Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
WANG Chun-Nong ZENG Ti-Ning LI Sheng-Nn LI Wn② LI Long-Fei CAO Fei YANG Zi-Hui
a (College of Pharmaceutical Sciences, Key Laboratory of Analytical Science and Technology of Hebei Province, Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, Hebei University, Baoding 071002, China)
b (Shan Dong Jinhuahai Biotechnology Co., Ltd Jinan 251400, China)
ABSTRACT A variety of new N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide compounds (8a-i)were synthesized through four steps from benzofuranol as raw materials. The crystal structure of compound 8a(C17H21N3O3S, Mr = 347.43) was measured by X-ray diffraction, which was classified as monoclinic system, Z =4, V = 1742.72(8) ?3, Dc = 1.324 Mg/m3, F(000) = 736, S = 1.03, μ = 0.21 mm-1, space group P21 with a =9.9177(3), b = 8.9519(2), c = 19.8679(5) ?, the final R = 0.035 and wR = 0.105 for 3873 observed reflections (I >2σ(I)). The X-ray structure presented N(3)-H(3)···N(2) and C(6)-H(6)···O(3) intermolecular hydrogen bonds,which acted as an important role in stabilizing the crystal structure. Additionally, preliminary biological assay on compound 8a showed good fungicidal activity in vivo, with the inhibition of 75% against Pseudoperonospora cubensis at 200 mg/L.
Keywords: N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide,preparation, crystal structure, antifungal activity; DOI: 10.14102/j.cnki.0254-5861.2011-3326
1 INTRODUCTION
Dihydrobenzofuran was a basic unit of many bioactive heterocycles, which has been used in medical and agricultural applications, such as antibacterial[1], antileishmanial[2,3],herbicide[4,5], insecticide[6], fungicide[7], etc. Meanwhile, the biological activities of thiadiazole derivatives have been studied in depth, such as antibacterial[8-13], insecticide[14-18],fungicide[19]and anti-inflammatory[20].
Shen and co-workers[21]synthesized seven thiazoles compounds containing 2,3-dihydrobenzofuran (1a-1g) (Fig. 1)and found that compounds 1a and 1f have great inhibition activity (59.8%, 57.9%) againstSclerotonia sclerotiorum,respectively, but have weak activity againstBotrytis cinerea,Alternaria alternata,Rhizoctonia solaniandErysiphe graminis.
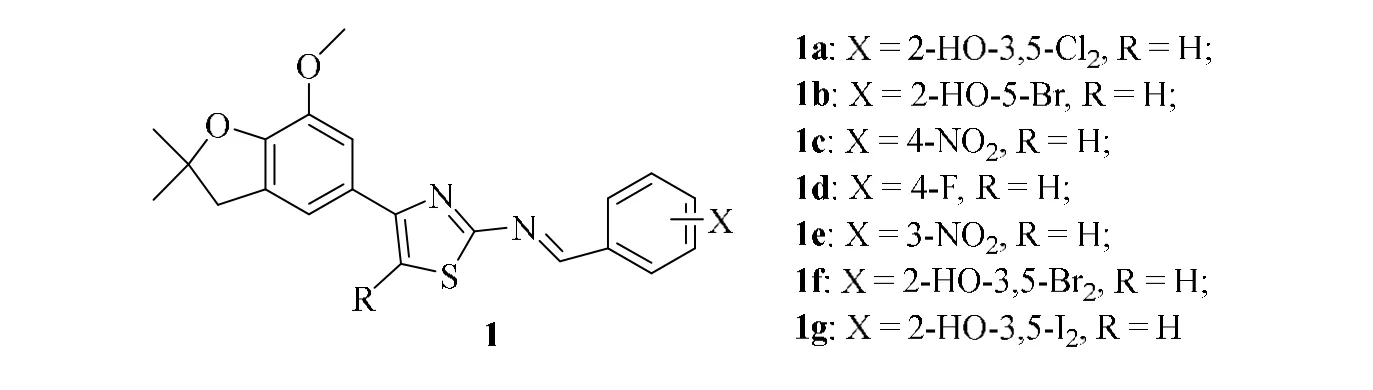
Fig. 1. Compound 1
Zou and co-workers[22]synthesized some novel 1,3,4-thiadiazole compounds (Fig. 2) and determined their fungicidal activity. The result showed that compounds 2m, 2n and 2p have the best fungicidal activity againstPuccinia reconditawith the inhibition of 90%. It could be concluded that with the increase of group hydrophobicity, the fungicidal activity of these compounds increased as well.

Fig. 2. Compound 2
Chen and co-workers[23]synthesized a few of 1,3,4-thiadiazoles compounds (Fig. 3) and evaluated their fungicidal activity. Compounds 3a and 3b had great fungicidal activity againstRhizoctonia solaniwith the inhibition of 90%~99%.

Fig. 3. Compound 3
Both dihydrobenzofuran compounds and 1,3,4-thiadiazole compounds have good fungicidal activity. Based on the active splice principle, a new series of compounds 8 were designed and synthesized by incorporating 1,3,4-thiadiazole into the side chain of 2,2-dimethyl-2,3-dihydrobenzofuran(Scheme 1), the new compounds were expected to achieve great antifungal activity so as to search for broad-spectrum and efficient antifungal drugs, which are of great significance for plant protection. Herein, the synthesis, characterization and bioactivity ofN-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl) amide compounds (8a-i) were reported.The preparation of compounds 8a-i is shown in Scheme 1.
2 EXPERIMENTAL
2. 1 Reagents and instruments
The whole reagents were obtained from professional reagent companies. Fusion points (oC) were tested on an SGWX-4B micromelting point apparatus (Shanghai Precision Instrument Technology Co., Ltd) and unadjusted. Nuclear Magnetic Resonance spectra were tested on a Bruker advanced instrument at 600 MHz and TMS as the interior label. The Bruker AXS SMART APEX II 4000 CCD diffractometer containing a graphite-monochromatic CuKα(λ=0.71073 ?) radiation at 273 K was used for crystal structure determination. Q EXACTIVE Mass Spectrometer (Thermo Fisher Scientific) was used to determine the molecular weight.
2. 2 Preparation
2. 2. 1 Preparation of compound 5
Benzofuranol (4, 9.2 mmol, 1.51 g) was put in DMF slowly at 0oC, then the admixture reacted at rt for 2 h. Additionally, DMF was dehydrated with NaH early. The color of the reaction solution varied from colorless to black. Ethyl chloroacetate (10.9 mmol, 1.34 g) and the appropriate amounts of KI were added to the mixture and refluxed for 7 h. Until the end of the reaction, the mixture was poured into crushed ice water and then extracted by AcoEt and washed by saturated brine. The obtained organic mixture was dried and evaporated. Then obtained coarse product was separated and purified to give compound 5 with yield of 89%.
2. 2. 2 Preparation of compound 6
Compound 5 (12 mmol, 3.00 g) and 20% NaOH solution(12 mL) in ethanol were reacted for 24 h at 60oC. Afterwards, the reaction solution was conditioned to be acidic (pH 1.5) by concentrated hydrochloric under an ice bath. Then the white solid obtained was filtered, washed by distilled water and freeze-dried for 5 h to give compound 6 with a yield of 89%.
2. 2. 3 Preparation of compound 7
Compound 6 (4.5 mmol, 1.00 g) and POCl3(22.5 mmol,3.45 g) were put in dioxane under an ice bath and then reacted for 0.5 h. And thiosemicarbazide (9 mmol, 0.82 g) was mixed lately. 2 h later, an appropriate amount of water was mixed in and the reaction solution was continued to reflux for 7 h. Until the end of the reaction, the mixture was cooled and adjusted to alkalinity (pH 11) by concentrated ammonia to get the crude product. After that, it was filtered, washed by water and freeze-dried for 5 h, then compound 7 was recrystallized from anhydrous EtOH with the yield of 87%.
2. 2. 4 Preparation of compounds 8a-i
When it was my turn, I sat with them at the little banquet table we had created in the waiting room. We talked for a while about dreams. The four children were telling me about what they wanted to be when they grow up. The six?year?old started the conversation. I want to be a nurse and help people, she declared.
Compound 7 (0.36 mmol, 0.10 g) and butyryl chloride(0.4 mmol, 0.04 g) in anhydrous DCM were added under an ice bath and then reacted for 0.5 h at rt. And pyridine (0.36 mmol, 0.03 g) was mixed lately. Until the end of the reaction,DCM was evaporated. Afterwards, the reaction solution was conditioned to be acidic by dilute hydrochloric, and the residue was extracted by AcoEt. The combined organic mixture was dried. Finally, AcoEt was evaporated, and the obtained coarse product was separated and purified to giveN-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide compounds (8a-i).
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-butyramide (8a): Yield 72%, m.p. 162~165oC.1H NMR(600 MHz, CDCl3)δ: 13.14 (s, 1H), 6.83 (dd,J= 14.5, 7.7 Hz, 2H), 6.71 (t,J= 7.7 Hz, 1H), 5.52 (s, 2H), 3.02 (s, 2H),2.71 (t,J= 7.4 Hz, 2H), 1.82 (dd,J= 14.7, 7.4 Hz, 2H), 1.52(s, 6H), 1.03 (t,J= 7.4 Hz, 3H).13C NMR (151 MHz,CDCl3)δ: 172.13, 162.04, 161.84, 148.19, 142.29, 129.32,120.49, 119.36, 114.97, 88.01, 66.14, 43.28, 38.18, 28.37,19.02, 13.82. HRMS-ESI m/z calcd. for C17H21N3O3S[M+H]+348.1376, found 348.1369.
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-benzamide (8b): Yield 74%, m.p. 203~205oC.1H NMR(600 MHz, CDCl3)δ: 11.54 (s, 1H), 8.14 (d,J= 7.7 Hz, 2H),7.64 (d,J= 7.3 Hz, 1H), 7.54 (t,J= 7.8 Hz, 2H), 6.84 (dd,J= 17.9, 7.7 Hz, 2H), 6.72 (t,J= 7.7 Hz, 1H), 5.55 (s, 2H),3.04 (s, 2H), 1.53 (s, 6H).13C NMR (151 MHz, CDCl3)δ:165.06, 162.64, 155.68, 147.79, 142.37, 133.52, 131.31,129.35, 129.16, 128.36, 120.56, 119.40, 115.01, 88.05, 66.32,43.32, 28.41. HRMS-ESI m/z calcd. for C20H19N3O3S[M+Na]+404.1039, found 404.1029.
4-Chloro-N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)benzamide (8c): Yield 67%, m.p. 215~217oC.1H NMR (600 MHz, DMSO)δ: 8.12 (d,J= 8.4 Hz, 2H), 7.46(d,J= 8.4 Hz, 2H), 6.90 (d,J= 8.1 Hz, 1H), 6.81 (d,J= 7.3 Hz, 1H), 6.71 (t,J= 7.7 Hz, 1H), 5.32 (s, 2H), 2.99 (s, 2H),1.42 (s, 6H).13C NMR (151 MHz, DMSO)δ: 167.83, 166.58,156.37, 147.32, 142.34, 136.78, 134.92, 130.01, 128.51,127.73, 120.13, 118.26, 114.09, 87.03, 65.62, 42.43, 27.87.HRMS-ESI m/z calcd for C20H18ClN3O3S [M+H]+416.0830,found 416.0821.
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-acetamide (8d): Yield 82%, m.p. 230oC carbonization.1H NMR (600 MHz, CDCl3)δ: 11.71 (s, 1H), 6.85~6.80 (m,2H), 6.71 (t,J= 7.7 Hz, 1H), 5.52 (s, 2H), 3.03 (s, 2H), 2.40(s, 3H), 1.52 (s, 6H).13C NMR (151 MHz, DMSO)δ:170.46, 158.55, 147.38, 142.04, 128.74, 120.23, 118.65,114.34, 87.24, 65.15, 42.40, 27.88, 23.49. HRMS-ESI m/z calcd. for C15H17N3O3S [M+H]+320.1063, found 320.1056.
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-propionamide (8e): Yield 79%, m.p. 188~190oC.1H NMR(600 MHz, CDCl3)δ: 12.76 (s, 1H), 6.82 (t,J= 8.0 Hz, 2H),6.71 (t,J= 7.7 Hz, 1H), 5.52 (s, 2H), 3.03 (s, 2H), 2.73 (q,J= 7.5 Hz, 2H), 1.52 (s, 6H), 1.30 (t,J= 7.5 Hz, 3H).13C NMR (151 MHz, CDCl3)δ: 172.69, 162.07, 161.87, 148.20,142.27, 129.34, 120.51, 119.39, 115.06, 88.03, 66.17, 43.29,29.58, 28.38, 9.28. HRMS-ESI m/z calcd. for C16H19N3O3S[M+H]+334.1220, found 334.1210.
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-4-methoxybenzamide (8g): Yield 78%, m.p. 192~194oC.1H NMR (600 MHz, CDCl3)δ: 8.16 (d,J= 8.7 Hz, 2H), 7.01 (d,J= 8.7 Hz, 2H), 6.86 (d,J= 8.1 Hz, 1H), 6.82 (d,J= 7.3 Hz,1H), 6.71 (t,J= 7.7 Hz, 1H), 5.55 (s, 2H), 3.89 (s, 3H), 3.03(s, 2H), 1.52 (s, 6H).13C NMR (151 MHz, CDCl3)δ: 164.65,163.90, 162.32, 148.25, 147.23, 142.42, 130.65, 129.34,123.54, 120.55, 119.38, 115.07, 114.37, 88.01, 66.39, 55.69,43.33, 28.39. HRMS-ESI m/z calcd. for C21H21N3O4S[M+Na]+434.1145, found 434.1136.
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-pivalamide (8h): Yield 79%, m.p. 167~169oC.1H NMR(600 MHz, CDCl3)δ: 9.29 (s, 1H), 6.82 (dd,J= 12.9, 7.8 Hz,1H), 6.71 (t,J= 7.7 Hz, 1H), 5.53 (s, 2H), 3.02 (s, 2H), 1.55(s, 9H), 1.34 (s, 6H).13C NMR (151 MHz, CDCl3)δ: 177.51,162.41, 161.39, 147.96, 142.23, 129.03, 120.34, 119.04,114.48, 114.45, 87.78, 65.81, 43.17, 39.54, 28.26, 27.07.HRMS-ESI m/z calcd. for C18H23N3O3S [M+H]+362.1533,found 362.1524.
N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)-cyclohexanecarboxamide (8i): Yield 84%, m.p. 183~186oC.1H NMR (600 MHz, CDCl3)δ: 12.99 (s, 1H), 6.83 (dd,J=24.1, 7.7 Hz, 2H), 6.71 (t,J= 7.8 Hz, 1H), 5.52 (s, 2H), 3.02(s, 2H), 2.82 (tt,J= 11.6, 3.3 Hz, 1H), 1.98 (d,J= 11.8 Hz,2H), 1.83 (dd,J= 10.2, 3.1 Hz, 2H), 1.73 (d,J= 13.0 Hz,1H), 1.58 (ddd,J= 24.8, 12.7, 3.2 Hz, 2H), 1.52 (s, 6H),1.49~1.41 (m, 2H), 1.34~1.25 (m, 1H).13C NMR (151 MHz,CDCl3)δ: 175.17, 162.23, 161.59, 148.16, 142.37, 129.26,120.45, 119.27, 114.65, 87.98, 66.07, 44.83, 43.31, 29.35,28.38, 25.76, 25.59. HRMS-ESI m/z calcd. for C20H25N3O3S[M+H]+388.1689, found 388.1681.
2. 3 X-ray structure determination
Compound 8a was dispersed in the mixture of AcoEt and MeOH (AcoEt:MeOH = 3:1) and kept for self-volatilization.The colorless crystals were obtained after 5 days with dimensions of 0.27mm × 0.23mm × 0.07mm. A total of 9905 reflections were collected within limits of 2.50°<θ<28.29°,4316 were independent (Rint= 0.0300) and 3873 were considered to be observed (I> 2σ(I)) and used for subsequent refinement. SADABS was used to correct absorption effects of incident and diffracted beams. The structure was solved directly bySHELXL-2018/2and Fourier difference technique was uesed to expand. All the hydrogen atoms were placed in the theoretical positions, and all the non-hydrogens were asymmetrically refined. The crystal structure was refined through full-matrix least-squares techniques onF2withSHELXL-2018/3[24]. All the reference data were as follows:

2. 4 Antifungal activity assay
Compounds 8a-i were tested for the control ofPseudoperonospora cubensis(Pseudoperonospora c.) andColletrichum orbiculare(Colletrichum o.) on the first true leaf of cucumber that was fully unfolded. In addition, compounds 8a-i were also tested for the control ofErysiphe graminis(Erysiphe g.) on wheat leaves at the third-leaf stage. Compounds 8a-i and the control drugs, cyazofamid and azoxystrobin were formulated in water (containing 0.1% Tween 80)to 200 mg/L solutions, which were sprayed to cucumber or wheat leaves using a three-dimensional crop sprayer with spray bulk of 1×103dm3/hm2and spray pressure of 1.5 kg/cm2. The treated plants were dried naturally in the shade for 24 h. The cucumber leaves were inoculated with the spore suspensions ofPseudoperonospora c.andColletrichum o.(3-5×106/mL) and then transferred to artificial climate chamber for cultivation (24±1oC, RH>90, no light). 24 h later, the plants were managed for normal in the greenhouse. Additionally, the wheat leaves were cultivated with the spores ofErysiphe g.and cultured in greenhouse.The antifungal activities of compounds 8a-i were evaluated 5~7 days after treatment. The results of survey consultedAManual of Assessment Keys for Plant Diseasescompiled by American Society of Plant Diseases for studying the fungicidal activity about compounds 8a-i. It was represented by 100-0, with "100" representing disease-free and "0" representing the most severe degree of disease.

Table 1. Part of Bond Distances (?) and Angles (°) of 8a
3 RESULTS AND DISCUSSION
In this study, 8a-i were prepared and their structures were confirmed by HRMS and NMR. Additionally, the structure of compound 8a was characterized through single-crystal X-ray diffraction, which was classified as monoclinic system with pace groupP21. The perspectives of compound 8a with atomic numbering scheme are given in Fig. 4. The part of bond distances and bond angles are showed in Table 1.
As showed in Fig. 4, there were two planes in the crystal structure of 8a: the phenyl ring (C(3)-C(4)-C(5)-C(6)-C(7)-C(8)) and the 1,3,4-thiadiazole ring (N(1)-N(2)-C(11)-S(1)-C(10)), and the dihedral angle between them is 46.2°.

Fig. 4. Crystal structure of compound 8a with atom labels
As outlined in Fig. 4 and Table 1, the bond distances of C(7)-O(2) and C(8)-O(1) are 1.3731(14) and 1.3634(14) ?,which are much shorter than the ordinary value of C-O bond(1.43 ?), because the lone-pair electrons of O(2) and O(1)are conjugated with theπbonds of the benzene ring and C(8)-O(1) is involved in the dihydrobenzofuran ring. The C(11)-N(3) and C(12)-N(3) bond distances are 1.3664(16)and 1.3671(16) ?, much shorter compared to the typical C-N bond (1.47 ?) as the lone-pair electrons of N(3) are conjugated with theπbonds of the thiadiazole ring and the carbonyl group. The bond distances of C(10)-S(1) and C(11)-S(1) are 1.7293(13) and 1.7216(11) ? severally, also shorter than the common value of C-S single bond (1.82 ?)due to the involvement of C(10)-S(1) and C(11)-S(1) in the formation of thiadiazole rings.

Fig. 5. Hydrogen bonding diagram
As shown in Fig. 5 and Table 2, an intermolecular hydrogen bond N(3)-H(3)···N(2) forming a one-dimensional chain structure can be seen between the amino and thiadiazole groups, the angle and distance of which are 176.7° and 2.9029(14) ? separately. And a weak V-type hydrogen bond is formed through intermolecular hydrogen bond C(6)-H(6)···O(3) between the benzene and carbonyl groups with the distance of 3.4059(16) ? and angle of 141°. The intermolecular hydrogen bonds played a significant role in stabilizing the structure.
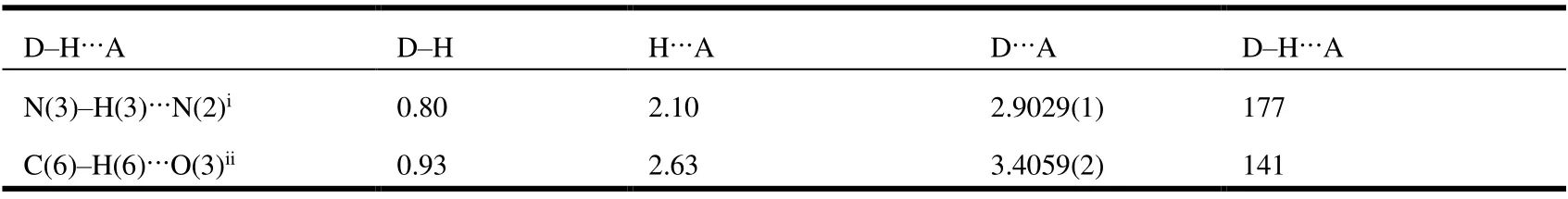
Table 2. Hydrogen Bond Distances and Angles of Compound 8a (? and °)
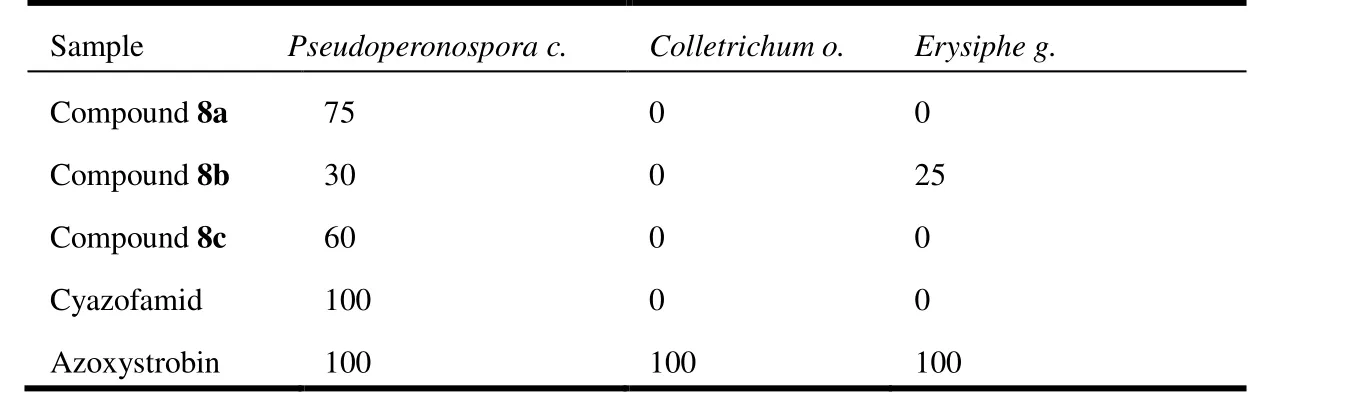
Table 3. Control Effect of Compounds 8 on Plant Pathogenic Bacteria (%, 200 mg/L)
According to antifungal activity assay, compounds 8a and 8c exhibited good fungicidal activity againstPseudoperonospora c.with the inhibition of 75% and 60% respectively,but had no inhibition activity againstErysiphe g. Additionally, compound 8b showed weak activity againstPseudoperonospora c.andErysiphe g., whereas none of compounds had activity againstColletrichum o.The compound that was substituted byn-propyl had the best antifungal activity againstPseudoperonospora c.When the substituted group was phenyl, the compound possessed weak antifungal activity. The antifungal activity of the compound was increased againstPseudoperonospora c., on account of the electron absorbing impact of thep-substituted phenyl group.If thep-substituted phenyl groups were electron-absorbing groups (-CH3and -OCH3), the antifungal activity of the compound would be lost. Therefore, 1,3,4-thiadiazol compounds 8a-c possessed good selectivity against fungal activity, and it was of great significance for further study of these compounds.
4 CONCLUSION
In summary, a new type of 1,3,4-thiadiazole compound with dihydrobenzofuran was prepared and characterized by spectroscopy, and the structure of compound 8a was confirmed by single-crystal X-ray diffraction. The antifungal activity assay showed that compounds 8a-c had certain activity against plant pathogenic bacteriain vivo.
- 結構化學的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①
- Manganese(II) and Copper(I) Compounds Based on Two Derivatives of Imidazo[1,5-a]pyridine: Synthesis,Structures, Magnetic Properties, and Catalytic Activity①

