patterning of leaves and in stomatal production(Jover-Gil et al.2012).Mutation of AGO1 leads to narrow leaves,dwarf seedlings,late flowering,and low fertility, developmental defects (Baumberger and Baulcombe 2005;Vaucheret 2008).But it remains unclear how AGO1 regulates stomatal development.
Here,we identified that 28y is a new loss-of-function allele of AGO1.Time-lapse analysis revealed that AGO1,like TMM,is another negative regulator in restricting asymmetric spacing divisions in SLGCs.We demonstrated that AGO1 acts downstream of TMM in stomatal production.Furthermore,we proposed that the upregulation of SPCH transcript in 28y mutants is partially mediated by an AGL16-dependent miRNA pathway.

Figure 1.Seedling and stomatal phenotypes of 28y mutant(A–C)28y mutant.A 14 d old 28y seedling(A).Inset,a mature 28y plant grown in soil.Differential interference contrast images showing clustered stomata from a 28y cotyledon(B)and a hypocotyl(C).White brackets indicate stomata in clusters.(D–F)Wild type.A 14 d old wild-type seedling(D).Patterned and spaced stomata in epidermis from a wild-type cotyledon(E)and a hypocotyl(F).Bars=200 mm(A,D),50 μm(B,C,E,F).
RESULTS
Overproduction of stomatal in 28y cotyledons and hypocotyls
Dwarf seedlings with dark green and narrow cotyledons were found in the segregating population of a natural mutant.These mutant plants could form a couple true leaves but could not produce seeds(Figure 1A,D).This line number is 28,thus we termed this mutant as 28y.Segregation analysis demonstrated that the mutation in the 28y mutant is recessive and monogenic(107 out of 435).To assay the stomatal phenotypes,we analyzed the stomatal density and index of 14 d old cotyledons and total stomatal number of hypocotyls.The stomatal density and index of wild type were 160±17/mm2and 31±0.9%,respectively.By contrast,28y mutant had an approximately double stomatal density of 337±63/mm2,and a higher stomata index of 44±4.6%.The total stomatal number in 28y hypocotyls was much more than that in wild type as well(Table 1).In addition,the stomata in the 28y mutant often formed clusters(Figure 1B,C,E,F;white brackets indicate stomata in direct contact).These results indicated that 28y negatively regulates stomatal production either in cotyledons or hypocotyls.
Excessive asymmetric spacing divisions in 28y hypocotyls
To investigate the abnormal stomatal pattern formation in the 28y mutant,we analyzed the developmental processes of stomata in wild type and 28y hypocotyls by time-lapse differential interference contrast(DIC)imaging.In wild-type hypocotyls,MMCs divided asymmetrically to produce a triangular meristemoid and a big sister cell,a SLGC(Figure 2A,B).Meristemoids differentiated into GMCs that then divided symmetrically to generate a pair of stomatal GCs.While the adjacent SLGCs differentiated into pavement cells(Figure 2C,D).
Mutation of 28Y did not affect MMC divisions in producing meristemoids and their big sister cells,SLGCs(Figure 2E,F).However,unlike wild type,SLGCs in the 28y mutant were able to acquire MMC character and executed asymmetric spacing divisions to produce satellite meristemoids and new SLGCs(Figure 2G).The satellite meristemoids then directly differentiated into GMCs and mature stomata eventually,but the new SLGCs continued spacing divisions to produce higher-order stomatal complexes(Figure 2H).Thus,the excessive spacing division that happens in SLGC is the cause of overproduction of stomata in 28y hypocotyls.
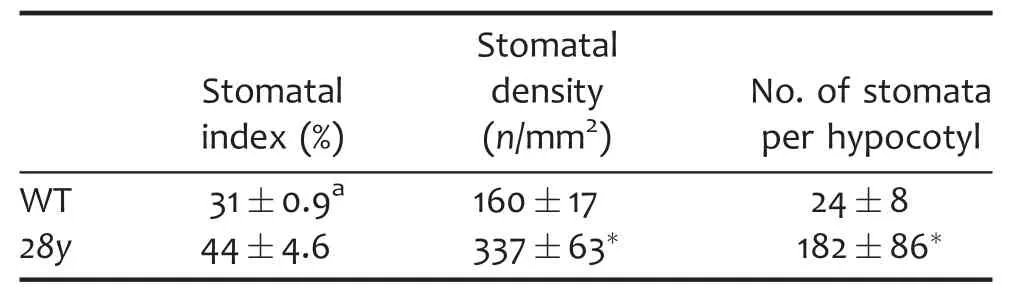
Table 1.Increased stomatal production in 28y mutant

Figure 2.Stomatal lineage tracing in hypocotyls(A–D)Stomatal development in a wild-type hypocotyl.Insets,illustrations of the development of stomatal lineage cells within the white frames.A meristemoid mother cell(MMC)divides asymmetrically to produce a triangular meristemoid(red)and a big sister cell,a stomatal lineage ground cell(SLGC).Meristemoid differentiates into a guard mother cell(GMC)(yellow)that then divide symmetrically to generate a pair of stomatal guard cells(GCs)(green).The adjacent SLGCs differentiate into epidermal pavement cells.(E–H)Higher-order stomatal complexes form in a 28y hypocotyl.Excessive asymmetric spacing divisions in SLGCs produce extra satellite meristemoids and stomata.Bar=20 μm(A–C,E–G),30 μm(D,H).
Ectopic stomatal lineage cells in 28y cotyledons
Owing to the narrow and thick cotyledons of the 28y mutant,it is difficult to trace the epidermal cell development by timelapse DIC imaging.We then use stomatal lineage marker genes to analyze the process of stomatal development in wild-type and 28y cotyledons.TMM encodes a leucine-rich repeat receptor-like protein controlling stomatal production and pattern(Nadeau and Sack 2002).TMM:TMM-GFP is highly expressed in stomatal lineage especially before and after stomatal asymmetric divisions,but not in mature GCs and pavement cells(Figure 3A–C).There was no obvious difference of TMM:TMM-GFP expression pattern between 28y and wild type at 1 d after seed germination(Figure 3A,D).The number of cells expressing TMM:TMM-GFP was gradually reduced in the wild-type cotyledons at 5 or 7 d after germination(Figure 3B,C).Due to the excessive asymmetric divisions occurred in SLGCs,the number of cells expressing TMM:TMM-GFP in 28y cotyledons was higher than that in the wild-type plants at the same developmental stage(Figure 3E,F).
In contrast to the expression pattern of TMM:TMM-GFP E1728 is only expressed in mature GCs.With the development of leaf and maturation of GCs,the number of cells expressing E1728 fluorescence reaches a constant level in wild-type cotyledons.At 7 d after germination,most of stomata completed their development and acquired their maturity in wild type(Figure 3G–I).However,the number of mature GCs in 28y cotyledons increased during the same observation period.The presence of immature GCs without E1728 fluorescence in 7 d old cotyledons indicated a high remaining activity of producing new stomata in 28y mutant(Figure 3J–L).

Figure 3.Ectopic stomatal lineage cells produced in 28y cotyledons(A–C)TMM:TMM-GFP is expressed in stomatal lineage cells and their neighbor sister cells in wild-type cotyledons,tracing at 3,5,and 7 d after germination.(D–F)Number of the epidermal cells expressing TMM:TMM-GFP in the 28y mutant is higher than that in wild-type cotyledons at the same stages.(G–I)Expression of a mature stomatal marker E1728 in wild-type cotyledons.(J–L)E1728 expression in 28y cotyledons.An arrow indicates a newly formed stoma without E1728 fluorescence in a 7 d old 28y cotyledon.Bar=20 μm.
spch is epistatic to 28y in regulating stomatal asymmetric division
SPCH encodes a bHLH transcription factor that is required for stomatal cell lineage initiation,asymmetric entry and spacing divisions(MacAlister et al.2007).In the loss-of-function spch-4 mutant,only pavement cells can be found in cotyledons or hypocotyls(Figure 4A,B).To investigate the interaction between 28y and SPCH in regulating stomatal production,28y was crossed with spch-4 mutant.Although the overall growth of spch-4 28y double mutant seedlings is similar to that of 28y single mutant(data not shown),no stoma was found in spch-4 28y epidermis from either cotyledons or hypocotyls,suggesting that spch-4 mutant is epistatic to 28y in stomatal initiation(Figure 4C,D).
In wild-type epidermis,expression of a translational reporter SPCH:SPCH-GFP is restricted to stomatal precursor cells and big neighbor cells.In an older organ,the SPCH:SPCHGFP expression decreased and finally disappeared in the big neighbor cells(Figure 4E).In the 28y mutant,the cells expressing SPCH:SPCH-GFP were often formed in big clusters,indicating that the epidermal cells acquired stomatal lineage fate(Figure 4F).This is in agreement with the upregulated SPCH transcript levels in 28y seedlings(Figure 4G).
28y is epistatic to tmm-1 in regulating stomatal production in hypocotyls
Stomatal phenotypes in tmm mutants are tissue specific(Bhave et al.2009).tmm mutants produce clustered stomata in leaves,but no stomata in hypocotyls(Figure 5A,B).28y was crossed with a null tmm-1 mutant to generate a tmm-1 28Y double mutant.Although the stomatal density and index have no significant difference between tmm-1 and tmm-1 28y double mutant cotyledons(Table 2),mutation of 28Y suppressed the formation of big stomatal clusters in the tmm-1 mutant,suggesting that 28Y may act downstream of TMM(Figure 5C,E).
Surprisingly,unlike the “no stomata” in tmm-1,the tmm-1 28y double mutant produced plenty of stomata in hypocotyls,indicating that 28y is epistatic to the tmm-1 mutant in regulating hypocotyl stomatal production(Figure 5B,D).Expression of SPCH:SPCH GFP in wild-type plants also displayed a moderate stomatal overproduction phenotypes in cotyledons and hypocotyls(Figure S1A,B).Most the epidermal cells were converted into stomata when SPCH:SPCH-GFP was introduced into tmm-1 cotyledons(Figure S1C).In hypocotyls,expression of SPCH:SPCH–GFP induced large stomatal clusters in tmm-1(Figure S1D).Because our real-time polymerase chain reaction(PCR)analysis showed a significant increase of SPCH transcriptional level in 28Y mutants(Figure 4I),we speculated that 28Y negatively regulates SPCH expression downstream of TMM.
28Y acts in a pathway parallel to BR-mediated stomatal regulation
The phytohormone BR positively regulates stomatal production through enhancing SPCH protein stability by phosphorylation.The stomatal production in hypocotyls largely increased when treated by the BR hormone brassinolide(BL)(Gudesblat et al.2012).An application of BL at a concentration of 1 μM significantly increased stomatal number in wild-type hypocotyls,whereas the BR synthesis inhibitor brassinazole(BRZ)inhibited stomatal production in hypocotyls(Figure S2A–C).
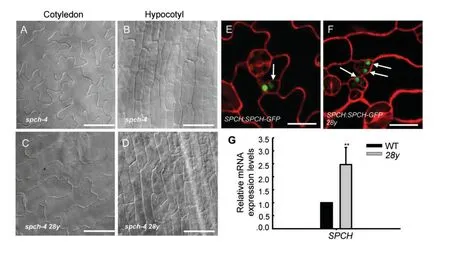
Figure 4.spch is epistatic to 28y in initiating stomatal lineage(A,B)No stomata occurred in a spch-4 cotyledon(A)and a hypocotyl(B).(C,D)Stomatal defects in an spch-4 mutant are not rescued by 28y.(E)SPCH:SPCH–GFP in wild-type epidermis is restricted mostly in meristemoids.An arrow points to a stomatal lineage ground cell(SLGC)with weak green fluorescent protein(GFP)fluorescence.(F)More cells expressing SPCH:SPCH–GFP are present in 28y epidermis,indicated by arrows.(G)SPCH transcript level was largely increased in 28y mutant compared with that in wild types(**P < 0.01 after Student’s t-test,28y vs wild type).Bars=50 μm(A–D),20 μm(E,F).

Figure 5.28y is epistatic to tmm in regulating hypocotyl stomatal production(A)Clustered stomata in tmm-1 cotyledons.(B)No stomata produced in tmm-1 hypocotyls.(C,D)Clustered stomata are present in tmm-1 28y double mutant cotyledons(C)as well as in their hypocotyls(D).Note that 28y is epistatic to tmm-1 in rescuing the “no stomata” phenotype of tmm-1 hypocotyls.(E)28Y mutation reduces the cluster complexity of tmm-1(**P < 0.01 after Student’s t-test,28y tmm-1 vs tmm-1).Bars=50 μm(A,C),20 μm(B,D).

Table 2.Stomatal densities and indexes in tmm-1 and tmm-1 28y mutants
To test whether the 28y gene regulates stomatal production through the BR-mediated SPCH signaling pathway,28y mutants were treated with BR and BRZ,respectively.We found that the number of stomata in 28y hypocotyls greatly increased from untreated 182±86 to 857±180(n=10)per hypocotyl after 1 μmol/L BL treatment(Figure S2D,E).By contrast,the stomatal number was largely reduced by BRZ-treatment to a level of 70±16(n=10)per hypocotyl(Figure S2F).Taken together,28Y and BR appear to act independently in regulating stomatal production in hypocotyls.
28Y functions at the early stage of stomatal development
MUTE is a bHLH transcription factor that is required for the meristemoid to GMC fate transition and for asymmetric amplifying division(Pillitteri et al.2007).In the mute mutant,the stomatal development is arrested at meristemoid stage but the amplifying asymmetric division is repeated,resulting in“rosettes” of larger daughter cells surrounding a triangular meristemoid at the center(Figure S3A,B).In mute 28y double mutant,no mature stomata were found in either cotyledons or hypocotyls.Presence of clusters of “rosettes” indicates an additive interaction between MUTE and 28y,indicating that 28y functions at early stages during stomatal development but requires MUTE for M-to-GMC differentiation during stomatal development(Figure S3C,D).
The MUTE:MUTE–GFP is expressed in the nuclei of late meristemoids and early GMCs,but is not in GCs and pavement cells(Figure S3E).Ectopic expression MUTE:MUTE–GFP in small cells next to an existing stoma was often found in 28y epidermis(Figure S3F).As we expected,there was a relatively high MUTE transcript level in 28y seedlings(Figure S3G).
28Y gene encodes AGO1 protein
To find the mutated gene in the 28y genome,we initiated mapbased cloning of 28y.The 28y mutant was crossed with Ler and F2 population for genetic mapping.The mutation was first delimited to a BAC clone F21D18 on chromosome 1.Analysis of genes in this fragment revealed the presence of AGO1(AT1G48410).As the phenotype of ago1 mutant alleles is highly similar to the 28y mutant,we then sequenced AGO1 in 28y.A single C-to-T substitution at the 7th base and a deletion of TAGCTGA between the 8th base and the 14th base of the 5th exon were found within the AGO1 gene in 28y,resulting in an early transcription termination(Figure 6A).
To confirm whether the stomatal phenotype is caused by AGO1 mutation,a point mutation line CS3854(ago1-8),obtained from the Arabidopsis Biological Resource Center(ABRC)seed stock center,was used for further analysis.Compared with the wild type,ago1-8 shows phenotypic characteristics similar to the 28y mutant,such as narrow and thick leaves,smaller seedling size,flat pavement cells without lobes,and excessive stomata in epidermis(Figure 6B,C).We crossed the heterozygous 28y mutant with heterozygous ago1-8 mutant.The phenotypic analysis of F1 seedlings demonstrated that 25%seedlings show similar phenotypes to 28y and ago1-8,indicating that 28y is allelic to ago1-8(Figure 6D,E).
Then,we generated transgenic lines carrying the GUS gene driven by the AGO1 promoter.A preferential expression of AGO1:GUS was found in stomatal lineage cells,consistent with the involvement of AGO1 in stomatal development(Figure 6F–J).
AGO1 is required to repress AGL16-mediated miRNA stomatal regulatory pathway
AGO1 acts in the miRNA pathway in regulating gene expression during many development processes(Vaucheret et al.2004).The miR824 regulates asymmetric division through repressing AGL16 mRNA in stomatal lineage cells(Kutter et al.2007).Mutation of miR824 or overexpression of the AGL16m form causes excessive asymmetric division and results in extra stomata in epidermis,a similar stomatal phenotype found in the 28y mutant.Real-time PCR results showed that the relative mRNA expression level of AGL16 is significantly increased in 28y mutants(Figure 7).This result suggested that AGO1 regulates stomatal division through participating in the miR824 pathway and repressing AGL16 expression,indicating that AGO1 is required to restrict AGL16-mediated regulation of stomatal development.
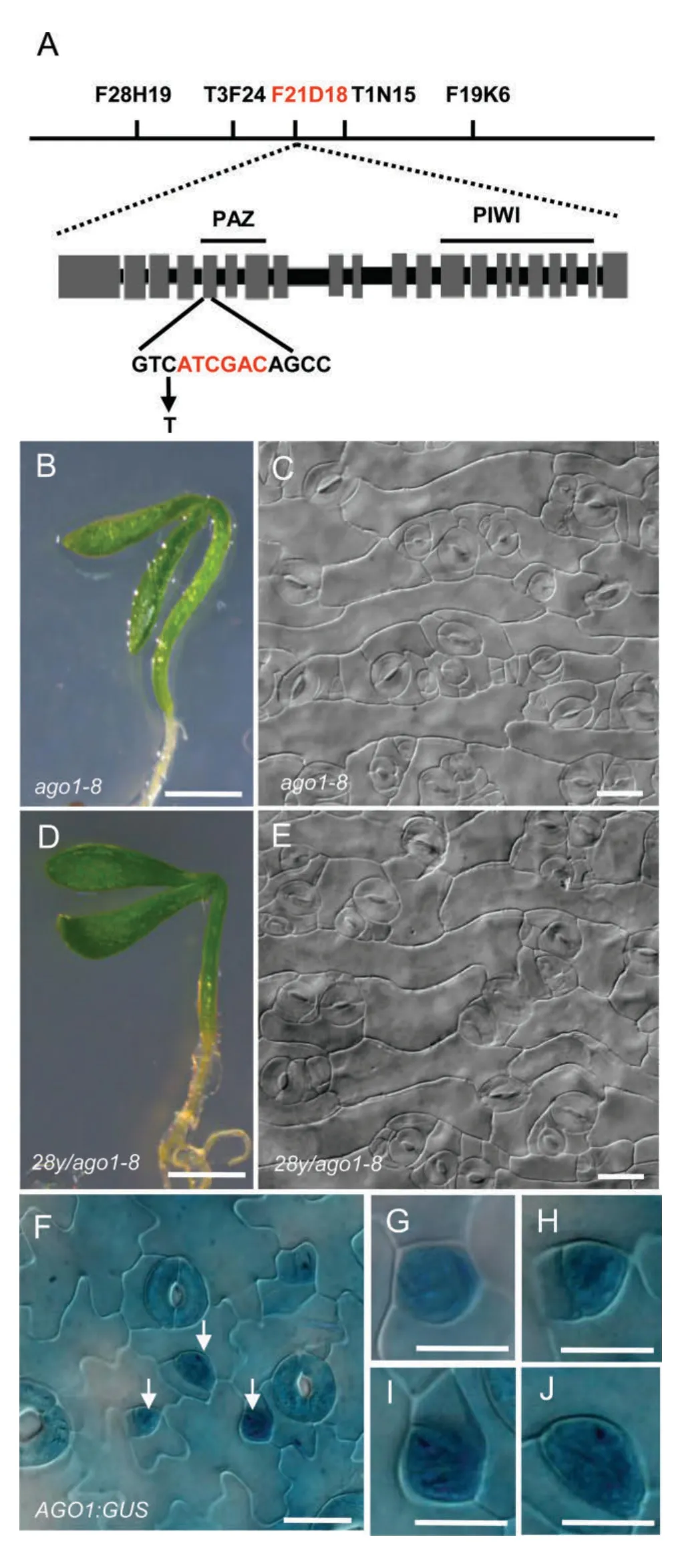
Figure 6.Mutation of AGO1 is the cause of the defective seedling growth and stomatal production in the 28y mutant(A)Mapping of the 28Y gene and physical structure of the 28Y/AGO1 gene.Letters above lines indicate the position of molecular markers.BAC at F21D18(red letters)contains the 28Y/AGO1 gene.The mutations in the 28y mutant are indicated in the bottom panel.28y has a T-to-A transition(arrow)and a deletion of bases(red letters),resulting in a premature termination of AGO1 transcript.Gray boxes indicate the exons.(B,C)ago1-8 seedlings and its cotyledon epidermis.(D,E)28y is allelic to ago1-8.A progeny seedling and the cotyledon epidermis of 28y and ago1-8 crosses.(F–J)AGO1:GUS broadly expressed in the epidermis(F),especially in stomatal lineage cells,including meristemoid mother cell(MMC)(G),meristemoids(H),guard mother cells(GMCs)(I),young guard cells(GCs)(J).Bars=200 mm(B,D),20 μm(C,E–J).
DISCUSSION
28y is a new strong allele of AGO1
AGO1 is the founding member of the Arabidopsis AGO gene family.In Arabidopsis,more than 100 ago1 alleles have been used to characterize the pleiotropic functions of AGO1 in many plant development processes,namely,embryonic meristem development,adventitious root formation,root radian patterning,leaf development,and venation patterning(Bohmert et al.1998;Vaucheret et al.2004;Miyashima et al.2009;Jover-Gil et al.2012).Although abnormal stomatal overproduction was observed in two intermediate alleles ago1-51/icu9-1 and ago1-52/icu9-2,the molecular mechanism has not been well characterized(Jover-Gil et al.2012).
ago1-8(EMS-induced mutation)is a strong allele exhibiting dwarf seedlings,and narrow and thickened rosette leaves(Lynn et al.1999).However,the phenotypes in 28y are stronger than those found in ago1-8.The premature termination of transcript of AGO1 in 28y would produce a truncated protein that lacks the PAZ and PIWI domains that are required for RNA recognition.
AGO1 is involved in regulation stomatal ground cell differentiation and division
The stomatal development is the most complex process of epidermal development including at least one asymmetric division and a symmetric division.There are three types of asymmetric division including entry division,amplifying division,and spacing division.Stomatal lineage ground cells are big daughter cells of new meristemoids after each kind of asymmetric divisions.The SLGCs can directly differentiate into jigsaw-puzzle-shaped pavement cells that interlock with neighboring cells.Stomatal lineage ground cells are able to acquire an MMC fate and then divide unequally to produce new satellite meristemoids and SLGCs(Bergmann and Sack 2007).Thus,asymmetric spacing divisions taken place in SLGCs initiate more stomatal lineage cells and produce more stomata in epidermis,leading to an increase of total number or density of stomata in epidermis.By contrast,each asymmetric amplifying division that has happened in meristemoids regenerates a meristemoid and produces another SLGC,resulting in a decrease of stomatal index,the ratio of the number of stomata to the total number of epidermal cells.A significant increase of stomatal density,but not stomatal index,in 28y cotyledons and a higher number of total stomata in 28y hypocotyls indicates a function of AGO1 in restricting stomatal spacing division instead of amplifying division.
Excessive asymmetric divisions occurred in 28y SLGCs lead to a significant increase of stomatal density.However,the narrow and small size of pavement cells in 28y may contribute as well to its increased stomatal density.But lineage tracing results demonstrated that excessive spacing asymmetric divisions in SLGCs are the major cause of overproduction of stomata in either cotyledon or hypocotyl epidermis.In addition,the lobe formation in 28y pavement cells are greatly reduced,indicating that AGO1 may be required as well for the cell shape control during pavement cell differentiation.Based on previous reports(Jover-Gil et al.2012)and our observations,AGO1 is not only required in restricting asymmetric division in SGLCs but also in promoting pavement cell differentiation.Therefore,AGO1 is playing as a key gene in regulating SLGC division and differentiation,providing a developmental flexibility of stomatal production and distribution in epidermis in response to ever-changing surrounding environments.

Figure 7.AGL16 transcript levels in 28y mutantsThe AGL16 relative mRNA expression levels are upregulated in 28y mutant seedlings(**P< 0.01 after Student’s t-test,28y vs wild-type seedlings).
AGO1 regulates stomatal spacing division though repressing SPCH expression
Our genetic analysis results demonstrate that AGO1 acts upstream of SPCH in stomatal production.SPCH is a key bHLH transcriptional factor required for stomatal lineage initiation(acquiring MMC fate)and subsequent asymmetric division(Lau and Bergmann 2012).We found that SPCH transcriptional level is negatively regulated by AGO1.AGO proteins are integral players in small RNA-mediated regulatory pathways.These small RNA-mediated AGO proteins regulate gene expression at various levels,such as by RNA cleavage,DNA methylation(Vaucheret et al.2004).Although a CpG island was predicted in SPCH cDNA(465–707,counting from ATG),no obvious difference in methylation level was found between wild-type and 28y plants(Figure S4),indicating that repression of the SPCH expression by AGO1 may not occur through methylation machinery.
It was shown that miR824-mediated regulation of AGL16 may act in stomatal development(Kutter et al.2007).AGL16 functions as a positive regulator in promoting spacing division.Indeed,an increased transcript level of AGL16 was found in 28y plants,which was also observed in ago1-52 mutant(Jover-Gil et al.2012).Here,we found that AGO1 is expressed widely in epidermis but displays a preferential high expression in MMCs,meristemoids,GMCs,and young GCs,but not in mature GCs.However,AGL16 mRNA was detected only in mature GCs(Kutter et al.2007).Thus,AGL16 transcript levels may be downregulated by AGO1 in stomatal precursor cells and immature GCs,but in mature GCs.Because AGO1 is proposed to restrict the division in SLGCs,the presence of miR824 in satellite meristemoids and GMCs raises the possibility that the regulating of AGO1 on AGL16 transcript is mediated by miR824,which may move from stomatal precursor cells to the neighboring SLGCs(Figure 8A).
Additional AGO1-mediated signaling pathway in regulating stomatal development
A current model of stomatal signaling pathway involves TMM/ERf-MAPK and BR pathways(Figure 8B).Signals of secreted peptide ligands,such as,EPF1,EPF2,STOMAGEN,and CHALLAH,are received by TMM and ERECTA family receptors.In turn,these signals are transduced via MAPK cascade,which regulates stomatal lineage entry through its control of the phosphorylation status of SPCH.The phytohormone BR regulates stomatal development also through SPCH phosphorylation.The degradation of SPCH induced by MPKs or BIN2-mediated phosphorylation prevents epidermal cells from entering into the stomatal cell lineage(Lampard et al.2008;Gudesblat et al.2012;Kim et al.2012).
Loss-of-function of TMM causes a region-specific stomatal phenotype.For instance,many excess stomata in direct contact form in tmm leaves,displaying “too many mouths”phenotype.However,no stoma is produced in tmm stems and hypocotyls(Geisler et al.1998).Extra spacing divisions and their misplaced division planes cause the formation of stomatal clusters in tmm mutants.Our phenotypic analysis of stomatal complexity in tmm-1 28y double mutants showed that AGO1 mutation suppressed the formation of big stomatal clusters in the tmm-1 mutant,suggesting a role of AGO1 in limiting spacing divisions downstream of TMM.The 28y complemented the no stomata phenotype in tmm hypocotyls,further confirming that AGO1 act downstream of TMM in stomatal production.Similarly,overexpression of SPCH(SPCH:SPCH-GFP)could compensate defects of stomatal production in tmm hypocotyls.Taken together,our results suggest that AGO1 may function downstream of TMM but upstream of SPCH in hypocotyl stomatal production.
SPCH activity is regulated by MAPK or BIN2-mediated protein phosphorylation in TMM/ERf or BR signaling pathways.In 28y hypocotyls,BR treatment has additive effects in promoting stomatal production and the BR inhibitor BRZ cannot completely suppress stomatal formation.Thus,we propose that a transcriptional regulation of SPCH by the AGO1-mediated miRNA pathway is an additional regulatory route to the known SPCH phosphorylation(Figure 8).
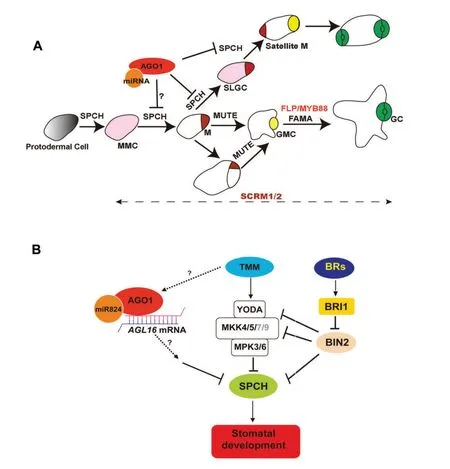
Figure 8.Scheme of AGO1 regulatory pathway in stomatal development(A)Stomata lineage initiates from meristemoid mother cell(MMC,in pink color).Meristemoid mother cell executes asymmetric entry division and produces a meristemoid(M,in brown color)and a big sister cell,a stomatal lineage ground cell(SLGC).SPCH,MUTE,FAMA,and FLP/MYB88 are transcriptional factors in regulating key transitions during stomatal development.Stomatal lineage ground cells can differentiate into a pavement cells.Stomatal lineage ground cells also can reacquire an MMC fate and initiate asymmetric spacing divisions to produce satellite meristemoids.AGO1 acts in restricting spacing divisions.(B)AGO1 functions downstream of TMM but independently from brassinosteroid(BR)-mediated SPCH phosphorylating pathway.Instead,AGO1 may be involved in AGL16-mediated miRNA post-transcriptional regulatory pathway.
MATERIALS AND METHODS
Plant genotypes and growth conditions
All genotypes were in Columbia(Col-0)and Ler ecotypic background.Seeds of ago1-8(CS3854)were obtained from ABRC.Arabidopsis thaliana were grown on half-strength Murashige–Skoog(MS)medium(pH 5.8)at 22–24°C under a 16:8 h light:dark cycle.For the BL(Sigma-Aldrich,St Louis,MO,USA)and BRZ(TCI,Shanghai,China)treatments,seeds were sown to the surface of medium supplemented with 1 μmol/L BL or 10 μmol/L BRZ,respectively.
Microscopy
Two week old cotyledons and hypocotyls were cleared as in Malamy and Benfey(1997).For fluorescence,cotyledons and hypocotyls were stained with 0.1%(w/v)propidium iodide,and green fluorescent protein fluorescence was imaged using a confocal laser-scanning microscope(FV1000-MPE;Olympus,Tokyo,Japan).
The density of epidermal cells and stomatal cells was measured within six regions located at the base,the middle,and top of each leaf using an Olympus BX51 microscope.Stomatal index was calculated as ratio of the number of stomata to the total number of epidermal cells.
For the time-lapse imaging of stomatal development in hypocotyls,the seedlings were sown on the surface of halfstrength MS medium solidified with 1%agar and grown vertically.After each time-point imaging,the water that remained on epidermis was soaked up by small pieces of filter paper and seedlings were placed back in the growth chamber.Images were taken with a×60 objective and a camera mounted on an Olympus BX51 microscope.
GUS staining
To obtain AGO1:GUS,the promoter DNA fragment obtained by PCR using the primer shown in Table S1 and was cloned into the pMD19-T vector(Takara,Dalian,China)for sequencing.The fragment was subcloned into a pCAMBIA1300 vector(CAMBIA,Canberra,ACT,Australia)and introduced into Col-0 plants.The transformants were selected on medium containing 25 mg/L hygromycin and confirmed by PCR.GUS staining was performed as in Lai et al.(2005).Seedlings were then cleared and observed using an Olympus BX51 microscope.
Quantitative reverse transcription PCR analysis
For quantitative reverse transcription(RT)-PCR analysis,total RNA from wild-type and 28y seedlings was extracted using TRNzol reagent(Tiangen Biotech,Beijing,China).Reverse transcription was performed using a Promega kit(Promega,Madison,WI,USA).TUBULIN2 was used as an internal control.Real-time quantitative PCR experiments were repeated independently three times.cDNA was amplified using SYBR Premix ExTaq(TaKaRa)with a Corbett Research Rotor-Gene 3000 Thermal Cycler.Primers specific to SPCH,MUTE,and AGL16 were used for quantitative RT-PCR indicated in the Table S1.
Map-based cloning and sequencing
The 28y mutant was crossed to the wild-type Ler.The F2 population was screened for ago1 mutants on the basis of defective phenotypes of seedlings.28y was first mapped within the BAC Clone F21D18.The mutations in 28y were identified by sequencing.
Methylation analysis
CpG island region and primers were analyzed as in Li and Dahiya(2002).The methylation reaction of 28y and wild-type DNA were done according to the manufacturer’s instructions(Zymo Research,Irvine,CA,USA),and then amplified by PCR using the primers shown in Table S1.The PCR products were cloned into the pMD19-T vector,and then were sequenced and analyzed.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China,30971652 and 31271463(J.L.),31071198(K.Y.),Hundred Talents Program and KSCX2-YW-N-073 from the Chinese Academy of Sciences.
Abrash EB,Bergmann DC(2010)Regional specification of stomatal production by the putative ligand CHALLAH.Development 137:447–455
Baumberger N,Baulcombe D(2005)Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs.Proc Natl Acad Sci USA 102:11928–11933
Bergmann DC(2004)Integrating signals in stomatal development.Curr Opin Plant Biol 7:26–32
Bergmann DC,Sack FD(2007)Stomatal development.Annu Rev Plant Biol 58:163–181
Bhave N,Veley K,Nadeau J,Lucas J,Bhave S,Sack F(2009)TOO MANY MOUTHS promotes cell fate progression in stomatal development of Arabidopsis stems.Planta 229:357–367
Bohmert K,Camus I,Bellini C,Bouchez D,Caboche M,Benning C(1998)AGO1 defines a novel locus of Arabidopsis controlling leaf development.EMBO J 17:170–180
Carrington JC,Ambros V(2003)Role of microRNAs in plant and animal development.Science 301:336–338
Casson SA,Hetherington AM(2010)Environmental regulation of stomatal development.Curr Opin Plant Biol 13:90–95
Geisler M,Yang M,Sack FD(1998)Divergent regulation of stomatal initiation and patterning in organ and suborgan regions of the Arabidopsis mutants too many mouths and four lips.Planta 205:522–530
Gudesblat GE,Schneider-Pizon J,Betti C,Mayerhofer J,Vanhoutte I,van Dongen W,Boeren S,Zhiponova M,de Vries S,Jonak C,Russinova E(2012)SPEECHLESS integrates brassinosteroid and stomata signalling pathways.Nat Cell Biol 14:548–554
Hara K,Kajita R,Torii KU,Bergmann DC,Kakimoto T(2007)The secretory peptide gene EPF1 enforces the stomatal one-cellspacing rule.Genes Dev 21:1720–1725
Hunt L,Gray JE(2009)The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development.Curr Biol 19:864–869
Jover-Gil S,Candela H,Robles P,Aguilera V,Barrero JM,Micol JL,Ponce MR(2012)The microRNA pathway genes AGO1,HEN1 and HYL1 participate in leaf proximal-distal,venation and stomatal patterning in Arabidopsis.Plant Cell Physiol 53:1322–1333
Kanaoka MM,Pillitteri LJ,Fujii H,Yoshida Y,Bogenschutz NL,Takabayashi J,Zhu JK,Torii KU(2008)SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation.Plant Cell 20:1775–1785
Khan M,Rozhon W,Bigeard J,Pflieger D,Husar S,Pitzschke A,Teige M,Jonak C,Hirt H,Poppenberger B(2013)Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein(MAP)kinase kinases,which control stomata development in Arabidopsis thaliana.J Biol Chem 288:7519–7527
Kim TW,Michniewicz M,Bergmann DC,Wang ZY(2012)Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway.Nature 482:419–422
Kutter C,Schob H,Stadler M,Meins F,Jr,Si-Ammour A(2007)MicroRNA-mediated regulation of stomatal development in Arabidopsis.Plant Cell 19:2417–2429
Lai L,Nadeau JA,Lucas J,Lee E.-K,Nakagawa T,Zhao L,Geisler MJ,Sack FD(2005)The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage.Plant Cell 17:2754–2767
Lampard GR,MacAlister CA,Bergmann DC(2008)Arabidopsis stomatal Initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS.Science 322:1113–1116
Lampard GR,Lukowitz W,Ellis BE,Bergmann DC(2009)Novel and expanded roles for MAPK signaling in Arabidopsis stomatal cell fate revealed by cell type-specific manipulations.Plant Cell 21:3506–3517
Lau OS,Bergmann DC(2012)Stomatal development:A plant’s perspective on cell polarity,cell fate transitions and intercellular communication.Development 193:3683–3692
Lee JS,Kuroha T,Hnilova M,Khatayevich D,Kanaoka MM,McAbee JM,Sarikaya M,Tamerler C,Torii KU(2012)Direct interaction of ligandreceptor pairs specifying stomatal patterning.Genes Dev 26:126–136
Li LC,Dahiya R(2002)MethPrimer:designing primers for methylation PCRs.Bioiformatics 18:1427–1431
Lynn K,Fernandez A,Aida M,Sedbrook J,Tasaka M,Masson P,Barton MK(1999)The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene.Development 126:469–481
MacAlister CA,Ohashi-Ito K,Bergmann DC(2007)Transcription factor control of asymmetric cell division that establish the stomatal lineage.Nature 445:537–540
Malamy JE,Benfey PN(1997)Organization and cell differentiation in lateral roots of Arabidopsis thaliana.Development 124:33–44
Miyashima S,Hashimoto T,Nakajima K(2009)ARGONAUTE1 acts in Arabidopsis root radial pattern formation independently of the SHR/SCR pathway.Plant Cell Physiol 50:626–634
Nadeau JA,Sack FD(2002)Control of stomatal distribution on the Arabidopsis leaf surface.Science 296:1697–1700
Ohashi-Ito K,Bergmann DC(2006)Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development.Plant Cell 18:2493–2505
Pillitteri LJ,Sloan DB,Bogenschutz NL,Torii KU(2007)Termination of asymmetric cell division and differentiation of stomata.Nature 445:501–505
Shpak ED(2013)Diverse roles of ERECTA family genes in plant development.J Integr Plant Biol 55:1238–1250
Sugano SS,Shimada T,Imai Y,Okawa K,Tamai A,Mori M,Hara-Nishimura I(2010)Stomagen positively regulates stomatal density in Arabidopsis.Nature 463:241–244
Vaucheret H(2008)Plant ARGONAUTES.Trends Plant Sci 13:350–358
Vaucheret H,Vazquez F,Crete P,Bartel DP(2004)The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development.Genes Dev 18:1187–1197
Yang M,Sack FD(1995)The too many mouths and four lips mutations affect stomatal production in Arabidopsis.Plant Cell 7:2227–2239
SUPPORTING INFORMATION
Additional supporting information can be found in the online version of this article:
Figure S1.Introduction of SPCH:SPCH-GFP induces large stomatal clusters in tmm-1 hypocotyls
(A,B)SPCH:SPCH-GFP gain-of-function phenotype in cotyledons(A)and hypocotyls(B).Brackets denote small cells produced by excessive stomatal asymmetric divisions.(C,D)Large stomatal clusters form in SPCH:SPCH-GFP tmm-1 cotyledons(C)and hypocotyls(D).Bar=20 μm
Figure S2.28Y and BR appear to act independently in regulating hypocotyl stomatal production
(A)Untreated wild-type hypocotyls.(B)BL treatment(1 μM)promotes stomatal production in wild-type hypocotyls.(C)BR-biosynthesis inhibitor BRZ(30 μM)greatly suppresses stomatal production in wild-type hypocotyls.(D)Untreated 28y hypocotyls.(E)Application of BL promotes stomatal production in 28y hypocotyls.(F)Stomatal production in 28y hypocotyls is suppressed by BRZ.Bar=50 μm
Figure S3.mute is epistatic to 28y in stomatal development
(A,B)Loss of MUTE function induces extra asymmetric amplifying divisions of meristemoids as well as meristemoid arrest in cotyledons(A)and hypocotyls(B).(C,D)Meristemoid clusters were found in mute 28y double mutant cotyledons(C)and hypocotyls(D).(E,F)MUTE:MUTE-GFP expression in wildtype and 28y mutant.(G)MUTE transcript levels was significantly increased in 28y mutant.Bar=20 μm
Figure S4.No significant difference of methylation levels between wild-type and 28y mutant
(A)Blue region indicated the predicted a CpG island in SPCH.(B)Methylation level was not significantly different between wildtype and 28y mutant
Table S1.Primer sequences used in this article
 Journal of Integrative Plant Biology2014年6期
Journal of Integrative Plant Biology2014年6期
- Journal of Integrative Plant Biology的其它文章
- A step-by-step protocol for formaldehyde-assisted isolation of regulatory elements from Arabidopsis thaliana
- Polycomb-group histone methyltransferase CLF is required for proper somatic recombination in Arabidopsis
- Rice MtN3/saliva/SWEET gene family:Evolution,expression profiling,and sugar transport
- Molecular characterization and expression analysis of Triticum aestivum squamosa-promoter binding protein-box genes involved in ear development
- BnWRI1 coordinates fatty acid biosynthesis and photosynthesis pathways during oil accumulation in rapeseed
- Genetic analysis of biomass and photosynthetic parameters in wheat grown in different light intensities
