New Insights into Folding Kinetics of α,ω Dye-Functionalized Poly(N-isopropylacrylamide)
Xio-yn Wng,Hi-yn Fn,Xio-dong Ye,b?,Shi-lin Liu,Gung-zho Zhng
a.Hefei National Laboratory for Physical Sciences at the Microscale,Department of Chemical Physics,University of Science and Technology of China,Hefei 230026,China
b.CAS Key Laboratory of Soft Matter Chemistry,University of Science and Technology of China,Hefei 230026,China
c.Faculty of Materials Science and Engineering,South China University of Technology,Guangzhou 510640,China
Two narrowly-distributed poly(N-isopropylacrylamide)(PNIPAM)samples were prepared via atom transfer radical polymerization(ATRP)with a novel dansyl functionalized initiator.The other end of the PNIPAM was functionalized by dabcyl group via click reaction.From the static fluorescence measurements,the fluorescence intensity of dansyl group and energy transfer efficiency between dansyl and dabcyl groups increased when the temperature increased from 36 ?C to 45 ?C,indicating that the microenvironment surrounding dansyl became hydrophobic and the distance between dansyl and dabcyl decreased.The kinetics of the conformational change of the dye-labeled PNIPAM was studied by a home-made laser-induced temperature jump device with fluorescent measurement.Our results revealed that the characteristic transition time was 3.8 and 5.8 ms for PNIPAM with degrees of polymerization of 85 and 142,respectively,indicating that the characteristic transition time was related to the chain length.Besides,characteristic transition time for the change of the energy transfer efficiency was 2.9 ms for PNIPAM with the degree of polymerization of 85,suggesting that the energy transfer efficiency change was faster than the fluorescence intensity change of dansyl group.
Key words: Fluorescence,Laser-induced temperature jump,Phase transition,Poly(N-isopropylacrylamide)
I.INTRODUCTION
Poly(N-isopropylacrylamide)(PNIPAM)can change its conformation from a random coil to a globule when the quality of the solvent switches from good to poor.It undergoes a reversible phase separation at a specific temperature(~32?C),which is named as the lowest critical solution temperature(LCST)[1].PNIPAM has been widely studied by diflerent techniques such as turbidimetry[2–4],laser light scattering[5–9],calorimetry[10–12],fluorescence[13–15],nuclear magnetic resonance[16],and infrared spectroscopy[17–19].Turbidity measurements showed that PNIPAM chains first collapse and then aggregate when the temperature is above the LCST[2,3].The coil-to-globule transition of the individual PNIPAM chains in dilute solutions was observed about two decades ago by Wu et al.using laser light scattering[8,20].Previous studies revealed that the collapse of PNIPAM chains is mainly entropydriven and caused by the hydrophobic interactions between isopropyl groups[3,8,17–19].
Moreover,the kinetics of conformational transition of PNIPAM after the temperature change also attracts much attention[21–27].Some studies[21,23]revealed that there were two stages during the phase transition:the formation of polymer segment“blobs”and the compactification of blobs to form globule.However,others claimed that there were three or four stages during the coil-to-globule transition.For example,Kuznetsov et al.[24]studied the polymer chain by Gaussian selfconsistent method and suggested a three-stage model including the fast nucleation on the polymer chain,the formation of clusters,and the coarsening of clusters.The four stages of the conformation transition were reported as pearling,bridge stretching,collapse of the pearl necklace,and the equilibrium collapsed configuration by Halperin and Goldbart[26].
Many experimental eflorts on the kinetics of phase transition of PNIPAM have been made since 1990s.For example,Xu et al.[28]studied the phase transition of pyrene-labeled PNIPAM in a mixture of water and methanol using stopped-flow fluorescence spectroscopy and concluded that there were two stages with characteristic time of 12 and 270 ms.But due to the dead time of a stopped flow apparatus,the early stage of the collapse less than millisecond can not be observed.Zhang et al.[29]used laser induced temperature jump device combined with time-resolved mid-infrared absorbance diflerence spectroscopy to observe the collapse of PNIPAM chains in deuterium oxide(D2O),and found that there were three stages in the conformational transition process,which were the formation of nuclei,the growth of nuclei into clusters,and the compulsively inverted transition process induced by the thermal relaxation process,with characteristic transition times of~1μs,~80μs,and 3 ms,respectively.Tsuboi et al.[30,31]studied the phase transition of PNIPAM by both transmittance and fluorescence measurement using laser induced temperature jump.They found that the phase transition completed within tens of milliseconds and the characteristic transition time observed from the fluorescence measurement was~35μs.Yushmanov et al.[32]studied the coil to globule collapse and intermolecular aggregation by temperature jump1H NMR spectroscopy and found that the collapse time of PNIPAM was shorter than one second.In our previous studies we used water soluble 8-anilino-1-naphthalensulfonic acid ammonium salt(ANS)or labelled dansyl group as fluorescent probe to investigate the collapse kinetics by laser-induced temperature jump and revealed that there were two stages in the conformational transition process,and the characteristic times of these two stages were~0.1 and~0.8 ms.However,the dyes in the PNIPAM chains are usually randomly labeled so that the position of the dyes is not well-defined[33–35].
There is another method to study the conformational transition of polymers in which the fluorescent probe is attached to the end of polymers.In this method,the position of fluorescent probe is well-defined.Furthermore,fluorescence resonance energy transfer(FRET)is used to obtain more information during the conformational transition when a pair of fluorescent probes including one donor and one acceptor is introduced to each end of one polymer chain.The energy transfer efficiency(E)from the donor to the acceptor is very sensitive to the distance between the donor and the acceptor at the ends of polymer chains[36],so FRET is a powerful tool to characterize the conformation of macromolecules and is used as spectroscopic ruler to describe the conformational change of polymer chains[37–40]and biomacromolecules[41–44].For example,Liu et al.[40]synthesized poly(methacrylic acid)(PMAA)with two diflerent fluorescent dye end groups using anionic polymerization and the grafting ratios of the end groups were relatively high.However,the conditions for anionic polymerization were very harsh and this method was only limited to a small range of monomers.Recently,Roth et al.[45]obtained the α,ω dye-functionalized poly(diethylene glycol methacrylate)using reversible addition-fragmentation chain transfer polymerization(RAFT),and the grafting ratios of the two dyes were 71%and 47%.
In this work,we used dansyl as the donor and 4-(dimethylaminoazo)benzene-4-carboxylic acid(dabcyl)as the acceptor to study the thermodynamics and kinetics of conformational transition of PNIPAM with welldefined positions of dyes.The PNIPAM was synthesized by atom transfer radical polymerization(ATRP)with a novel initiator containing a dansyl group.The dansyl group was linked to the initiator by a sulfonamide group and was stable during the following reactions.The dabcyl group as the acceptor in the FRET pair was linked to the other end of PNIPAM via click chemistry.
II.EXPERIMENTS
A.Materials
Tetrahydrofuran(THF,Sinopharm)was pretreated with potassium hydroxide(KOH)and then distilled after being refluxed over metal sodium.Dichloromethane(DCM,Sinopharm),triethylamine(TEA,Sinopharm),and isopropanol(Sinopharm)were distilled over CaH2prior to use.N,N-dimethylformamide(DMF,Sinopharm)was first dried with anhydrous magnesium sulfate and then distilled under a reduced pressure.Tris[2-(dimethylamino)ethyl]amine(Me6TREN)was synthesized following a procedure described in Ref.[46].Copper(I)bromide(CuBr,Alfa Aesar,98%)and copper(I)chloride(CuCl,Alfa Aesar,97%)were washed with glacial acetic acid to remove soluble oxidized species,filtrated,washed with ethanol,and dried undervacuum. MonomerN-isopropylacrylamide(NIPAM, Eastman Kodak) was recrystallized three times from a mixture of toluene/n-hexane.N,N,N′,N′,N′′-pentamethyldiethylenetriamine(PMDETA,Sigma,99%),2-chloropropionyl chloride(Aladdin,97%),propargylbromide(Aladdin,98%),triethylene glycol(Sinopharm),4-dimethylaminoazo benzene-4-carboxylic acid(dabcyl,Fanbo biochemical,95%),sodium hydride(NaH,Sigma,99%),sodium azide(NaN3,Sigma,99%),N,N-dimethyl-4-aminopyridine(DMAP,Aladdin,99%),and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride(EDC·HCl,Aladdin,98%)were used as received.
B.Characterization

Scheme 1 Synthesis of PNIPAM with dansyl and dabcyl groups as two functional groups.
The sedimentation experiments were performed on a Proteomelab XL-A/I analytical ultracentrifuge(Beckman Coulter Instruments)equipped with a UV-Vis absorbance optics.Each experiment was conducted at 20.0?C with a rotational speed of 56000 r/min at a wavelength of 250 nm.The data were analysed with the software SEDFIT[47–50].The UV-Vis spectra andfluorescent spectra were performed on a Unico UV/Vis 2802pcs spectrophotometer and a Hitachi F4600 fluorescence spectrophotometer,respectively.The1H nuclear magnetic spectra(1H NMR)were recorded on Bruker DMX-400 spectrometer using deuterated chloroform(CDCl3)as solvent and tetramethylsilane(TMS)as an internal standard.The gel permeation chromatography(GPC)was performed on a Waters 1515 apparatus equipped with three Waters styragel columns(HR2,HR4,HR6)and a refractive index detector(RI,Wyatt WREX-02,using a conventional calibration with linear polystyrene standards),and with THF as an eluent.The infrared(IR)spectra were performed on a NICOLET 8700 Fourier transform infrared spectrometer.The details about the laser-induced temperature jump instrument were reported elsewhere[3,31,33,51–55].Briefly,a pulsed 1.54-μm infrared laser beam was used for the temperature jump experiments.A 200-W high-pressure mercury lamp(Shanghai Hualun Bulk Factor)with a transmitting filter(245?400 nm)was used as the source for excitation of dansyl group.Thefluorescence was collected with a photomultiplier tube(Hamamatsu R928)and recorded on a Tektronix oscilloscope(TDS 3054B)[33–35,56].The synthesic process of PNIPAM with dansyl and dabcyl groups as two functional groups is shown in Scheme 1.
C.Sample preparation
1.Synthesis of the initiator dansyl-Cl(b)
In a 100 mL three-neckflask, compound a was prepared from dansylchloride, and 2,2′-(ethylenedioxy)bis(ethylamine)[35](1 g,2.62 mmol)was dissolved in 40 mL THF,and KOH(0.7 g,12.5 mmol)was added.The solution was kept in a bath of ice and water under the protection of N2flow.2-Chloropropionyl chloride(0.4 g,3.17 mmol)dissolved in 15 mL THF was added dropwise. After stirring overnight at room temperature,the yellow suspension was centrifuged to remove the salt and then the solution was evaporated.The raw product was dissolved in DCM and washed with dilute hydrochloric acid solu-tion,water,saturated sodium bicarbonate solution and water,successively.The organic part was dried with anhydrous sodium sulfate overnight,and then concentrated and subjected to silica gel column chromatography(ethyl acetate:methanol=30:1)to give a high purity product(b).1H NMR(400 MHz,CDCl3):δ/ppm 8.54(d,1H),8.30(d,1H),8.23(dd,1H),7.57?7.50(m,2H),7.18(d,1H),7.02(s,1H),5.57(t,1H),4.38(m,1H),3.55?3.39(m,10H),3.09(m,2H),2.89(s,6H),1.68(d,3H).
2.Synthesis of dabcyl-alkyne(e)
Compound c containing one alkyne group and one hydroxyl group was first prepared.NaH(6 g),THF(80 mL)and triethylene glycol(19.23 mL,0.144 mol)were added into a one-neck flask and stirred for 30 min.Then propargyl bromide(7.5 mL,0.096 mol)was added dropwise over a period of 1.5 h under stirring.The reaction was quenched by water after 11 h.The final product was obtained after purification by silica gel column chromatography.1H NMR(400 MHz,CDCl3)of compound c:δ/ppm 4.20(d,1H),3.73?3.59(m,12H),2.42(t,1H),2.40(s,1H).
Compound e was synthesized by an esterification reaction.Dabcyl(d)(0.7955 g,2.96 mmol),compound c(0.7384 g,3.93 mmol),DMAP(86.9 mg,0.71 mmol)and DCM(80 mL)were added to a 150 mL threeneck flask.The reaction system was stirred under N2protection in an ice-water bath. EDC·HCl(0.72 g,3.75 mmol)in DCM(20 mL)was added dropwise.After the addition of EDC·HCl,the mixture was kept at room temperature and stirred overnight.The desired product was obtained after purification by silica gel column chromatography.1H NMR(400 MHz,CDCl3)of compound e:δ/ppm 8.17?8.15(m,2H),7.92?7.85(m,4H),6.77?6.75(m,2H),4.50(t,2H),4.20(d,2H),3.86(t,2H),3.75?3.68(m,8H),3.11(s,6H),2.42(t,1H).
3.Synthesis of dansyl-PNIPAM-dabcyl
A mixture containing NIPAM(2.53 g,0.0224 mol),the initiator b(40.7 mg,8.64×10?5mol),Me6TREN(0.056 g,2.42×10?4mol)and isopropanol(15 mL)was added to a sealing tube,and degassed through three freeze-pump-thaw cycles. The CuCl(16 mg,1.6×10?4mol)was added when the mixture was frozen and then the tube was sealed under vacuum. The reaction was carried out for 11 h at 0?C.The copper catalyst was removed with a neutral Al2O3column and the solution was precipitated into ether.Then dansyl-PNIPAM-Cl(0.61 g)was obtained with a 24%yield.Dansyl-PNIPAM-Cl(0.3 g,3.1×10?5mol),NaN3(0.04 g,6.2×10?4mol)and DMF(5 mL)were added to a 10 mL one-neck flask. The mixture was stirred at 45?C for 48 h. DMF was evaporated under a reduced pressure. DCM was added,thorough stirred,centrifuged to remove the excess NaN3and then precipitated into ether.Then dansyl-PNIPAM-N3was obtained.Compound e(184 mg,4.19×10?4mol),PMDETA(200 μL,9.62×10?4mol)and DMF(25 mL)were added to a 100 mL threeneck flask,bubbled with nitrogen for 1 h and then CuBr(59.1 mg,4.10×10?4mol)was added.Dansyl-PNIPAM-N3(81.4 mg,8.39×10?6mol)dissolved in DMF(2.5 mL)was added by a single channel syringe pump in 5 h at a temperature of 60?C,and the reaction system was further carried out for 20 h.The copper catalyst was removed with a neutral Al2O3column and the solvent was evaporated under a reduced pressure.The raw product was dissolved in a small amount THF and then precipitated into ether.The precipitation was repeated several times to remove the free dabcyl.Then dansyl-PNIPAM-dabcyl(16 mg)was obtained with a 20%yield.
III.RESULTS AND DISCUSSION
In this study,we used dansyl group as a fluorescent probe to investigate the change of the microenvironment of PNIPAM chain in aqueous solutions.Dabcyl and dansyl groups were used as a fluorescent pair in FRET methods,and the distance between the donor and the acceptor can be estimated from the efficiency of energy transfer[39,57–59].Here,a dabcyl group was covalently connected to the other end of PNIPAM to study the thermodynamics and kinetics of the change of the end-to-end distance of PNIPAM chains which is temperature dependent.Since dabcyl group has no fluorescent emission,we can obtain the information of energy transfer efficiency using the change of the fluorescent intensity of the donor dansyl group itself.On the other hand,it is well known that the solubility of polymer in aqueous solutions decreases when the hydrophobic chromophores are covalently attached to the polymers[60].In order to reduce the interference of chromophores on polymer solubility,the content of chromophores should be as low as possible.For example,the content of dansyl is 0.06%in the study of PNIPAM labelled with dansyl by Asano et al.[61].Our previous work also shows that the molar ratio of PNIPAM unit to dansyl group should be more than 370 to avoid the labeling eflect of the dansyl group[35].Therefore,we inserted a hydrophilic segment between the PNIPAM chain and the dansyl group to decrease the eflect of the hydrophobic dansyl group on the solubility of PNIPAM chains and avoid the aggregation of PNIPAM chains,as shown in Scheme 1.Moreover,according to the power law relation between the radius of gyration(Rg)and weight average molar mass(Mw)of PNIPAM(Rg=0.0224×Mw0.54nm)reported by Kubata et al.[62],energy transfer efficiency is not sensitive to the conformational change of PNIPAM when the degree of polymerization of PNIPAM is larger than 200since the F¨orster radius between dansyl and dabcyl is~3 nm,so we synthesized two PNIPAM samples with degree of polymerization less than 200[57,59].

TABLE I Characterization of fluorescent-labeled PNIPAM samples.

FIG.1 GPC curves of dansyl-PNIPAM85-Cl and dansyl-PNIPAM142-Cl monitored by a RI detector.
Dansyl-PNIPAM-ClwassynthesizedbyATRP method using an initiator(compound b)containing a dansyl group with a hydrophilic segment,as shown in Scheme 1.PNIPAM with one dansyl group at the end of the polymer(dansyl-PNIPAM-Cl)was used as the reference compound.Further azidation substitution reaction of dansyl-PNIPAM-Cl by sodium azide in DMF led to dansyl-PNIPAM-N3. Dabcyl group was introduced to the end of PNIPAM by click reaction using the compound e containing both an alkyne group and a dabcyl group.The characterization results of two series of PNIPAM samples with diflerent molecular weights are summarized in Table I.The molecular weights of dansyl-PNIPAM85-Cl and dansyl-PNIPAM142-Cl were measured by GPC(FIG.1).The results show that the number-average molecular weights are 8200 and 13800 g/mol and both samples are narrowly distributed,as shown in FIG.1.The subscripts 85 and 142 denote the degree of polymerization and were calculated by the results of NMR,which will be discussed later.

FIG.2 IR spectra of dansyl-PNIPAM142-N3and dansyl-PNIPAM142-dabcyl.
FIG.2 shows the IR spectra of PNIPAM with a repeating unit of 142 before and after click reaction.The peak of azide at the wavenumber of~2030 cm?1disappeared after click reaction,indicating the high reaction efficiency of click chemistry.The existence of dabcyl groups at the end of PNIPAM was confirmed by1H NMR,as shown in FIG.3.FIG.3 shows that the characteristic peaks located at 7.4?8.6 ppm(a)and~4.0 ppm(peak c)are attributed to the protons of dansyl group and-CH?(CH3)2.Therefore,degree of polymerization of dansyl-PNIPAM142-Cl can be calculated according to the area ratio of these peaks.The calculated Mnwas 16100 g/mol,which was close to the value measured by GPC.The functionality of dabcyl group of dansyl-PNIPAM142-dabcyl is~81%calculated from the area ratio of the peak h to the peak c.The calculated Mnof shorter PNIPAM was 9600 g/mol and the functionality of dabcyl group was~80%.We also measured the UV/Vis absorbance spectra of dansyl-PNIPAM85-dabcyl and dansyl-PNIPAM142-dabcyl in aqueous solutions to determine the functionality of dabcyl group,as shown in FIG.4.Because the absorbance at 472 nm is only from the absorbance of dabcyl group(see FIG.S1 in supplementary materials),using the absorbance of dabcyl in water at 472 nm the functionality of dabcyl group was also determined as 90%and 73%for dansyl-PNIPAM85-dabcyl and dansyl-PNIPAM142-dabcyl,respectively[63].It is clear that the results calculated from UV/Vis spectra are slightly diflerent from the value measured from1H NMR spectra,presumably due to a relatively larger error of the integration of the dabcyl peak during the calculation.So the value of the functionalities of dabcyl group calculated from the UVVis absorbance spectra was used in the following discussion.
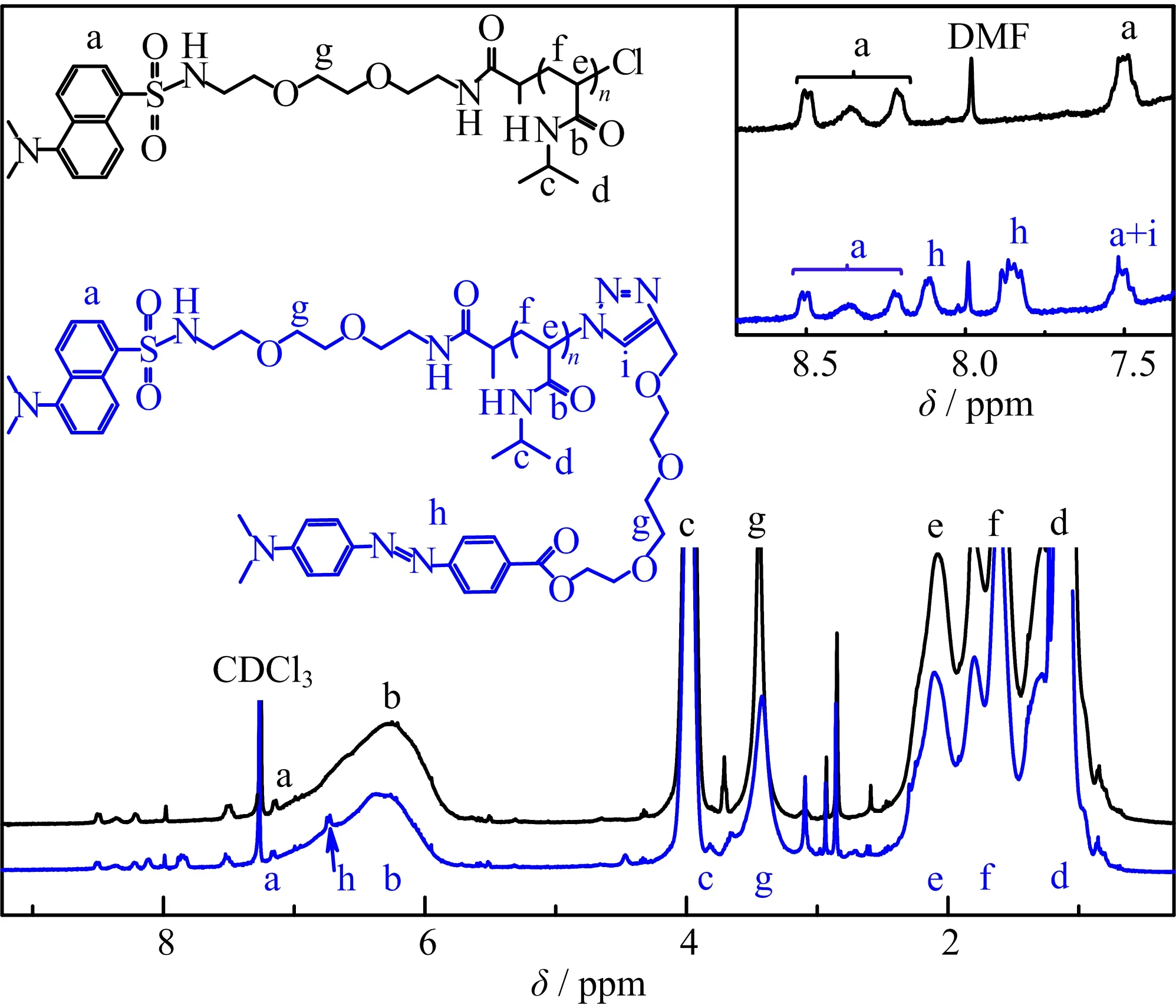
FIG.31H NMR spectra of dansyl-PNIPAM142-Cl(black solid line)and dansyl-PNIPAM142-dabcyl(blue solid line)with CDCl3as the solvent.

FIG.4 The UV-Vis spectra of dansyl-PNIPAM85-dabcyl and dansyl-PNIPAM142-dabcyl in aqueous solutions at room temperature,where the concentration of each sample was 0.25 mg/mL.
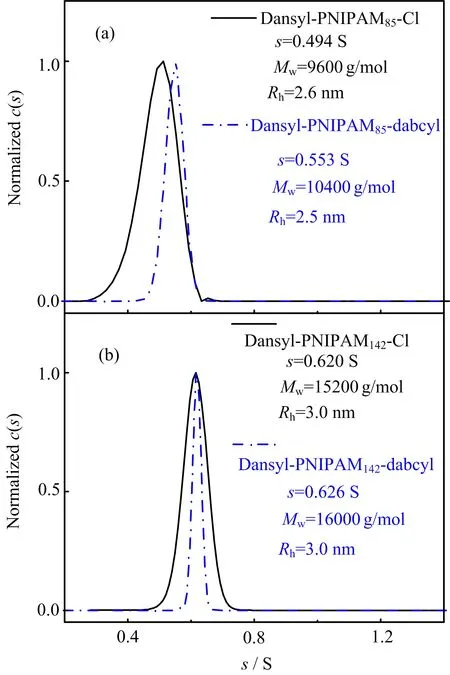
FIG.5 Normalized sedimentation coefficient(s)distributions of(a)dansyl-PNIPAM85-Cl and dansyl-PNIPAM85-dabcyl,(b)dansyl-PNIPAM142-Cl and dansyl-PNIPAM142-dabcyl in water,where the concentration of each sample was 0.25 mg/mL.
The sedimentation coefficient distributions of these samples were measured by analytical ultracentrifuge(AUC),as shown in FIG.5. The sedimentation coefficients are narrowly distributed,indicating that there are no aggregates in the aqueous solutions when the temperature was 20?C.The measured molecular weights of dansyl-PNIPAM85-Cl and dansyl-PNIPAM142-Cl using AUC are 9600 and 15200 g/mol,which are close to the results measured by GPC.Moreover,the sedimentation coefficient increases from 0.494 S to 0.553 S and from 0.620 S to 0.626 S for dansyl-PNIPAM85-Cl and dansyl-PNIPAM142-Cl,indicating the successful attachment of the dabcyl group.FIG.5 also shows the distribution of sedimentation coefficient becomes narrower after the attachment of the dabcyl group,presumably because PNIPAM chain slightly changes its conformation due to the hydrophobicity of dabcyl group[49].
We measured the transmittance of dansyl-PNIPAM85-Clanddansyl-PNIPAM85-dabcyl(FIG.6(a))during a heating process,and found that the transmittance remains unchanged at the temperature lower than 35?C,and then decreases sharply at temperature above 35?C due to the dehydration and aggregation of PNIPAM chains.Moreover,there was no diflerence between the curves of dansyl-PNIPAM85-Cland dansyl-PNIPAM85-dabcyl,indicatingthat the introduction of dabcyl group did not change the properties of PNIPAM chains.A similar phenomenon can be found from the curves of dansyl-PNIPAM142-Cl and dansyl-PNIPAM142-dabcyl.

FIG.6 Temperature dependences oftransmittance at650nmfor(a)dansyl-PNIPAM85-Clanddansyl-PNIPAM85-dabcyland(b)dansyl-PNIPAM142-Cland dansyl-PNIPAM142-dabcyl,where the concentration of each sample was 0.25 mg/mL.
The fluorescence emission spectra of dansyl group ofdansyl-PNIPAM85-Cl,dansyl-PNIPAM85-dabcyl,dansyl-PNIPAM142-Cl,and dansyl-PNIPAM142-dabcyl measured at a temperature range from 32?C to 46?C,are shown in FIG.7 and FIG.8.At temperatures lower than 36?C,there was no significant change in the emission intensity of dansyl-PNIPAM85-Cl,suggesting that the microenvironment surrounding the dansyl group is hydrophilic and remains unchanged below 36?C.The emission intensity of dandyl-PNIPAM85-Cl increased with the increasing of the temperature,and a blue shift of the emission spectra was observed as well,indicating that the microenvironment surrounding the dansyl group became hydrophobic when the temperature is above 36?C due to the dehydration and aggregation of the PNIPAM chains.The fluorescence emission intensity of dansyl-PNIPAM85-dabcyl is lower than that of dansyl-PNIPAM85-Cl at 32?C due to the occurrence of energy transfer from dansyl group to dabcyl group.The emission intensity of dansyl-PNIPAM85-dabcyl decreases slightly when the temperature increases from 32?C to 39?C and then it increases when the temperature is above 39?C,indicating the opposing effects of energy transfer from dansyl to dabcyl and the hydrophobic microenvironment of dansyl,i.e.energy transfer eflect dominates when T is below 39?C and hydrophobic microenvironmental eflect dominates at higher temperature.

FIG.7 Temperature dependence of the fluorescence emission spectra of(a)dansyl-PNIPAM85-Cl and(b)dansyl-PNIPAM85-dabcyl in aqueous solutions,where the concentration of each sample was 0.25 mg/mL and λex=325 nm.

FIG.8 Temperature dependence of the fluorescence emission spectra of(a)dansyl-PNIPAM142-Cl and(b)dansyl-PNIPAM142-dabcyl in aqueous solutions,where the concentration of each sample was 0.25 mg/mL and λex=325 nm.

FIG.9 Temperature dependence of FRET efficiency between dansyl and dabcyl on(a)dansyl-PNIPAM85-dabcyl and(b)dansyl-PNIPAM142-dabcyl in water,where the concentration of each sample was 0.25 mg/mL.The FRET effi ciency was calculated by(I1?I2)/(f·I1),where I1and I2 were the fluorescence intensity of dansyl-PNIPAM-Cl and dansyl-PNIPAM-dabcyl,and f represents the functionality of dabcyl group.
In the heating process,the change in emission intensity of dansyl-PNIPAM142-Cl is similar to that of dansyl-PNIPAM85-Cl,and the emission spectra also show a blue shift. The fluorescence emission intensity of dansyl-PNIPAM142-dabcyl is the same as that of dansyl-PNIPAM142-Cl at 32?C,revealing that there is no energy transfer between dansyl and dabcyl because of the higher size of PNIPAM with longer chain length.The emission intensity of dansyl-PNIPAM142-dabcyl is nearly a constant at temperature below 36?C and starts to increase when the temperature is above 36?C,which is also similar to the emission spectra of dansyl-PNIPAM142-Cl.The increase in fluorescence emission intensity of dansyl-PNIPAM142-dabcyl is less than that of dansyl-PNIPAM142-Cl,due to the increase in the FRET efficiency.
The energy transfer efficiency at each temperature was calculated by(I1?I2)/(f·I1),where I1and I2are the fluorescence intensity of dansyl-PNIPAM-Cl and dansyl-PNIPAM-dabcyl,and f represents the functionality of dabcyl groups,the results are plotted in FIG.9[39].The energy transfer efficiency of dansyl-PNIPAM85-dabcyl increases continuously from the beginning of the heating process,and that of dansyl-PNIPAM142-dabcyl starts to increase from 35?C.The increase in the FRET efficiency between dansyl and dabcyl is caused by the decrease of distance between the two dyes and the increase of F¨orster radius(R0)between dansyl and dabcyl which depends on the quantum yield of dansyl group.
The kinetics of the conformational change of PNIPAM was studied by a home-made laser-induced temperature jump device combined with fluorescent measurement[33–35]. The time dependence of fluorescence intensity of dansyl-PNIPAM85-Cl and dansyl-PNIPAM142-Cl and the FRET efficiency between dansyl and dabcyl after temperature jump are shown in FIG.10.The result shows that both the intensity of dansyl and the energy transfer efficiency of PNIPAM with shorter chain length reach a plateau more quickly,i.e.the PNIPAM sample with a polymerization degree of 85 levels o ffat~18 ms and the longer PNIPAM sample levels o ffat~26 ms,indicating the chain length dependence of the conformational change of PNIPAM chain.
All the curves in FIG.10 can be well fitted with a single-exponential function,and the characteristic transition time(τ)is(3.8±0.1)ms and(5.8±0.2)ms for the change of fluorescence intensity of dansyl on dansyl-PNIPAM85-Cl and dansyl-PNIPAM142-Cl,respectively,indicating that the collapse of longer PNIPAM chain is slower,presumably due to the fact that the collapse of the longer PNIPAM chain contains two stages including the formation of“pearls” and the coarsening of the pearls while the collapse of the shorter PNIPAM only contains the formation of “pearls”. Moreover,for the dansyl-PNIPAM85-Cl and dansyl-PNIPAM85-dabcyl samples,the change of the energy transfer(τ85,ET=(2.9±0.1)ms)is faster than the change of fluorescence intensity(τ85,dansyl=(3.8±0.1)ms)of dansyl group,indicating that the collapse of the PNIPAM85probably due to that the formation of “pearl” is earlier than the microenvironmental change surrounding the dansyl group. According to our previous studies[34,35],there are two stages in the folding process of PNIPAM with molecular weight higher than 1×106g/mol,that is,the formation of pearls and the coarsening.The characteristic time of the formation of pearls is~0.1 ms and remains unchanged when the chain length increases,while that of the coarsening process increases when the degree of polymerization of PNIPAM increases.The characteristic time measured in this study is longer than that of the coarsening of the“pearls”for PNIPAM chains with molecular weight higher than 1×106g/mol in our previous studies(τ=0.5 ms using covalently attached dansyl fluorophores and τ=0.8 ms using free ANS probes),presumably due to the diflerence of both the molecular weight and the position of the dyes in the polymers.Furthermore,FIG.10 also shows that the characteristic time of energy transfer of dansyl-PNIPAM142-dabcyl is τ142,ET=(5.7±0.2)ms,which is close to that of the change of fluorescence intensity of dansyl groups(τ142,dansyl=(5.8±0.2)ms),indicating the concurrence of these two changes.FIG.11 shows the schematic diagram of conformational transition of PNIPAM during heating process.

FIG.10 Time dependence of temperature jump-induced change of fluorescence intensity of(a)dansyl-PNIPAM85-Cl(black solid line)and(b)dansyl-PNIPAM142-Cl(black solid line),and the FRET efficiency between dansyl and dabcyl on(a)dansyl-PNIPAM85-dabcyl(blue solid line)and(b)dansyl-PNIPAM142-dabcyl(blue solid line).The yellow solid lines represent single exponential fitting curves.The concentrations of the samples were 0.25 mg/mL,and the initial temperature was~35?C for all samples.

FIG.11 Schematic diagram of conformational transition of PNIPAM during heating process.
IV.CONCLUSION
Two series of poly(N-isopropylacrylamide)(PNIPAM)samples with dansyl and dabcyl fluorescent dyes at two ends were synthesized using atom transfer radical polymerization. A novel initiator with dansyl group as the donor provided PNIPAM with an αend dansyl group.The functionality of dansyl group was~100%. While the acceptor dabcyl group on the ω-end of PNIPAM was introduced via click reaction. The functionality of dabcyl group was determined by the UV-Vis absorption spectroscopy(>70%)and1H NMR spectroscopy.The fluorescence intensity change of dansyl group and energy transfer efficiency between dansyl and dabcyl groups at diflerent temperatures were investigated using static fluorescence measurements.The energy transfer efficiency and thefluorescence intensity of dansyl increased in the heating process. When the temperature increased from 32?C to 35?C,the energy transfer efficiency of dansyl and dabcyl on dansyl-PNIPAM85-dabcyl increased,while that of dansyl-PNIPAM142-dabcyl remained unchanged.Then a home-made laser induced temperature jump instrument combined with fluorescence measurement was used to study the kinetics of the folding process of PNIPAM chains.The dehydration of the end group of PNIPAM chain and the change of end-to-end distance were investigated at the same time.The result shows that the characteristic time of fluorescence intensity of dansyl is 3.8 ms for dansyl-PNIPAM85-Cl and 5.8 ms for dansyl-PNIPAM142-Cl,indicating that the characteristic time is related to the molar mass of PNIPAM,while the characteristic time of energy transfer effi ciency on dansyl-PNIPAM85-dabcyl is 2.9 ms which is less than that of fluorescence intensity of dansyl group,illustrating that the distance between dansyl and dabcyl changes faster than the change of the microenvironment of dansyl group.
Supplementary materials:The UV-Vis absorbance spectra of compound a,dansyl-PNIPAM142-Cl and dansyl-PNIPAM142-dabcyl are shown in FIG.S1.
V.ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China(No.21674107 and No.21274140)and the Fundamental Research Funds for the Central Universities(No.WK2340000066).
[1]H.G.Schild,Prog.Polym.Sci.17,163(1992).
[2]S.Fujishige,K.Kubota,and I.Ando,J.Phys.Chem.93,3311(1989).
[3]M.G.Heskins and J.E.Guillet,J.Macromol.Sci.A 2,1441(1968).
[4]O.Sedl′a?cek,P.?Cernoch,J.Ku?cka,R.Konefal,P.?St?ep′anek,M.Vetr′?k,T.P.Lodge,and M.Hrub′y,Langmuir 32,6115(2016).
[5]C.Wu and S.Q.Zhou,Phys.Rev.Lett.77,3053(1996).
[6]C.Wu and S.Q.Zhou,Macromolecules 28,8381(1995).
[7]C.Wu and S.Q.Zhou,Macromolecules 28,5388(1995).
[8]X.H.Wang,X.P.Qiu,and C.Wu,Macromolecules 31,2972(1998).
[9]K.Kubota,S.Fujishige,and I.Ando,J.Phys.Chem.94,5154(1990).
[10]Y.W.Ding and G.Z.Zhang,Macromolecules 39,9654(2006).
[11]H.Inomata,S.Goto,K.Otake,and S.Saito,Langmuir 8,687(1992).
[12]H.G.Schild and D.A.Tirrell,J.Phys.Chem.94,4352(1990).
[13]F.M.Winnik,Polymer 31,2125(1990).
[14]R.Walter,J.Ri?cka,C.Quellet,R.Nyflenegger,and T.Binkert,Macromolecules 29,4019(1996).
[15]F.M.Winnik,Macromolecules 23,233(1990).
[16]H.Ohta,I.Ando,S.Fujishige,and K.Kubota,J.Polym.Sci.Part B 29,963(1991).
[17]Y.Maeda,T.Nakamura,and I.Ikeda,Macromolecules 34,1391(2001).
[18]A.Percot,X.X.Zhu,and M.Lafleur,J.Polym.Sci.Part B 38,907(2000).
[19]Y.Maeda,T.Higuchi,and I.Ikeda,Langmuir 16,7503(2000).
[20]X.D.Ye,K.J.Zhou,and C.Wu,Acta Polym.Sin.1389(2017).
[21]P.G.De Gennes,J.Physique Lett.46,639(1985).
[22]A.Est`eve,A.Bail,G.Landa,A.Dkhissi,M.Brut,M.D.Rouhani,J.Sudor,and A.M.Gue,Chem.Phys.340,12(2007).
[23]A.Y.Grosberg,S.K.Nechaev,and E.I.Shakhnovich,J.Phys.France 49,2095(1988).
[24]Y.A.Kuznetsov,E.G.Timoshenko,and K.A.Dawson,J.Chem.Phys.104,3338(1996).
[25]N.Kikuchi,J.F.Ryder,C.M.Pooley,and J.M.Yeomans,Phys.Rev.E 71,061804(2005).
[26]A.Halperin and P.M.Goldbart,Phys.Rev.E 61,565(2000).
[27]A.Byrne,P.Kiernan,D.Green,and K.A.Dawson,J.Chem.Phys.102,573(1995).
[28]J.Xu,Z.Y.Zhu,S.Z.Luo,C.Wu,and S.Y.Liu,Phys.Rev.Lett.96,027802(2006).
[29]S.H.Zhang,Y.Chen,H.Li,and Y.X.Weng,Chin.J.Chem.Phys.22,447(2009).
[30]Y.Tsuboi,Y.Yoshida,K.Okada,and N.Kitamura,J.Phys.Chem.B 112,2562(2008).
[31]Y.Tsuboi,Y.Yoshida,N.Kitamura,and K.Iwai,Chem.Phys.Lett.468,42(2009).
[32]P.V.Yushmanov,I.Fur′o,and I.Iliopoulos,Macromol.Chem.Phys.207,1972(2006).
[33]X.D.Ye,Y.J.Lu,S.L.Liu,G.Z.Zhang,and C.Wu,Langmuir 23,10366(2007).
[34]X.D.Ye,Y.J.Lu,L.Shen,Y.W.Ding,S.L.Liu,G.Z.Zhang,and C.Wu,Macromolecules 40,4750(2007).
[35]C.L.Li,X.D.Ye,Y.W.Ding,and S.L.Liu,Chin.J.Chem.Phys.25,389(2012).
[36]E.Haas,M.Wilchek,E.Katchalski-Katzir,and I.Z.Steinberg,Proc.Nat.Acad.Sci.USA 72,1807(1975).
[37]L.Stryer and R.P.Haugland,Proc.Nat.Acad.Sci.USA 58,719(1967).
[38]L.Stryer,Annu.Rev.Biochem.47,819(1978).
[39]G.J.Liu and J.E.Guillet,Macromolecules 23,1388(1990).
[40]G.J.Liu,J.E.Guillet,E.T.B.Al-Takrity,A.D.Jenkins,and D.R.M.Walton,Macromolecules 24,68(1991).
[41]L.E.Morrison and L.M.Stols,Biochemistry 32,3095(1993).
[42]S.S.Ghosh,P.S.Eis,K.Blumeyer,K.Fearon,and D.P.Millar,Nucleic.Acids Res.22,3155(1994).
[43]K.Cai and V.Schirch,J.Biol.Chem.271,27311(1996).
[44]N.Ota,K.Hirano,M.Warashina,A.Andrus,B.Mullah,K.Hatanaka,and K.Taira,Nucleic.Acids Res.26,735(1998).
[45]P.J.Roth,M.Haase,T.Basch′e,P.Theato,and R.Zentel,Macromolecules 43,895(2010).
[46]J.Queflelec,S.G.Gaynor,and K.Matyjaszewski,Macromolecules 33,8629(2000).
[47]P.Schuck,Biophys.J.78,1606(2000).
[48]P.Schuck,M.A.Perugini,N.R.Gonzales,G.J.Howlett,and D.Schubert,Biophys.J.82,1096(2002).
[49]X.Y.Wang,X.D.Ye,and G.Z.Zhang,Soft Matter 11,5381(2015).
[50]Y.T.Gao,S.Wu,and X.D.Ye,Soft Matter 12,5959(2016).
[51]D.H.Turner,G.W.Flynn,N.Sutin,and J.V.Beitz,J.Am.Chem.Soc.94,1554(1972).
[52]D.H.Turner,G.W.Flynn,S.K.Lundberg,L.D.Faller,and N.Sutin,Nature 239,215(1972).
[53]S.Ameen,Rev.Sci.Instrum 46,1209(1975).
[54]A.Kazzaz,S.Ruschin,I.Shoshan,and G.Ravnitsky,IEEE J.Quantum Elect.30,3017(1994).
[55]A.P.Williams,C.E.Longfellow,S.M.Freier,R.Kierzek,and D.H.Turner,Biochemistry 28,4283(1989).
[56]Y.J.Lu,X.D.Ye,J.F.Li,C.L.Li,and S.L.Liu,J.Phys.Chem.B 115,12001(2011).
[57]B.D.Adair and D.M.Engelman,Biochemistry 33,5539(1994).
[58]M.Miki and C.G.dos Remedios,Biochem.Int.22,125(1990).
[59]X.Wang,M.L.De Vocht,J.De Jonge,B.Poolman,and G.T.Robillard,Protein Sci.11,1172(2002).
[60]H.Menzel,R.Kr¨oger,and M.L.Hallensleben,J.Macromol.Sci.Part A 32,779(1995).
[61]M.Asano,F.M.Winnik,T.Yamashita,and K.Horie,Macromolecules 28,5861(1995).
[62]K.Kubota,S.Fujishige,and I.Ando,Polym.J.22,15(1990).
[63]C.C.Lee,C.J.Kuo,T.P.Ko,M.F.Hsu,Y.C.Tsui,S.C.Chang,S.Yang,S.J.Chen,H.C.Chen,M.C.Hsu,S.R.Shih,P.H.Liang,and A.H.J.Wang,J.Biol.Chem.284,7646(2009).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Imaging HNCO Photodissociation at 201 nm:State-to-State Correlations between CO(X1Σ+)and NH(a1?)
- Energy-Transfer Processes of Xe(6p[1/2]0,6p[3/2]2,and 6p[5/2]2)Atoms under the Condition of Ultrahigh Pumped Power
- Ultrafast Investigation of Excited-State Dynamics in Trans-4-methoxyazobenzene Studied by Femtosecond Transient Absorption Spectroscopy
- Strong Current-Polarization and Negative Diflerential Resistance in FeN3-Embedded Armchair Graphene Nanoribbons
- Unexpected Chemistry from the Homogeneous Thermal Decomposition of Acetylene:An ab initio Study
- Direct Observation of Transition Metal Dichalcogenides in Liquid with Scanning Tunneling Microscopy
