Renewable p-Xylene Production by Co-catalytic Pyrolysis of Cellulose and Methanol
Chi Tng,Li-jun Zhu,Ming-hui Fn,Qun-xin Li?
a.Department of Chemical Physics,Key Laboratory of Urban Pollutant Conversion,Chinese Academy of Sciences,Anhui Key Laboratory of Biomass Clean Energy,University of Science and Technology of China,Hefei 230026,China
b.Anhui Key Laboratory of Tobacco Chemistry,Anhui Tobacco Industrial.Co.,Ltd.,Hefei 230088,China
This work developed a one-step process for renewable p-xylene production by co-catalytic fast pyrolysis(co-CFP)of cellulose and methanol over the diflerent metal oxides modified ZSM5 catalysts.It has been proven that La2O3-modified ZSM5(80)catalyst was an eflective one for the production of bio-based p-xylene.The selectivity and yield of p-xylene strongly depended on the acidity of the catalysts,reaction temperature,and methanol content.The highest p-xylene yield of 14.5 C-mol%with a p-xylene/xylenes ratio of 86.8%was obtained by the co-CFP of cellulose with 33wt%methanol over 20%La2O3-ZSM5(80)catalyst.The deactivation of the catalysts during the catalytic pyrolysis process was investigated in detail.The reaction pathway for the formation of p-xylene from cellulose was proposed based on the analysis of products and the characterization of catalysts.
Key words:Cellulose,p-Xylene,Catalytic fast pyrolysis,La2O3-modified ZSM5
I.INTRODUCTION
As an important chemical intermediate,p-xylene has been mainly used for the production of terephthalic acid(TPA)that is an important aromatic carboxylic acid for the synthesis of resins and fibers[1].p-Xylene can be produced from various catalytic transformations such as the disproportionation of toluene,the alkylation of benzene or toluene,and the isomerization of C8 aromatics along with a series of separation processes[2,3].Currently,there has been great interest in producing this commodity chemical more sustainably from biomass sources[4,5].
Cellulose,a crystalline polymer consisting of β-1,4-linked D-glucose units,can be obtained from various biomass sources such as wood,straw,grasses,and algae[6]. Together with the massive amount of lignocellulosic biomass produced and accumulated on the earth,cellulose is the most important bioresource,and can be utilized as renewable materials for the production of biofuels and biochemicals[7–17].Among various routes for transformation of cellulose to bio-based chemicals,fast pyrolysis[8?11]and catalytic fast pyrolysis of cellulose[12–17]have received considerable interest.Cellulose pyrolysis is believed to proceed in two stages:the formation of volatile and non-volatile intermediates via depolymerization of cellulose,and the cracking of intermediates into final products including gas,liquid(tar and wood vinegar or pyroligneous acid)and solid(char and coke)products[8].Generally,the liquid oxygenates obtained from the pyrolysis of cellulose include anhydrosugars(mainly levoglucosan),acids,furans,ketones,aldehydes,and alcohols,due to the glucose polymer of cellulose[9–11].Pyrolysis of cellulose lower than 300?C mainly involves the reduction in degree of polymerization,the formation of free radicals,elimination of water,and leaving char residue[10].Over 300?C,cellulose pyrolysis involves both depolymerization and fragmentation reactions,leading to the increase in organic liquid products[10].Generally,the depolymerization process produces anhydro-oligosaccharides,monomeric anhydrosugars and derivatives,furans and cyclopentanones.And the fragmentation process creates linear carbonyls,alcohols,esters,and other products[11].
Catalytic fast pyrolysis(CFP)can transform cellulose into valuable liquid products[12].The catalytic pyrolysis of cellulose to aromatics over zeolites involves a complex pathway:(i)the formation of anhydrous sugars from the cellulose,(ii)the dehydration of these sugars to furanics,and(iii)the acid-catalysed decarbonylation,decarboxylation,dehydration and oligomerisation of furanics to aromatic products[13–16].Mihalcik et al.[14]studied the catalytic pyrolysis of cellulose usingfive zeolite catalysts.They found that the ZSM-5(23)catalyst was the most eflective catalyst for producingaromatics.The framework and Si/Al ratio of the catalysts play a major role in their ability to eflectively convert the oxygenates(like furans,acids and anhydrous sugars)into aromatics.Mullen et al.[15]investigated the production of aromatics via catalytic pyrolysis of cellulose with Fe-modified ZSM-5.They reported that the highest carbon yield of~18%aromatics was achieved with 1.4wt%Fe-ZSM-5 from cellulose.Rezaei et al.[16]reported that fast pyrolysis of cellulose strongly depended on the pore structure and acidity of the catalysts.To increase aromatics yield and reduce coke yield,there are several reports on the production of aromatics chemicals by co-catalytic pyrolysis of biomass and additives(such as low-density polyethylene)[17,18].Even though cellulose can be utilized as renewable materials for the production of biochemicals,the biggest challenge for the production of bio-based chemicals is how to improve the yield and selectivity of target product,along with reducing coke and catalyst deactivation[19,20].The lack of selective chemistry might be a serious problem for eventual commercialization,and thus,further study on the optimization of catalysts and controlling reaction pathways is required.
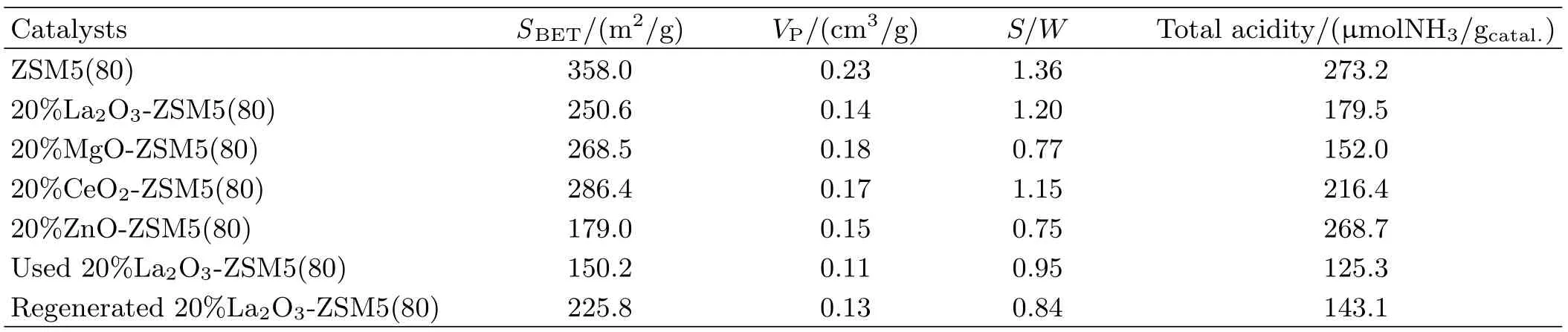
TABLE I Characterizations of the catalysts.
In this work,we developed a one-pot process that cellulose was able to be directionally converted into p-xylene by coupling the catalytic pyrolysis of cellulose into aromatic monomers,the alkylation of light aromatics to xylenes and the isomerization of m-xylenes and o-xylenes to p-xylene over metal oxidesmodified ZSM5(80)catalysts. The influences of the catalysts acidity,methanol content and reaction temperature on the selectivity and yield of p-xylene were investigated in detail.
II.MATERIALS AND METHODS
A.Cellulose and catalysts
The cellulose sample from Sigma/Aldrich was made up of 45.04wt%C,6.21wt%H,48.74wt%O,and 0.01wt%N.ZSM5(80)catalyst stems from the Catalyst Plant of Nankai University.The metal oxides modified ZSM5(80)catalysts including La2O3-ZSM5(80),MgO-
B.Catalytic pyrolysis measurement
ZSM5(80),CeO2-ZSM5(80),and ZnO-ZSM5(80)were gained using the impregnation route[2].
For the catalyst characterizations,the crystallinity of zeolites was investigated by X-ray diflraction on a Philips diflractrometer. Surface area of catalyst was obtained from the nitrogen adsorption-desorption isotherms(Coulter-SA-3100 analyzer).The acid characteristics of the catalyst samples were characterized by temperature programmed desorption of ammonia with the detailed procedure as described in our previous work[21].Coke deposited on the used catalyst was tested by temperature programmed oxidation with a Q5000IR thermogravimetric analyzer(USA).The results of the catalyst characterizations are shown in Table I.
One-step conversion of cellulose into aromatic compounds was carried out by the catalytic cracking of cellulose in a tubular catalytic cracking reactor over the metal oxides modified ZSM5(80)catalysts,as described in detail in our previous work[21].Before each test,the catalyst with the weight ratio of 2:1 between catalyst and biomass was loaded in the reactor.On reaching a setup temperature,cellulose sample was fed into the catalytic bed with a feed hopper installed in the top of the reactor.Methanol was also added by a liquid injection pump for the experiments of co-catalytic pyrolysis of cellulose and methanol.
The collected gas was measured through a GCSP6890 gas chromatograph.The organic liquid products were separated from the aqueous phase with ethyl ether.Analysis of liquid products was conducted with a Thermo Trace GC/ISQ GC/MS.The GC oven was programmed from the room temperature(25?C)to 280?C at 10?C/min and kept at 280?C for 10 min.Major products were quantified using calibration standards of GC/MS.Product yield was obtained in terms of molar carbon yield,and the moles of carbon in a product are divided by the moles of carbon in the reactant.The yield and selectivity of the products were calculated based on Eq.(1)?Eq.(3)as described in our previous work[21].

FIG.1 The XRD characterizations for the ZSM5(80),La2O3-ZSM5(80),MgO-ZSM5(80),CeO2-ZSM5(80),and ZnO-ZSM5(80)catalysts.

where Yiis the overall carbon yields,Yjis the yield of a product,and Skis the selectivity of a product;the units of Yi,Yj,and Skare all in unit of C-mol%.
III.RESULTS AND DISCUSSION
A.Characterization of catalysts
FIG.1 shows the XRD patterns of the unmodified ZSM5(80)and the metal oxides modified ZSM5(80)catalysts. The characteristic peaks for the unmodifi ed ZSM5(80)are at 2θ=8.0?,9.0?,23.1?,and 24.5?,corresponding to typical characteristic peaks of ZSM5[22].Similar characteristic peaks of the MFI structure were also observed for the unmodified ZSM5(80)and the metal oxides modified ZSM5(80)catalysts.The crystallinity for the modified catalysts was obviously reduced,which was also reported in Refs.[23,24].No obvious characteristic peaks corresponding to MgO and La2O3species were identified from MgO-ZSM5(80)and La2O3-ZSM5(80)respectively,indicating that MgO and La2O3might be highly dispersed on the catalyst surface.
To investigate the changes of acidity and acid sites,the NH3-TPD analyses were performed for unmodified ZSM5(80)and modified ZSM5(80)catalysts with diflerent metal oxides.
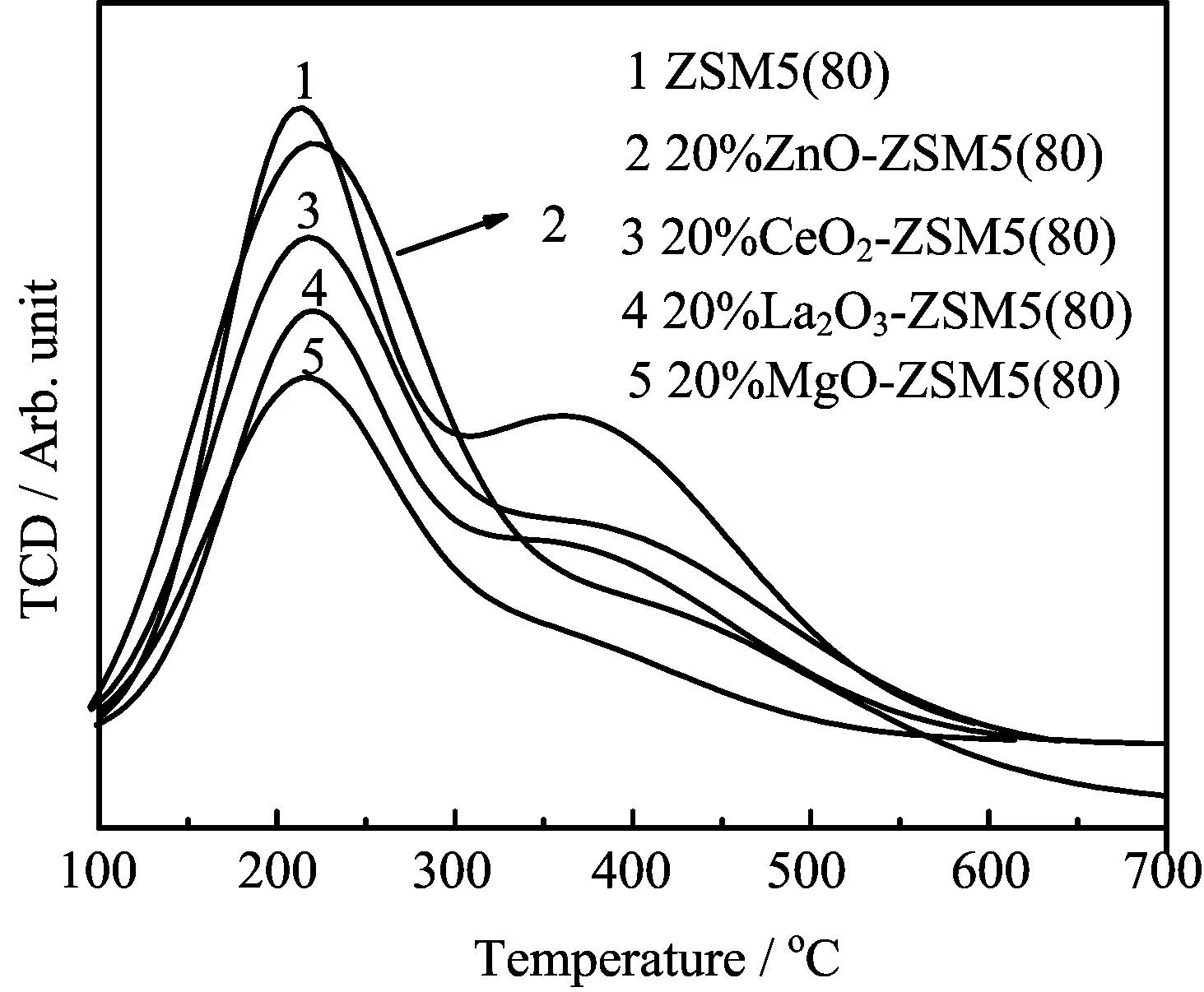
FIG.2 NH3-TPD profiles of five catalysts ZSM5(80),La2O3-ZSM5(80),MgO-ZSM5(80),CeO2-ZSM5(80),and ZnO-ZSM5(80)catalysts.
The NH3-TPD characterizations are shown in FIG.2 and Table I.Total acidity was determined by the ammonia amount desorbed from the catalyst surface.Compared with ZSM5(80),the acidity of all modifi ed ZSM5(80)catalysts was significantly reduced by adding the metal oxides into ZSM5(80).For example,the acidity of ZSM5(80)was 273.2μmolNH3/gcatal.,and the acidity of 20%La2O3-ZSM5(80)decreased to 179.5μmolNH3/gcatal..As can be seen from FIG.2,ZSM5(80)exhibited two desorption peaks near 250 and 400?C,corresponding to ammonia desorbed from the weak acid site and strong acid site,respectively[25]. Similar double peaks were also found for La2O3-ZSM5(80)and CeO2-ZSM5(80),while the high-temperature desorption peak corresponding to the strong acid site almost disappeared for MgO-ZSM5(80)and ZnO-ZSM5(80).Furthermore,the ratio of strong acid site to the weak acid site(S/W ratio)can be determined by fitting the NH3-TPD patterns into two Gaussian components[25].The S/W ratio for ZSM5(80)was 1.36,and the values for the catalysts modified by the metal oxides of La2O3,MgO,CeO2,and ZnO decreased to 1.20,0.77,1.15,and 0.75 respectively.
B.Screening of catalysts for transformation of cellulose into p-xylene
Table II shows the catalytic transformation of cellulose into aromatics including p-xylene over the diflerent metal oxides modified ZSM5(80)catalysts.Introduction of the metal oxides to ZSM5(80)improved the ratio of organic liquid products and suppressed the formation of coke during the catalyst pyrolysis process.When ZSM5(80)was used,the organic liquid products consisted primarily of xylenes and toluene,with a small amount of benzene,ethylbenzene,trimethylbenzene,furans and low oligomers.However,the metal oxides modified ZSM5(80)significantly increased the desired p-xylene yield,accompanied by the decrease in the yields of benzene,toluene,and polycyclic aromatics such as naphthalene,methylnaphthalene,and indene.
For the five diflerent catalysts,the selectivity of p-xylene decreased in the following order: La2O3-ZSM5(80)>MgO-ZSM5(80)>ZnO-ZSM5(80)>CeO2-ZSM5(80)>ZSM5(80). La2O3-ZSM5(80)catalyst presented the highest p-xylene yield of 14.5 C-mol%with a p-xylene/xylenes ratio of 86.8%(T=450?C,cellulose/methanol=2:1(in mass ratio),and catalyst/cellulose=2:1(in mass ratio)).The above results implied that adding the metal oxides into ZSM5(80)promoted the alkylation of benzene and toluene to form xylenes,and the isomerization of m-xylene and o-xylene to p-xylene.In view of the NH3-TPD results,the increase in the yield and selectivity of p-xylene using the diflerent metal oxides modified ZSM5(80)catalysts should be in association with the alteration of the a cid strength and the strong acid sites.

TABLE II Catalytic pyrolysis of cellulose into p-xylene over the diflerent metal oxides modified ZSM5(80)catalysts.a
C.Influences of reaction conditions on catalytic pyrolysis of cellulose to p-xylene
FIG.3 shows influence of temperature on the production of p-xylene by the co-catalytic pyrolysis of cellulose and methanol over the La2O3-ZSM5(80)catalyst(cellulose/methanol=2:1(in mass ratio)and catalyst/cellulose=2:1(in mass ratio)). On increasing temperature,the yields of organic liquid products and solid residues decreased,and the yield of gas was enhanced due to the second catalytic cracking of organics.Reaction temperature also significantly aflected the yields and selectivity of the individual aromatic product.The main product observed in the temperature range of 450?550?C was p-xylene,together with a smaller amount of other aromatics including benzene,toluene,ethylbenzene,and the heavier C8+aromatics(e.g.,trimethylbenzene,naphthalene,m-naphthalene,and indene).The yields of heavier C8+aromatics were reduced with temperature increasing,due to the second catalytic pyrolysis of heavier aromatics to form light aromatics.Furthermore,increasing temperature tended to decrease both the p-xylene and overall xylenes yields due to the catalytic cracking of xylenes.The selectivity of p-xylene decreased from 47.2 C-mol%to 30.4 C-mol%with temperature increasing from 450?C to 600?C.The ratio of p-xylene to xylenes was also reduced at higher temperatures.It has been shown that the alkylation of benzene/toluene to xylenes and the isomerization of m-xylene and o-xylene to p-xylene generally occur at the lower temperatures below 450?C when using zeolite as a catalyst[2,3].Accordingly,it seems to be unfavourable for the alkylation and isomerization reactions at high temperatures,leading to the decrease in the p-xylene selectivity in the catalytic pyrolysis of cellulose.In addition,it was noticed that the intermediate of furans was further converted to aromatics,especially at higher temperatures[26].As the temperature increased,both polycyclic aromatics and coke tended to decrease due to the reduction of the polymerization of aromatics.

FIG.3 Influence of temperature on the production of p-xylene by the co-catalytic pyrolysis of cellulose and methanol over the 20%La2O3-ZSM5(80)catalyst(cellulose/methanol=2:1(in mass ratio)and catalyst/cellulose=2:1(in mass ratio)).
Co-catalytic pyrolysis of cellulose with methanol also prominently aflected the yield and selectivity of p-xylene.FIG.4 shows the influence of the methanol content on the production of p-xylene by the cocatalytic pyrolysis of cellulose and methanol over the La2O3-ZSM5(80)catalyst.It was observed that the cocatalytic pyrolysis decreased the yield of solid residue(coke)and improved the yield of aromatics. The yield of coke/char in the absence of methanol was 35.5 C-mol%,while the yeild of solid residue greatly reduced to 18.9 C-mol%by co-feeding cellulose with 50wt%methanol.The yield of the organic liquid products from the co-catalytic pyrolysis was higher than that in the absence of methanol.Besides,co-catalytic pyrolysis prominently improved the yield of xylenes and selectivity of p-xylene in aromatics.In the absence of methanol,the yields of benzene,toluene,and p-xylene obtained from the catalytic pyrolysis of cellulose were only 1.7,5.5,and 6.5 C-mol%,respectively.For co-feeding cellulose and 33wt%methanol,the yield of p-xylene increased significantly to 14.5 C-mol%,accompanied by the decrease in the yields of benzene and toluene.Increasing the methanol content tends to increase the selectivity of p-xylene,which goes through a maximum of 47.2 C-mol%at the biomass/methanol mass ratio of 2:1. It should be pointed out that p-xylene can be formed from cellulose or methanol alone(FIG.4). However,the p-xylene yield was not linearly increased with methanol content increasing,which shows that methanol has a certain synergistic eflect on the formation of p-xylene during catalytic cracking of cellulose. Present work found that more desired p-xylene was formed in the the co-catalytic pyrolysis of cellulose and methanol.The improvement of the xylenes yield and p-xylene selectivity in the co-feed process was due to that methanol produced methyl radicals,and then benzene and toluene formed from catalytic pyrolysis of cellulose were alkylated to xylenes. Meanwhile,the metal oxides modified ZSM5(80)catalysts(like 20%La2O3-ZSM5(80))enhanced the isomerization of m-xylene and o-xylene to p-xylene due to the modulation of the acidity and strong acid sites.There are several previous studies reported on co-catalytic pyrolysis of biomass or its derivatives(like bio-oil)with methanol over ZSM-5 catalysts[27–29].In all these investigations,it has been shown that the yields of aromatics were improved and the coke yield was reduced for co-catalytic pyrolysis.Similar improvement was also observed in the present work.The ratio of hydrogen to carbon eflective(H/Ceff)of the feed can be improved during co-catalytic pyrolysis,and a higher(H/Ceff)favors to produce more aromatics and reduce the coke amount[28].
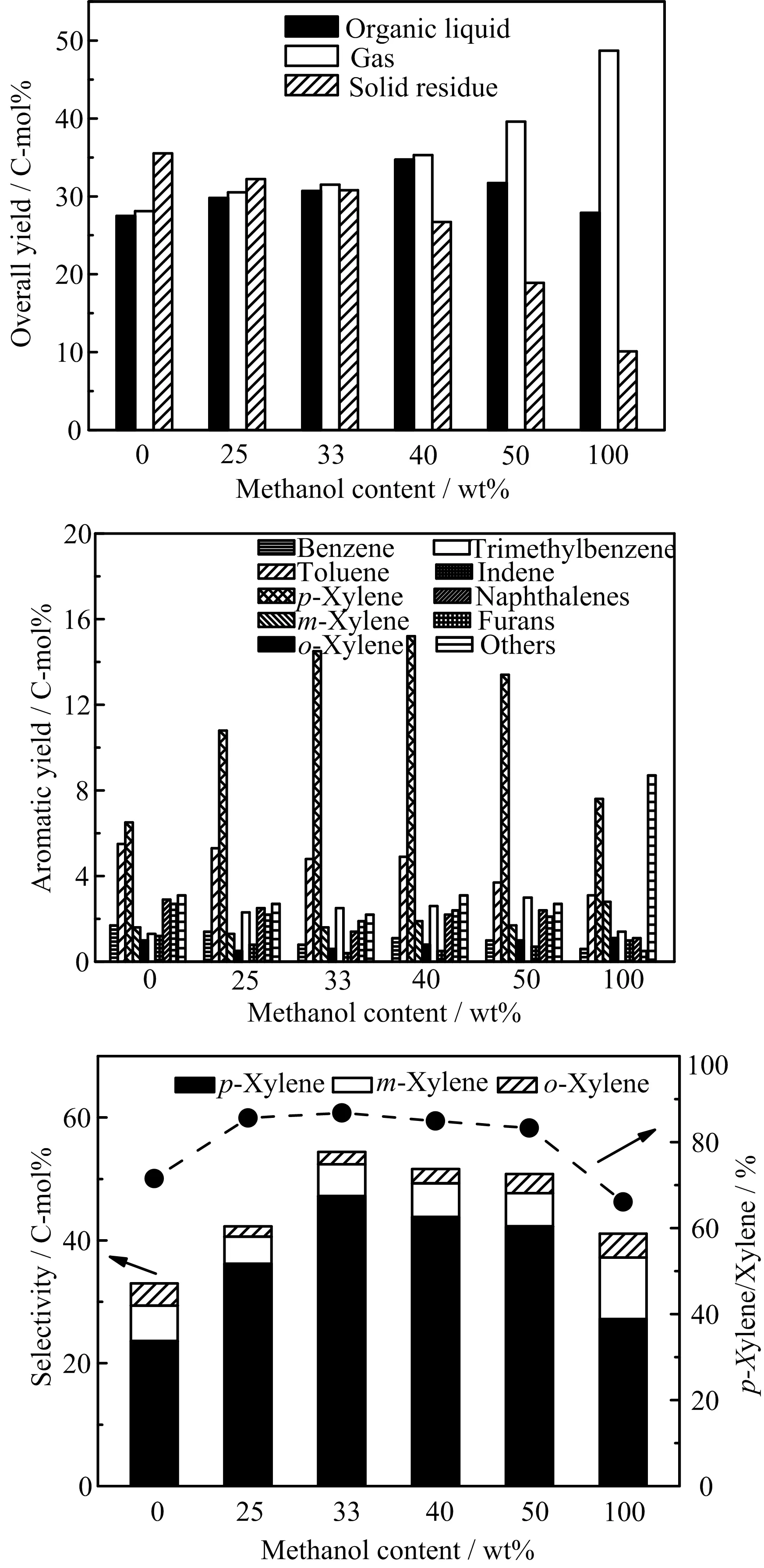
FIG.4 Influence of the methanol content on the production of p-xylene by the co-catalytic pyrolysis of cellulose and methanol over the 20%La2O3-ZSM5(80)catalyst(T=450?C and catalyst/cellulose(in mass ratio)=2:1).

FIG.5 Stability and reproducibility of catalyst during the co-catalytic pyrolysis of cellulose and methanol over 20%La2O3-ZSM5(80)(T=450?C,cellulose/methanol=2:1(in mass ratio),and catalyst/cellulose=2:1(in mass ratio)).
D.Catalyst stability and reaction mechanism
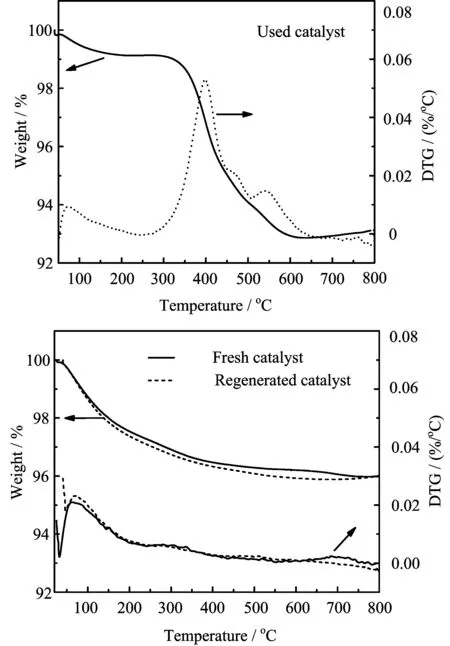
FIG.6 TGA profiles and DTG(diflerential thermogravimetric analysis)curves from three samples: the fresh 20%La2O3-ZSM5(80)catalyst,used one,and regenerated catalyst.
The stability test of 20%La2O3-ZSM5(80)catalyst during the co-catalytic pyrolysis of cellulose and methanol was run at 450?C,and the regeneration of catalyst was conducted in oxygen at 600?C.After continuously used for three rounds,the catalyst deactivation was obviously observed(FIG.5).To understand catalyst deactivation,the formation of the coke on the used catalyst was characterized by the TPO/DTG analyses with a thermogravimetric analyzer(FIG.6).The coke content on the used catalyst was about 6.4 gc./gcatal.,indicating that the coke was deposited externally on the zeolite and inside the micropores of the zeolite.For the used catalyst,two peaks in the DTG curve were distinguished in the range of 350?450 and 500?600?C,respectively.The first profi le in the lower temperature region was attributed to a thermal origin coke formed from the pyrolysis of biomass,which burned easily since the coke was deposited outside the catalyst particles[27].The second profile in the higher temperature region was attributed to the catalytic origin coke,which was mainly generated by the polymerization of aromatic compounds formed from the catalytic pyrolysis of cellulose,followed by dehydrogenation inside the acid catalyst[16,27].Moreover,the acidity of the fresh 20%La2O3-ZSM5(80)obtained from NH3-TPD was 179.5μmol NH3/gcatal.,and decreased to 125.3μmol NH3/gcatal.after 3 h(FIG.7).S/W ratio for the used catalyst was reduced remarkably to 0.95.In turn,the deactivation of catalyst was due to coke deposition as well as the acidity reduction[30].

FIG.7 NH3-TPD profiles of(a)fresh 20%La2O3-ZSM5(80),(b)used catalyst for co-catalytic pyrolysis of the cellulose and methanol at 450 ?C for 3 h,(c)regenerated catalyst by the coke burn-o ffat 600 ?C,and(d)comparison of three samples.The dash lines in FIG.7(a)?(c)stand for fitting the NH3-TPD patterns into two Gaussian components.

FIG.8 Reaction pathways for the co-catalytic pyrolysis of cellulose and methanol to p-xylene over the metal oxides modified ZSM5(80)catalysts.
Finally,the main reaction pathway for the catalytic pyrolysis of cellulose into the aromatics(including p-xylene)was proposed(FIG.8),according to the products identified,the catalyst characterizations,and the previous work[8–16].The transformation mainly included several cascade reactions:(i)the catalytic pyrolysis of cellulose into furans,(ii)the deoxygenation(namely decarboxylation,decarbonylation,and dehydration)of furans into olefin intermediates in hydrocarbon pool,(iii)the aromatization of olefins into C6-C9 monocyclic aromatics,(iv)the alkylation of light aromatics(like benzene and toluene)to xylenes,(v)the isomerization of m-xylene and o-xylene to p-xylene,and(vi)the polymerization of aromatics to polycyclic aromatics and coke.In the reactions(i)?(iii),the catalytic pyrolysis of cellulose into the low-carbon aromatic monomers was realized over the acidic sites,which in-volved the formation of oxygenates(like furan,furfural,and other carbohydrates)by cellulose pyrolysis followed by deoxygenation,catalytic cracking,and aromatization of the cellulose-derived olefins[10].The reaction step(iv)involved the selective production of xylenes by the alkylation reactions of low carbon aromatics(mainly benzene and toluene)using methanol as alkylating agent. The metal oxides modified ZSM5(80)catalysts(like La2O3-ZSM5(80))were proven to be highly active catalysts for the alkylation of benzene and toluene to form xylenes,which tuned the acidity of the zeolite catalysts and the S/W ratio.The subsequent isomerization of xylenes in the reaction(v)resulted in the isomerization of m-xylene and o-xylene to p-xylene over the same metal oxides modified ZSM5(80)catalysts.In particular,the 20%La2O3-ZSM5(80)catalyst presented highest selectivity of p-xylene in aromatic productions.The formation of naphthalenes in reaction(vi)also occurred over the acidic sites of the zeolite,and reducing the acidity of the zeolite and adding methanol can inhibit eflectively the production of polycyclic aromatics.
IV.CONCLUSION
Present work shows that cellulose is a potential renewable raw material for production of bio-based p-xylene by co-CFP of cellulose and methanol over the metal oxides modified ZSM5(80)catalysts.The catalytic transformation mainly included four reaction steps:(i)pyrolysis of cellulose into oxygenates,(ii)catalytic pyrolysis of oxygenates into aromatics,(iii)alkylation of light aromatics into xylenes using methanol as alkylating agent,and(iv)isomerization of m-xylene and o-xylene into p-xylene.The La2O3-modified ZSM5(80)catalyst,which tuned the acidity and the S/W ratio in the zeolite,was proved to be highly active for the alkylation of benzene and toluene into xylenes and the isomerization of m-xylene and o-xylene to p-xylene.The highest p-xylene yield of 14.5 C-mol%with a p-xylene/xylenes ratio of 86.8%was obtained by the co-catalytic pyrolysis of cellulose with 33wt%methanol over 20%La2O3-ZSM5(80)catalyst.Carbon deposited on the zeolite was the primary cause of catalyst deactivation,and the activity of the used catalysts was almost recovered by the removal of coke.Present process potentially provides a available way for the production of bio-based p-xylene using renewable cellulose.
V.ACKNOWLEDGMENTS
This work was supported by the National Key Basic Program of China(2013CB228105).
[1]R.A.F.Tom′as,J.C.M.Bordado,and J.F.P.Gomes,Chem.Rev.113,7421(2013).
[2]K.Gao,S.Li,L.Wang,and W.Wang,RSC Adv.5,45098(2015).
[3]M.O.Daramolaa,A.J.Burgera,A.G.Fendlerb,S.Miachonb,and L.Lorenzena,Appl.Catal.A 386,109(2010).
[4]J.Zhu,J.Wang,and Q.Li,Chin.J.Chem.Phys.26,477(2013).
[5]D.I.Collias,A.M.Harris,V.Nagpal,I.W.Cottrell,and M.W.Schultheis,Ind.Biotechnol.10,91(2014).
[6]O.Nechyporchuk,M.N.Belgacem,and J.Bras,Ind.Crops Prod.93,2(2016).
[7]A.J.Foster,J.Jae,Y.T.Cheng,G.W.Huber,and R.F.Lobo,Appl.Catal.A 423-424,154(2012).
[8]H.Kawamoto,Curr.Org.Chem.20,2444(2016).
[9]P.D.Muley,C.Henkel,K.K.Abdollahi,C.Marculescu,and D.Boldor,Energ.Convers.Manage.117,273(2016).
[10]Q.Lu,X.Yang,C.Dong,Z.Zhang,X.Zhang,and X.Zhu,J.Anal.Appl.Pyrolysis 92,430(2011).
[11]D.K.Shen and S.Gu,Bioresour.Technol.100,6496(2009).
[12]K.Wang,K.H.Kim,and R.C.Brown,Green Chem.16,727(2014).
[13]V.Srinivasan,S.Adhikari,S.A.Chattanathan,M.Tu,and S.Park,Bioenerg.Res.7,867(2014).
[14]D.J.Mihalcik,C.A.Mullen,and A.A.Boateng,J.Anal.Appl.Pyrolysis 92,224(2011).
[15]C.A.Mullen and A.A.Boateng,ACS Sustainable Chem.Eng.3,1623(2015).
[16]P.S.Rezaei,H.Shafaghat,and W.M.A.W.Daud,RSC Adv.5,65408(2015).
[17]G.Zhou,J.Li,Y.Yu,X.Li,Y.Wang,W.Wang,and S.Komarneni,Appl.Catal.A 487,45(2014).
[18]C.Dorado,C.A.Mullen,and A.A.Boateng,Appl.Catal.B 162,338(2015).
[19]J.Grams and A.M.Ruppert,Energies 10,545(2017).
[20]J.Li,Y.Yu,X.Li,W.Wang,G.Yu,S.Deng,J.Huang,B.Wang,and Y.Wang,Appl.Catal.B 172-173,154(2015).
[21]Z.Zhang,P.Bi,P.Jiang,M.Fan,S.Deng,Q.Zhai,and Q.Li,Energy 90,1922(2015).
[22]L.Wang,H.Lei,Q.Bu,S.Ren,Y.Wei,L.Zhu,X.Zhang,Y.Liu,G.Yadavalli,J.Lee,S.Chen,and J.Tang,Fuel 129,78(2014).
[23]L.Zhang,J.Gao,J.Hu,W.Li,and J.Wang,Catal.Lett.130,355(2009).
[24]J.Lee,U.G.Hong,S.Hwang,M.H.Youn,and I.K.Song,Fuel Process.Technol.109,189(2013).
[25]Z.Song,A.Takahashi,N.Mimura,and T.Fujitani,Catal.Lett.131,364(2009).
[26]Y.Cheng and G.W.Huber,Green Chem.14,3114(2012).
[27]A.G.Gayubo,B.Valle,A.T.Aguayo,M.Olazar,and J.Bilbao,Energy Fuels 23,4129(2009).
[28]H.Zhang,T.R.Carlson,R.Xiao,and G.W.Huber,Green Chem.14,98(2012).
[29]Y.Zhang,M.Fan,R.Chang,and Q.Li,Chin.J.Chem.Phys.30,588(2017).
[30]A.G.Gayubo,A.T.Aguayo,A.Atutxa,R.Prieto,and J.Bilbao,Energy Fuels 18,1640(2004).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年6期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年6期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Imaging HNCO Photodissociation at 201 nm:State-to-State Correlations between CO(X1Σ+)and NH(a1?)
- Energy-Transfer Processes of Xe(6p[1/2]0,6p[3/2]2,and 6p[5/2]2)Atoms under the Condition of Ultrahigh Pumped Power
- Ultrafast Investigation of Excited-State Dynamics in Trans-4-methoxyazobenzene Studied by Femtosecond Transient Absorption Spectroscopy
- Strong Current-Polarization and Negative Diflerential Resistance in FeN3-Embedded Armchair Graphene Nanoribbons
- Unexpected Chemistry from the Homogeneous Thermal Decomposition of Acetylene:An ab initio Study
- Direct Observation of Transition Metal Dichalcogenides in Liquid with Scanning Tunneling Microscopy
