Surface modification of silicone rubber by CF4 radio frequency capacitively coupled plasma for improvement of flashover
Chenxu WANG(王晨旭),Bo ZHANG(張波),Sile CHEN(陳思樂),Yuhao SUN(孫宇豪),Xiong YANG(楊雄),Yanan PENG(彭雅楠),Xingyu CHEN(陳星宇) and Guanjun ZHANG(張冠軍),*
1 State Key Laboratory of Electrical Insulation and Power Equipment,School of Electrical Engineering,Xi’an Jiaotong University,Xi’an 710049,People’s Republic of China
2 School of Electrical and Information Engineering,Anhui University of Technology,Ma’anshan 243032,People’s Republic of China
Abstract The flashover performance of insulating materials plays an important role in the development of high-voltage insulation systems.In this paper,silicone rubber(SIR)is modified by CF4 radio frequency capacitively coupled plasma(CCP)for the improvement of surface insulation performance.The discharge mode and active particles of CCP are diagnosed by the digital single-lens reflex and the spectrometer.Scanning electron microscopy and x-ray photoelectron spectroscopy are used for the surface physicochemical properties of samples,while the surface charge dissipation,charge accumulation measurement,and flashover test are applied for the surface electrical characteristics.Experimental results show that the fluorocarbon groups can be grafted and the surface roughness increases after plasma treatment.Besides,the surface charge dissipation is decelerated and the positive charge accumulation is inhibited obviously for the treated samples.Furthermore,the surface flashover voltage can be increased by 26.67% after 10 min of treatment.It is considered that strong electron affinity of C-F and increased surface roughness can contribute to deepening surface traps,which not only inhibits the development of secondary electron emission avalanche but also alleviates the surface charge accumulation and finally improves the surface flashover voltage of SIR.
Keywords:silicon rubber,CF4,radio frequency capacitively coupled plasma,surface modification,flashover
1.Introduction
Silicone rubber(SIR)is widely used in electrical engineering due to its excellent mechanical strength and insulation properties[1].Generally,combination insulation systems are inevitably formed in high-voltage power systems when the solid insulating materials contact the vacuum or air.The weakest link of insulation systems mainly depends on the interfaces,along which the flashover strength is usually much lower than the bulk electrical breakdown strength[2].Therefore,improving the surface insulating strength of electrical materials has become a hot issue in the high-voltage engineering field.In recent years,direct fluorination has been proved to be an effective method for improving the surface flashover withstanding strength.Liuet altreated the low-density polyethylene samples in the mixed gas of F2and N2and found that the micro-size fluorinated layer was formed on the surface,the increased roughness and conductivity are helpful for the improvement of surface flashover in vacuum[3].Wanget alapplied the same method on the epoxy resin(ER)filled with different ratios of Al2O3grain.It was observed that the surface charge dissipation is accelerated after direct fluorination treatment[4].Zhouet alfurther investigated the surface electron trap and secondary electron emission characteristics of ER and polyethylene(PE)after direct fluorination.It is concluded that the reduction of surface secondary electron yield has a direct link with the improvement of flashover voltage.Besides,they also built a comprehensive flashover suppression model based on direct fluorination[5].Although the method of direct fluorination is effective,the usage of F2,as a kind of toxic gas with high activity,has a potential threat to the humans and environment.
In recent years,plasma surface modification has attracted wide attention because of its high efficiency and environmental friendliness[6-8].Chenet alutilized the He/CF4atmospheric pressure plasma jet for the surface treatment of ER samples and considered that the enhancement of flashover strength is the result of physical and chemical effects[9,10].Based on this result,they also found that the proper addition of CO2can decrease the fluorine-to-carbon ratio(F/C)in plasma,which is helpful for the formation of fluorinated layers[11].The surface conductivity of treated samples by He/CO2/CF4atmospheric pressure plasma decreases by two orders of magnitude and the surface charge accumulation is inhibited obviously,which contributes to the improvement of flashover voltage.Shaoet alalso reported that dielectric barrier discharge using Ar/CF4mixed gas at atmospheric pressure can increase the surface roughness and introduce the fluorocarbon group,which can reduce the surface secondary electron emission coefficient and improve the flashover voltage of PMMA[12].
Radio frequency capacitively coupled plasma(CCP)can produce active species with high energy uniformly in a large area[13].Driven by the industrial radio frequency power with mature technology,it also has an economic advantage.Therefore,it is usually applied in surface modification and microelectronic processing[14-16].The previous study has demonstrated that RF discharge in CF4gas can improve the hydrophobicity of SIR[17],but the studies about the improvement of surface flashover withstanding strength are hardly found.Therefore,in this work,we explore the discharge characteristic of CF4CCP and utilize it for modifying the surface of SIR.Besides,the physicochemical changes,and charge accumulation and dissipation behavior on SIR samples surface for different treatment time are investigated.Combined with the surface flashover test,the mechanism of improving the surface withstanding strength by CF4CCP is studied.
2.Experiment details
2.1.Material
Silicon rubber samples(Shandong Anlan Power Technology Co.,China)are composed of the polydimethylsiloxane(PDMS)matrix and inorganic filler(Al(OH)3and SiO2),which are cut into 50 mm×50 mm×1 mm slices.They are washed in an ultrasonic cleaner by deionized water before plasma treatment for 30 min,soaked in absolute alcohol for 10 min,then dried in a vacuum drying oven at 60°C for 12 h.
2.2.CF4 CCP surface treatment arrangement
The plasma surface treatment setup is shown in figure 1.The CCP reactor consists of symmetrical stainless-steel electrodes with a diameter of 14 cm and a gap of 2 cm.A 13.56 MHz RF power supply(VERG-300,Zhongshan K-mate Electronics Co.,Ltd)is used to generate low-temperature plasma and the SIR samples are placed on the grounded electrode.To remove the impact of impurity gas,the cavity is washed with highpure CF4(99.99%)three times before processing the material.Controlling the mass flowmeter and mechanical pump,the working gas can be maintained at different pressures.Moreover,the discharge voltage and current are measured by a high voltage probe(Tektronix P6015A)and a current probe(Pearson 2877)respectively,and recorded by a digital oscilloscope(WaveSurfer 104MXs-B,LeCroy).For further analysis of CCP discharge mode and discharge products,CCP discharge images are captured by a digital single-lens reflex(Canon 60D)and the emission spectra is probed by a triple grating spectrometer(Andor SR-303I-A).
2.3.Surface characterizations
The surface morphology of SIR samples is observed by scanning electron microscope(SEM,MAIA3 LMH,TESCAN).The surface chemical composition is analyzed by x-ray photoelectron spectroscopy(XPS,Thermo Fisher ESCALAB Xi+),and the XPS data is fitted by Thermo Fisher Scientific Avantage 5.979.
The surface potential and surface flashover are measured in a stainless-steel vacuum chamber,as can be seen in figure 2.The chamber is equipped with a mechanical pump and a molecular pump,which can form the cascade structure and maintain the air pressure below 10-3Pa.The high-voltage electrode is applied with negative standard lightning impulse voltage(1.2/48 μs)generated by a Marx generator and the grounded electrode is connected with the chamber,while the electrode distance is fixed to 10 and 5 min for the surface potential measurement and flashover voltage test respectively.Moved by the displacement platform,surface potential on samples is detected by a Kelvin probe and displays on an electrostatic voltmeter.The distance between the probe and the sample surface is 2 mm,and the measurement range is 48 mm×30 mm.Surface flashover voltage is characterized by three parameters,the first breakdown voltage(Ufb),conditioned voltage(Uco)and hold-off voltage(Uho).Firstly,the samples are continuously applied with a low voltage(-10 kV)five times.If the flashover does not happen,the voltage will increase by 1 kV each time until the flashover happened,which is called the first breakdown voltage(Ufb).Then the voltage is increased until the flashover happened five times in five tests,which is called the conditioned voltage(Uco).Finally,the voltage will decrease gradually until the flashover does not happen,which is called hold-off voltage(Uho).
Isothermal surface potential decay(ISPD)measurement is carried out in the thermal tank set at 50 °C in figure 3.Negative corona discharge can be generated from the needle tip electrode,which is applied with the negative polarity voltage(-15 kV).Under the relatively uniform electric field between the metal mesh electrode and grounded electrode,the charged particles could be deposited on the sample evenly.The charging time is 15 min and the samples are moved under the electrostatic probe after turning on the electrostatic voltmeter.
3.Experimental results
3.1.Discharge characteristic of CCP
Figure 4(a)shows the discharge images when the output power is set as 150 W and the gas pressure is 100 Pa,200 Pa,and 300 Pa respectively.When the gas pressure is 100 Pa,the diffuse discharge can be observed in the whole electrode space.According to previous researches on CCP characteristics,the discharge state is maintained at the DA model.Under this condition,a large amount of electrons multiplies repeatedly in the electric field of the sheath and drifts rapidly in the plasma.Increasing the gas pressure to 200 Pa,the discharge began to transform to γ mode gradually.Due to the contraction of plasma sheaths,positive ions will bombard the electrode and generate secondary electrons,which will accelerate the electron impact near the plasma sheath.Therefore,the obvious negative glow region and Faraday dark region can be observed near electrodes.Subsequently,when the gas pressure is further increased to 300 Pa,the negative glow region gradually shrinks and a distinct positive column region appears in the center of the electrode because of the impact of high energy electrons in the plasma region.The discharge waveform at 200 Pa and 150 W can be seen from figure 4(b),the peak voltage and current are 204 V and 1.5 A respectively and the phase shift between voltage and current is 67.3°.Because of its stable discharge and intense bombardment effect,this discharge condition is selected for plasma surface treatment.
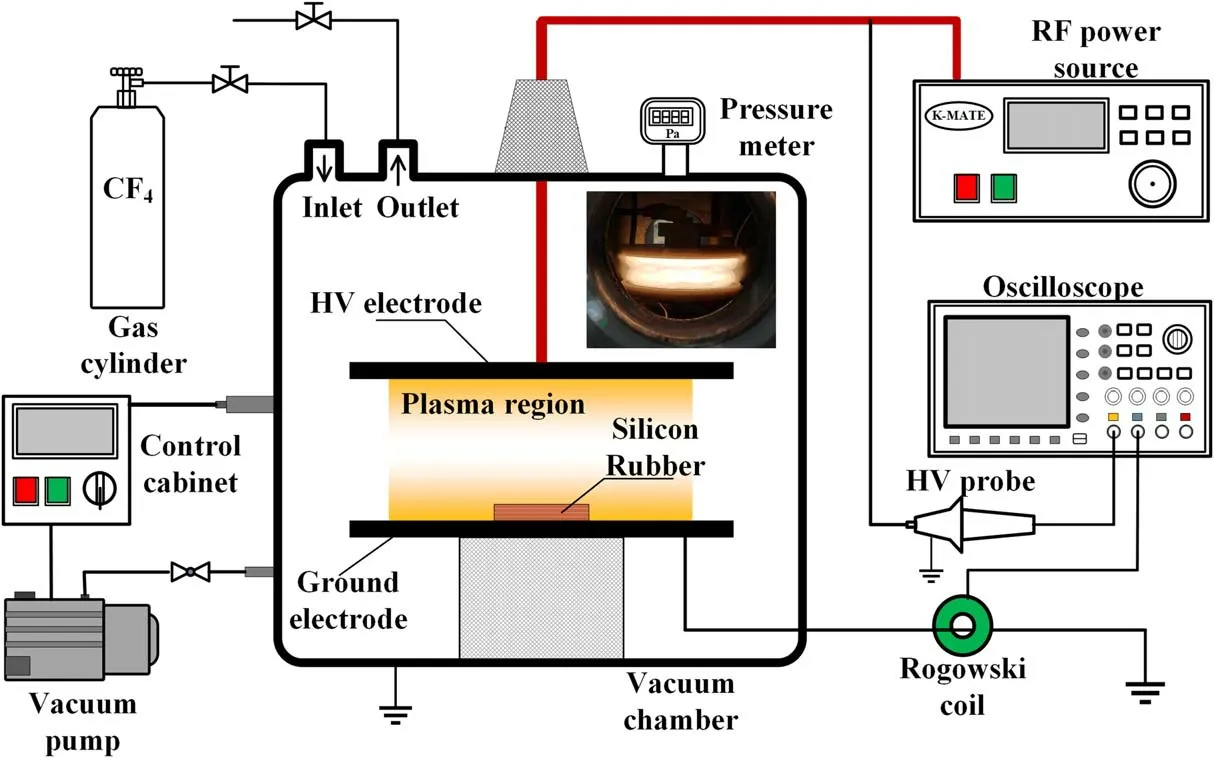
Figure 1.CCP treatment setup.
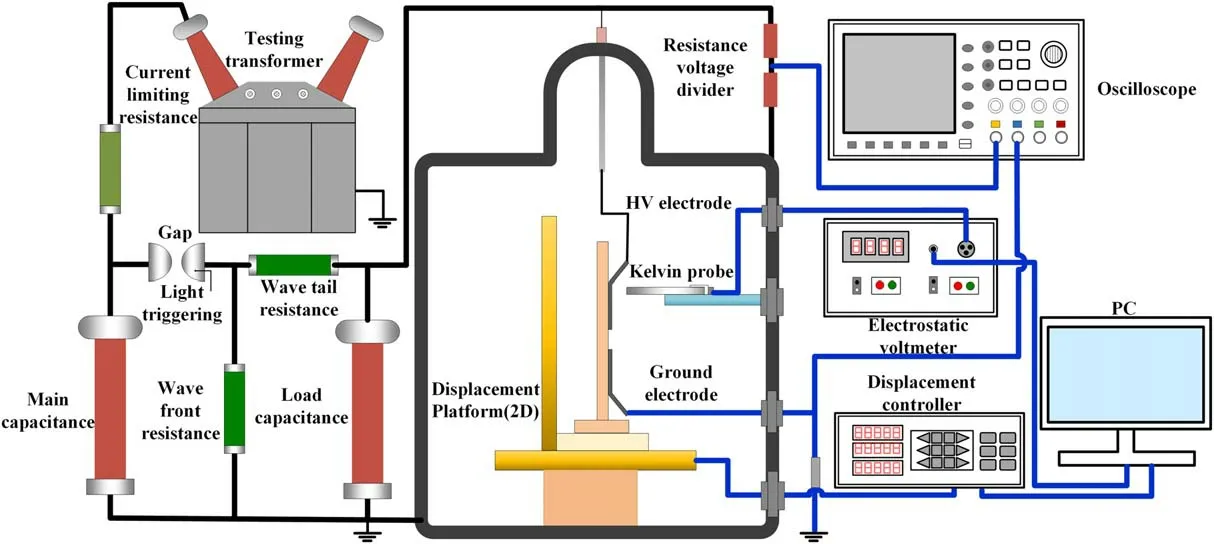
Figure 2.Surface charge accumulation and surface flashover voltage measurement setup.
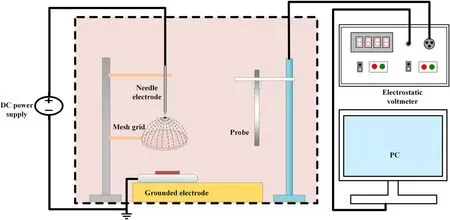
Figure 3.ISPD measurement setup.
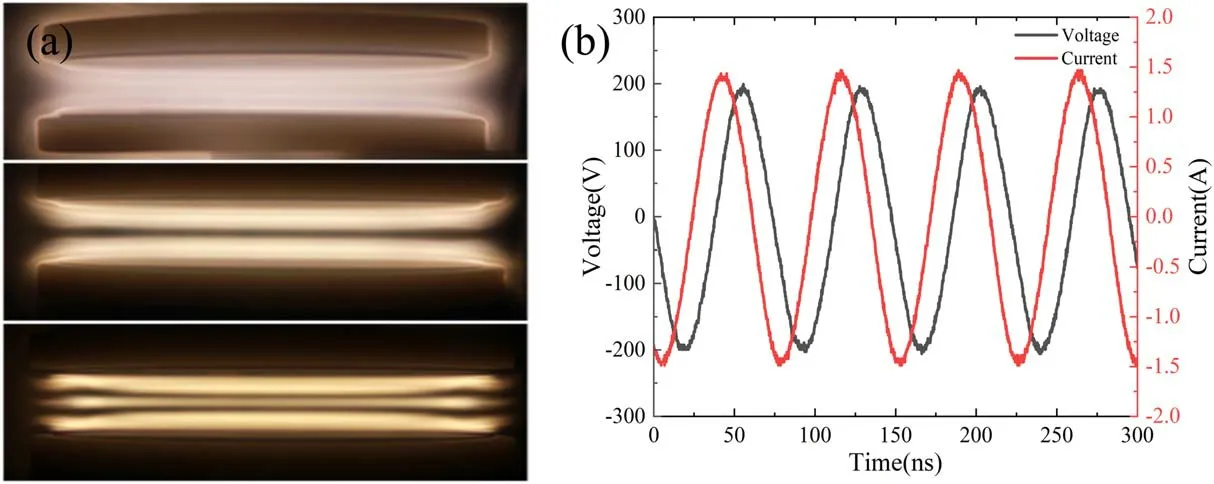
Figure 4.Discharge properties of CCP.(a)Discharge image of CCP at 100 Pa,200 Pa and 300 Pa;(b)discharge waveforms of CCP at 200 Pa.
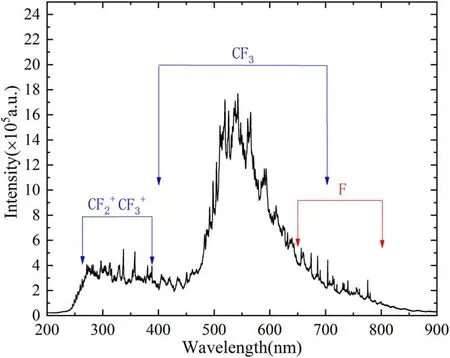
Figure 5.Emission spectra of CCP at 200 Pa.

Figure 6.SEM image of SIR surface at different treatment time(×5000).(a)0 min,(b)5 min,(c)10 min,(d)15 min,(e)20 min and(f)the image of cross-section layers.
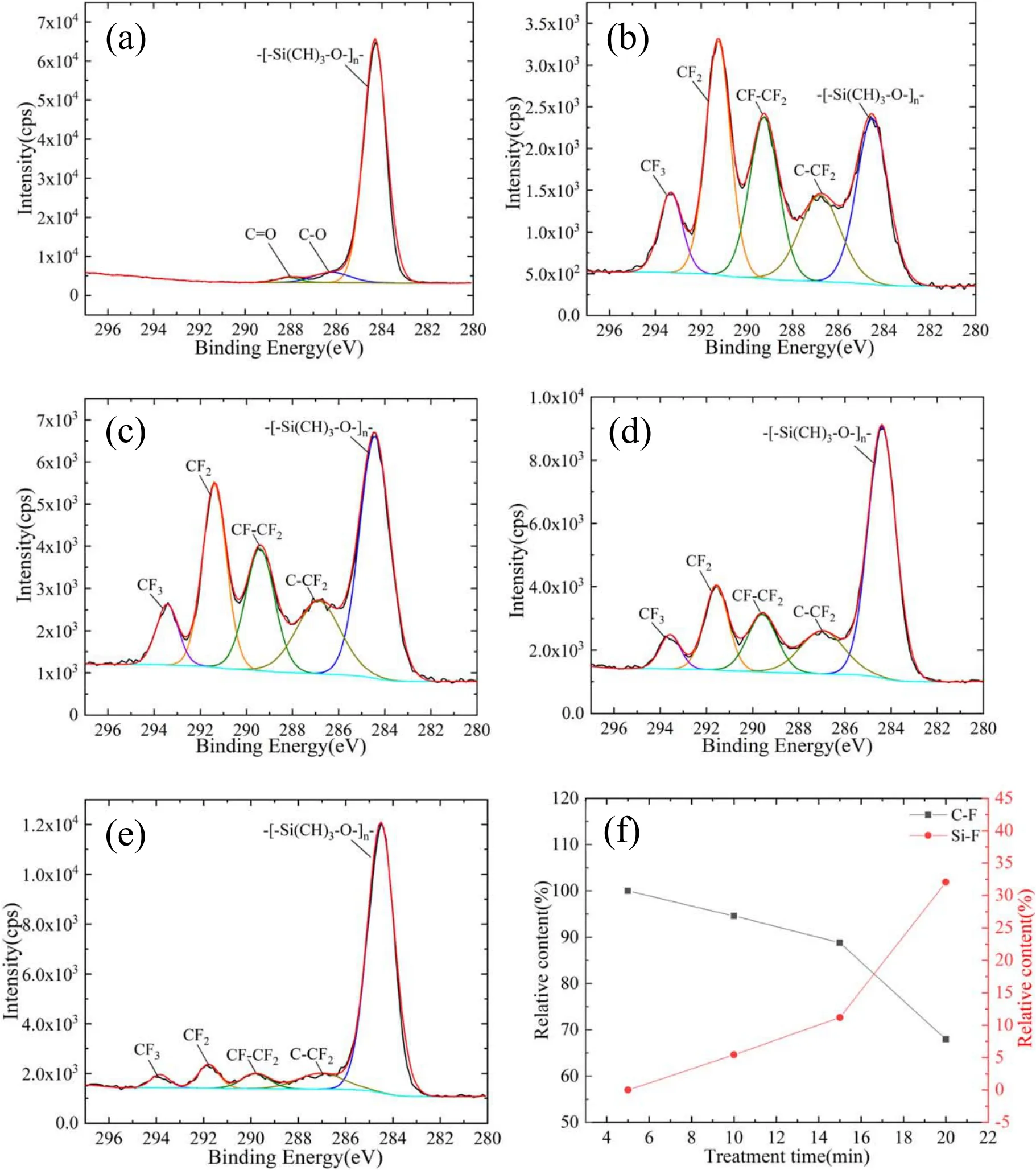
Figure 7.Peak-fitting for C1s of SIR at different treatment time.(a)0 min,(b)5 min,(c)10 min,(d)15 min,(e)20 min,and(f)is the relative content of C-F and Si-F bond.

Figure 8.Surface potential decay curve and trap distribution.
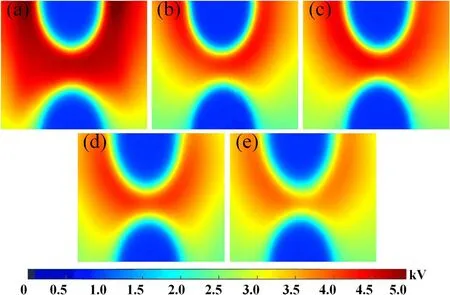
Figure 9.Surface potential distribution under different treatment time.(a)0 min,(b)5 min,(c)10 min,(d)15 min,(e)20 min.The applied voltage is -17 kV and the pulse number N=20.
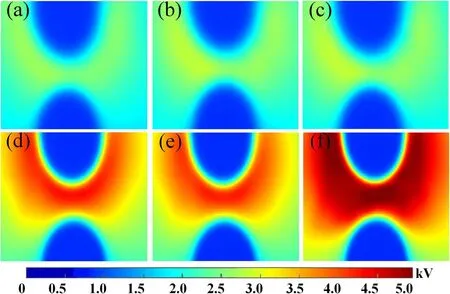
Figure 10.Surface potential distribution under different pulse number N.(a) N=10,(b) N=20,(c) N=30 for the virgin samples.(d)N=10,(e) N=20,(f) N=30 for the samples treated by 20 min.The applied voltage is -14 kV.
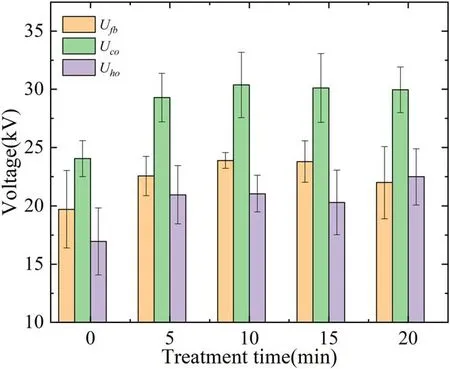
Figure 11.Flashover voltage of SIR samples under different treatment time.
The optical emission spectra of CCP are presented in figure 5.Necessarily,the wavelength of the spectrometer is calibrated by the standard line spectrum of a mercury argon lamp.The continuous spectra ranging from 220 to 380 nm are responsible forandin the UV region and other continuous spectra ranging from 400 to 700 nm are related to CF3[18].Besides,most of the sharp line spectra observed between 650 and 800 nm are from F atoms[19].
According to the results of spectroscopic diagnosis,the main reaction equations involved in CF4CCP are as follows[20]:
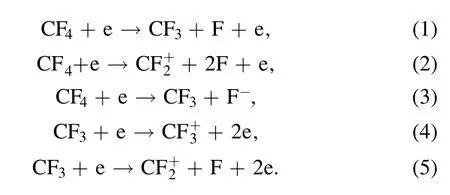
3.2.Surface morphology and chemical composition
The surface morphology of SIR samples treated at different time is demonstrated in figure 6,the surface of virgin samples is relatively smooth without obvious particles and defects in figure 6(a),but the sample surface treated for 5 min has obvious wrinkles and cracks and the texture becomes rough in figure 6(b).When the treatment time comes to 10 min,a large number of micron and sub-micron particles appear on the surface of samples,which is shown in figure 6(c).Furthermore,particle diameter increases with longer treatment time and some small holes can also be observed.Increasing the treatment time to 20 min,dense and uniform micron-sized particles can be found on the surface of samples in figure 6(e),while obvious particle agglomeration can be found.The surface morphology changes can be attributed to plasma bombardment and chemical grafting.Specifically,the chemical interaction and collisions between ion particles and electrons induce ion scattering,the bombardment and strain in different directions will lead to the roughness of wrinkles on samples[21].Besides,the bombardment effect is also helpful for the side-chain cleavage(Si-C bond and C-H bond in Si-CH3),and even breaks the main-chain structure(Si-O bond in[-Si-O-Si-]n).Therefore,the fluorocarbon groups in plasma are more inclined to graft on the sample surface,gradually form the agglomeration of fluorocarbon particles,and more inorganic filler(SiO2/Al(OH)3)existing in the PDMS matrix will be exposed due to the destruction of surface structure.The chemical structure changes will be discussed in the following part.
The surface chemical composition changes on SIR samples treated at different time are shown in table 1.The untreated samples are composed of C,O,and Si,which are roughly consistent with the PDMS matrix and slightly affected by inorganic filler.After 5 min CCP treatment,the content of the F element increases rapidly,while those of the C,O,and Si decrease.This result is attributed to the surface chemical grafting of fluorocarbon groups from CCP plasma.In addition,combining the SEM images displayed above,it can be inferred that the side-chain cleavage caused by plasma bombardment will enhance the grafting process.With further treatment time(10-20 min),small amounts of Al element can be observed and the contents of C and F come to decrease while those of Si,O,and Al increase gradually.This result can come from two aspects.On one hand,the sustained plasma bombardment leads to the ablation of PDMS and accelerates the filler exposure,which introduces many Si,O,and Al elements.On the other hand,the bombardment effect of plasma may cause stripping of the fluorocarbon group grafted on the surface.
In order to further investigate the chemical state of C1s and F1s.The peak-fitting of C1s peak and the relative contents of the C-F and Si-F bond are shown in figure 7.Therelated bonds for C element mainly include C-H,C-O/C-CF2,C=O,CF-CF2,CF2and CF3,and their binding energy is at 284.3 eV,286.6 eV,288.3 eV,289.3 eV,291.0 eV and 293.0 eV respectively[22,23].Simply,the F element only exists in C-F and Si-F.The functional groups on untreated samples mainly include C-H and small amounts of C-O,C=O,the latter may come from impurities pollution.Treated by 5 min,the content of C-CF2/CF-CF2/CF2/CF3dramatically increases and the C-H group decreases in figure 7(b).Notably,the content of C-F is far more than that of Si-F.It can be described that CF3and CF2,as the primary product of CCP,certainly have more chance to graft on the surface,and form the new structure as[-SiFx(CH3)2-x-O-]nor

Table 1.The chemical composition of SIR.
[-SiFx(CFyH3-y)2-x-O-]n(x≤2,y≤3).However,with a longer treatment time,the content of the fluorocarbon group decreases gradually,which demonstrates that the chemical grafting has been inhibited due to the appearance of more inorganic filler.Besides,the Si-CFnstructure is also easily subject to the destruction of plasma bombardment and forms a more stable structure of Si-F,owing to stronger bond dissociation energy in Si-F.Therefore,the Si-F bond has excessed 30% in F elements after 20 min treatment,as illustrated in figure 7(f).
3.3.Surface trap distribution and charge accumulation
The surface potential decay curve of samples treated at different treatment time is shown in figure 8(a).The initial potential is -6 kV,and the surface decay time to 80% of the initial potential needs 736 s,944 s,1749 s,3064 s,and 8765 s respectively.The results demonstrate that the surface charge dissipation can be inhibited after CCP treatment and the increased inhibition effect can be found with the extension of processing time.The decaying rate of the surface potential on samples treated for 20 min is less than 10% of that of the untreated samples.
According to the isothermal surface potential decay model(ISPD)[24],the electron trap distribution on the SIR samples can be calculated simply,it is assumed that the trap distribution is uniform and the de-trapped charge cannot be captured again.The calculation equation of electron trap energy level and electron trap density is as follows:
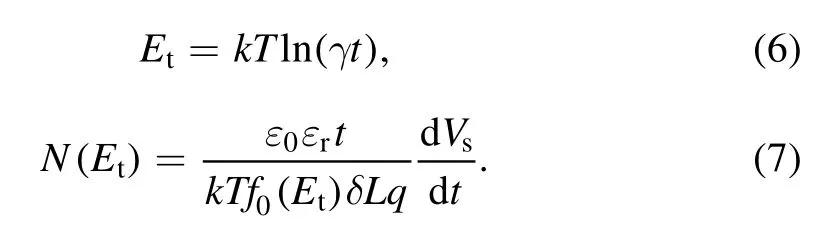
wherekis the Boltzmann constant,Tis the absolute temperature,γ is the escape frequency of a trapped electron,set as 4.17×1013s-1,ε0is the permittivity of the vacuum,εris the relative permittivity of the dielectric,qis Coulomb’s quantity of electron,δ is the thickness of the top charge layer,usually set as 2 mm,Lis the thickness of the sample,and the rate of initial occupancy of traps isf0(Et),which is determined by the injection of electrons.
The surface electron trap distribution of SIR samples treated at different time is shown in figure 8(b).For the virgin samples,the central electron trap energy level is 0.913 eV and the corresponding trap density is 3.38×1021eV-1·m-3.In contrast,the surface central trap energy level and trap density will increase obviously for the treated sample.The central electron trap energy level is at 0.926 and 0.948 eV for the samples treated for 5 and 10 min and their trap density is about 3.5×1021eV-1·m-3.When the treatment time is increased to 20 min,the central trap energy level is more than 1.0 eV and the corresponding trap density can reach 3.5×1021eV-1·m-3.
Previous researchers have demonstrated that the surface electron traps inside polymer are mainly formed by physical defects and chemical defects[25].On one hand,the CCP treatment will make the surface rougher and some electrons can be blocked due to the physical barriers.On the other hand,the grafting of fluorocarbon functional groups has formed many C-F structures with negative electron affinity,which will attract the electron.Therefore,the physicochemical changes on the sample surface increase the electron trap energy level and density.
The surface potential distribution of SIR samples for different treatment time is shown in figure 9.The impulse voltage at -17 kV is applied 20 times on the SIR samples consecutively.It is clearly found that the positive potential has appeared on the regions of the sample surface,which indicates the accumulation of positive charge.For the untreated samples in figure 9(a),high potential can be observed on the whole sample and the maximum potential is 4.8 kV near the cathode.When the treatment time increases,as shown in figures 9(c)-(e),the surface potential begins to reduce and the high potential region is contracted to the cathode area.Especially,the surface potential has decreased to the most extent and the maximum potential is only 3.1 kV after 20 min plasma treatment.
The different number of pulses at the voltage of-14 kV is applied on the samples untreated and treated for 20 min and the surface potential distribution is shown in figure 10.The maximum voltage is 3.9 kV after 10 pulses on the virgin sample surface and it has little change after 20 pulses.However,the surface potential has a rapid rise when the pulse number is increased to 30,distinctly,the maximum potential can reach 5 kV and the surface charge shows an obvious diffusion trend.After plasma treatment for 20 min,the charge accumulation on the surface of treated samples has decreased obviously.The maximum surface potential is 2.3 kV after 10 pulses.With the increase of pulse number,the maximum surface potential hardly changes.
3.4.Surface flashover characteristic
The result of vacuum surface flashover of SIR samples is shown in figure 11.TheUfb,Uco,andUhoare 19.71 kV,24.05 kV,and 16.96 kV respectively for the origin samples.Comparatively,theUfb,Uco,andUhoof treated samples all have been improved significantly.When the treatment time is 10 min,UfbandUcohave increased by 21.2% and 26.67%and reach their maximum of 23.88 and 30.4 kV.With the increase of treatment time,UfbandUcocome to decrease,which may be attributed to the excessive treatment.However,theUhohas some differences fromUfbandUco.Although it has a considerable raise compared to the original value,this voltage does not show a stable trend.This result may be attributed to the insulation damage after multiple flashovers happed on the surface.
4.Discussion
The surface charge accumulation and flashover voltage of SIR samples have been evidently improved by CCP treatment.The results show that plasma treatment has multiple effects on the physical morphology and chemical composition of samples surface.
The cooperating effect and competitive relation both exist between chemical grafting and physical bombardment during the process of CCP treatment on the SIR samples.When the treatment time is shorter than 10 min,the cooperative effect is obvious.Specifically,the plasma bombards the superficial layer and causes the cleavage of side chains of PDMS,which is more conducive for the chemical grafting of fluorocarbon groups.However,with the increase of treatment time,the competitive relation appears gradually,the long-time plasma bombardment can make the stripping of fluorocarbon group grafted on samples.Furthermore,the exposure of fillers can also impede the grafting reaction.Therefore,the surface roughness of SIR samples increases obviously and the relative content of F elements decreases with the increase of plasma treatment time.
Previous studies show that increased roughness and the introduction of C-F bonds can improve the surface flashover voltage[26,27].According to the theory about vacuum flashover,surface flashover starts with primary electrons emitted from the cathode triple junction(CTJ)and is accelerated by the applied external field.The secondary electron emission will be induced by the collision of primary electrons on dielectric and gradually forms the secondary electronic emission avalanche(SEEA)towards the anode.This course will leave positive charges and unleash desorbed gas molecules on the sample surface.When the surface field strength is high enough,gas ionization finally results in the breakdown across the vacuum-insulator interface.Therefore,in this work,the improvement of surface flashover can be considered from two aspects.On one hand,as shown in figure 8(b),the physicochemical changes on samples surface treated by CCP,the grafting of fluorocarbon group,and the increased roughness will increase the electron trap energy level and density.When primary electrons are emitted from CTJ,they can be captured by the fluorocarbon group with strong electron affinity and blocked by the surface barrier,which will inhibit the development of SEEA.
On the other hand,the polarity and distribution of surface charges will change the surface electric field,then alter the trajectory of electrons,and finally affect the surface flashover voltage[28].The accumulation of positive charges near the cathode will strengthen the local electric field,especially the normal electric field,which will introduce secondary electron avalanche and decrease flashover voltage.From figures 9 and 10,the maximum surface potential on the treated samples has decreased by 35.42% compared with the virgin samples and there is no obvious accumulation effect after multiple pulses.Therefore,the decrease of the positive charges will increase the surface flashover voltage.
5.Conclusion
CF4CCP is utilized to improve the surface flashover withstanding strength of SIR.The discharge of CCP is maintained at γ model,where the plasma contains amounts of active particles,as CF+3,CF+2,CF3and F,and has a bombardment effect for the sample surface.The physicochemical changes and the electrical property on the sample surface are investigated and some main conclusion is as follows:
1.The chemical grafting and physical bombardment has increased the content of F on the surface and improved the surface roughness.The short-time bombardment is conducive to the grafting of fluorocarbon groups,but long-time treatment would lead to the striping of fluorocarbon groups.
2.The surface potential decay has been inhibited and the electron trap energy level is increased,which will suppress the emission of primary electrons and the development of SEEA.
3.The surface flashover on SIR samples is not only related to the second electron emission avalanche but affected by the surface charge accumulation.Suppressing the accumulation of positive charge near the cathode will improve the surface flashover voltage.
Acknowledgments
This work is supported by National Natural Science Foundation of China(Nos.11775175,U1766218,51827809)and Natural Science Research Fund of Higher Education of Anhui Province(No.KJ2020A0246).
ORCID iDs
 Plasma Science and Technology2022年2期
Plasma Science and Technology2022年2期
- Plasma Science and Technology的其它文章
- Investigation of short-channel design on performance optimization effect of Hall thruster with large height-radius ratio
- Multi-layer structure formation of relativistic electron beams in plasmas
- Mode structure symmetry breaking of reversed shear Alfvén eigenmodes and its impact on the generation of parallel velocity asymmetries in energetic particle distribution
- Interaction between energetic-ions and internal kink modes in a weak shear tokamak plasma
- Investigation of the compact torus plasma motion in the KTX-CTI device based on circuit analyses
- Anomalous transport driven by ion temperature gradient instability in an anisotropic deuterium-tritium plasma
