Visible light-driven oxidant-free dehydrogenation of alcohols in water using porous ultrathin g-C3N4 nanosheets
Wei Zhang,Jiajun Wang,Zewei Liu,Yibing Pi,Rong Tan
National & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources,Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research(Ministry of Education),College of Chemistry and Chemical Engineering,Hunan Normal University,Changsha,410081,China
Abstract Graphitic carbon nitride(g-C3N4)is a fascinating photocatalyst for solar energy utilization in photo-catalysis.Nevertheless,it often suffers from moderate photo-catalytic activity due to its low specific surface area and fast recombination rate of photogenerated electrons upon photo-excitation.Herein,we overcome the bottlenecks by constructing a porous g-C3N4 nanosheet (PCNS) through a simple thermal oxidation etching method.Benefited from its porous layer structure,the obtained PCNS exhibits large specific surface area,efficient separation of photogenerated charge carriers,as well as high exposure of active sites.As a result,it is robust and universal in visible light-driven dehydrogenation of alcohols in water under oxidant-free condition.Almost quantitative yields(>99%)of various valuable carbonyl compounds were obtained over PCNS,while bulk g-C3N4 was far less efficient.Moreover,the photo-catalyst was highly stable and could be facilely recovered from the aqueous system for efficient reuse.The easy preparation and excellent performance made PCNS a promising and competitive photocatalyst for the solar applications.
Keywords: Photo-catalysis;Porous g-C3N4 nanosheets;Visible light irradiation;Oxidant-free dehydrogenation;Alcohols
1.Introduction
The efficient use of solar energy in chemical transformation has been considered as an environmentally friendly and energy sustainable strategy in response to energy and environmental issues.Photo-catalysis is of great potential in realizing this strategy.Apart from using solar light as the only energy input,this approach often makes chemical transformations efficient under mild conditions (room temperature and/or atmospheric pressure),which was a benefit for energy-saving and industrial applications [1-3].Dehydrogenation of alcohols under oxidant-free condition is an important transformation in chemical industry not only due to the atom-economic production of value-added carbonyl compounds,but also because it provides an opportunity to release H2from alcohols [4-7].Several semiconductor materials,such as titanium (IV) dioxide [8,9],have proven efficiency in this transformation.However,the wide band gaps (ca.3.2 eV) make them only absorb ultraviolet light [10],which accounts for only 5% of the total solar energy.It is undesirable for the practical application of solar energy in photochemical processes.Integration of nanoscale plasmonic metals (gold,palladium,or nickel,etc.) with the semiconductors could expand the absorption edge of photo-catalysts into visible region [8,11-14].But the employment of metals was also unsatisfied for industrial applications since this potentially led to product contamination in addition to an increase in process cost.The design of a metal-free photo-catalyst that efficiently promotes dehydrogenation of alcohols under visible light irradiation is desired.To our knowledge,such a catalytic system has rarely been reported to date.
As a metal-free polymer semiconductor,g-C3N4has recently been drawn much attention in the field of visible light-driven catalysis due to its ease of preparation,moderate band gap(~2.7 eV),and favorable chemical stability[15-18].However,its catalytic efficiency is often limited by the low specific surface area(ca.8 m2g-1),fast charge recombination,and poor visible light harvesting[19,20].Nanostructure design of g-C3N4has rapidly developed in recent years to solve these shortcomings.Among them,two-dimensional (2D) g-C3N4nanosheets have received much attention because of its enlarged specific surface area which increased surface-active sites and light harvesting [16,21-23].Furthermore,the 2D layered structure possessed more charge carriers with respect to the bulk counterpart [16],which inherently boosted the photo-catalysis.Porous engineering was also proved to boost the photo-catalytic activity of g-C3N4.With a narrower bandwidth than bulk g-C3N4,porous g-C3N4could broaden the visible light absorption range [19,24-26].More importantly,the featured porous structure shortened the diffusion length of photoexcited charge carriers [27-29],and enhanced the light harvesting due to multiple scattering effects [15].Indeed,mesoporous g-C3N4(mpg-C3N4) has been reported to improve the photo-catalytic efficiency by 10 times as compared with bulk g-C3N4in visible light-driven water splitting [15].Inspired by respective superiorities of 2D nanosheet and porous g-C3N4,we decided to introduce pore structure into g-C3N4nanosheets to make oxidant-free dehydrogenation of alcohols efficient under visible light irradiation.
Herein,the porous g-C3N4nanosheet ofPCNSwith an average pore diameter of ca.24.3 nm and thickness of ca.5.0 nm were synthesized through thermal polymorization of dicyandiamide and followed thermal oxidation “etching” of the bulk g-C3N4in atmosphere.Compared with bulk g-C3N4,PCNSpossessed a larger surface area (182.4 m2g-1),improved efficiency of charge transfer,and accelerated separation of photogenerated electron-hole pairs.As a consequence,it behaves as the efficient photo-catalyst in visible light-driven oxidant-free dehydrogenation of alcohols in water,giving a wide range of carbonyl compounds with almost quantitative yields (>99%),while,bulk g-C3N4was far less efficient.Moreover,as a heterogeneous photo-catalyst,PCNScould be facilely recovered and reused for at least seven times without loss of efficiency.This approach used solar light as the energy input,and enabled the dehydrogenation of alcohols under oxidant-free condition at room temperature in water,which is of great interest from the viewpoint of energy savings and sustainable chemical engineering.
2.Experimental section
2.1.Materials and reagents
Dicyandiamide was purchased from Sigma-Aldrich.Benzyl alcohol,2-phenylethanol,diphenylmethanol,4-methoxybenzyl alcohol,4-bromobenzyl alcohol,4-chlorobenzylalcohol,4-methylbenzyl alcohol and 2-methylbenzyl alcohol were obtained from Aladdin.Other Common reagents were available from local suppliers.All of solvents were purified by standard procedures.
2.2.Analytical method
Fourier transform-infrared (FT-IR) spectra of the samples were recorded on AVATAR 370 Thermo Nicolet spectrophotometer with a resolution of 4 cm-1.X-ray photoelectron spectroscopy (XPS) spectra were conducted on a Thermo ESCALAB 250XI electron spectrometer and calibrated by C 1s binding energy at 286.8 eV.X-ray diffraction (XRD)measurements were performed on a Philips X'PERT-Pro-MPD diffractometer using Cu Kαradiation (λ=1.5418 ?).Elemental analyses of nitrogen and carbon were carried out on Elementar Vario EL cube.Morphology of samples was observed using a transmission electron microscope (TEM,Microscope JEM-2100 F)and atomic force microscope(AFM,Bruker Dimension ICON).Nitrogen adsorption-desorption isotherms were obtained at -196°C on a Micromeritics Tristar 3000 analyzer.All samples were degassed under 1.33×10-4Pa at 80°C overnight prior to measurements.UVvis diffuse reflectance spectra(UV-vis DRS)were recorded by Cary 7000 spectrometer equipped with an integrating sphere.Photoluminescence (PL) spectra of samples were taken on F-4600 fluorescence spectrometer (Hitachi,Japan) under an excitation of 318 nm.Fluorescence spectra of the supernatant liquid were measured with a fluorescence spectrophotometer(Shimadzu RF-5300PC).An aqueous solution containing 3 mmol L-1terephthalic acid (TA) was prepared,and thenPCNS(50 mg)was suspended in this TA solution(3.0 mL)in a 1 cm×1 cm Pyrex glass cell.The sample cell was placed on the magnetic stirrer in the dark box,and the suspension was stirred at 25°C under the visible light irradiation(λ >420 nm).These experiments were performed for each sample irradiated at various periods,that is 1,2,3,4 and 5 h.Photocurrent-time curves were recorded on a CHI-660 E(Shanghai Chenhua) electrochemical workstation with a standard three-electrode cell setup under a periodic visible light illumination (Xe lamp,300 W,Perfectlight,China).Ag/AgCl electrode and platinum plate were used as the reference and counter electrodes,respectively,while the working electrodes were prepared by coating the test samples on an indium-tin oxide glass according to a typical slurry method[20].Na2SO4aqueous solution (0.5 mol L-1) was served as the electrolyte during the photoelectrochemical test.Electrochemical impedance spectroscopy(EIS)spectra were recorded in the frequency range from 0.05 Hz to105Hz at a sinusoidal ac perturbation of 5 mV.
2.3.Preparation of PCNS
ThePCNSwas synthesized through a two-step approach including thermal polymorization of dicyandiamide to bulk g-C3N4and the followed thermal oxidation etching of the obtained bulk g-C3N4in static air,as shown in Scheme 1.
Synthesis of bulk g-C3N4.Dicyandiamide (0.12 mol,10 g)was heated at 550°C in a tube furnace for 4 h with a heating rate of ca.2.3°C min-1,and then cooled to room temperature.The resultant agglomerates were milled into powder in a mortar,giving bulk g-C3N4as a yellow powder.FT-IR (KBr):3418,3155,3077,2920,1636,1559,1541,1459,1405,1317,1237,1206,1134,889,807 cm-1.Nitrogen content:60.96 wt%,carbon content: 34.23 wt%.
Synthesis of PCNS.Bulk g-C3N4(0.4 g) was placed in an open ceramic container and was heated at 520°C for 4 h in muffle with a heating rate of 10°C min-1.After cooled to room temperature,thePCNSwas obtained as a light yellow powder.The volume ofPCNSwith the same weight is almost 10 times larger than that of bulk g-C3N4,as shown in Scheme 1.It indicated a fluffy state of the delaminated nanosheets.FT-IR(KBr):3418,3253,3167,3088,1636,1575,1541,1458,1411,1320,1244,1209,1134,1011,890,810 cm-1.Nitrogen content: 57.48 wt%,carbon content: 33.79 wt%.
2.4.General procedure for oxidant-free dehydrogenation of alcohols
The oxidant-free dehydrogenation reaction of alcohols was carried out in a cylindrical quartz vessel equipped with a Xenon lamp (300 W,PLS-SXE300).Catalyst (50 mg) was dispersed into a mixture of alcohols (0.1 mmol) and water(5 mL) in the vessel.The system was purged with an argon flow for at least 30 min before irradiation to ensure the removal of oxygen.Reaction mixture was then stirred at 25°C under the visible light irradiation (λ >420 nm).GC analysis was employed to monitor the photo-catalytic dehydrogenation progress.After reaction,the reaction solution was extracted with ethyl acetate (3 × 10 mL),and analyzed by the GC-6890 N Agilent gas chromatograph equipped with a flame ionization detector (FID).PCNScatalyst was separated by centrifugation,washed with ethyl acetate,and dried under vacuum for the recycling experiment.
3.Results and discussion
3.1.Preparation of catalyst
Visible light-driven photo-catalysis,in which solar light is used as the energy input,is highly desirable for chemical transformation.The g-C3N4which possesses a narrow band gap energy (~2.7 eV) is an attractive photo-catalyst for the visible light-driven systems [15-18].However,the small specific surface area,as well as high electron-hole recombination rate,often limits its catalytic efficiency [19,20].Constructing g-C3N4with a 2D layered structure is recognized as an effective method for enhancing the catalytic performance,since it dramatically enlarges the surface area,increases the number of surface reactive sites,and especially,prolongs the charge carrier lifetime [21-23].Porous g-C3N4is also favorable for the visible light-driven photo-catalysis.Apart from facilitating mass transfer,the porous structure also enhances the light harvesting ability thanks to its large surface and multiple scattering effects[24-26].With those points in mind,we decided to construct porous ultrathin g-C3N4nanosheets ofPCNSto realize the visible light-driven oxidant-free dehydrogenation of alcohols in water.
The synthesis ofPCNSwas illustrated in Scheme 1.Bulk g-C3N4was first synthesized by thermal polymerization of dicyandiamide precursor at 550°C for 4 h.It underwent subsequent thermal oxidation “etching” at 520°C for 4 h in static air,giving thePCNSas light yellow powder.Most hydrogen-bond cohered strands of polymeric melon units in the layers (particularly for short ones) were not stable enough against (or reactive to) oxidation process in air during the“etching” process.They were thus gradually oxidized away from the bulk material [27,28],resulting in the exfoliation of bulk g-C3N4into 2D nanosheets.Additionally,the thermal etching treatment also generates gases,such as H2O and CO2,which probably act as bubble-soft-template and give rise to porous g-C3N4products,as reported in Ref.[29].

Scheme 1.Schematic illustration of synthetic strategy to PCNS.

Scheme 2.Proposed mechanisms for the visible light-driven oxidant-free dehydrogenation of alcohols in water over PCNS.
3.2.Composition and morphology of PCNS
The chemical compositions ofPCNSand bulk g-C3N4were investigated by FT-IR spectra,as shown in Fig.1a.As expected,PCNSshows the characteristic breathing modes of aromatic CN hetero cycles at 810 cm-1,typical stretching modes of C=N in triazine ring at 1636 cm-1,as well as the stretching mode of N-H at 3418 cm-1[22,26,30].These characteristic bands are similar to that of bulk g-C3N4,which implies that the featured graphitic structure is remained after the thermal oxidation treatment.It is worth noting that obvious signals associated with C-O and N-O vibration are not detected inPCNS,suggesting the negligible O-containing group onPCNSsurface.

Fig.1.FT-IR spectra (a),XPS survey spectra (b),high-resolution N 1s spectrum (c),and C 1s spectrum (d) of bulk g-C3N4 and PCNS.
XPS was employed to gain further insights into surface chemical changes during the thermal oxidation treatment(Fig.1b-d).Bulk g-C3N4displays C 1s,N 1s,and O 1s signals in the survey spectrum(Fig.1b).The oxygen signal may come from adsorbed H2O on the g-C3N4surface [30],and it almost disappears in the XPS survey spectrum ofPCNSdue to the high temperature treatment in open atmosphere.Apart from the O 1s single,the XPS survey spectrum ofPCNSis similar to that of bulk g-C3N4(Fig.1b).It suggests that the chemical states of both carbon and nitrogen elements inPCNSare the same as that in the bulk g-C3N4.While,we noticed that,the surface atomic ratio of N to C decreases from 1.31 for bulk g-C3N4to 1.28 forPCNS,suggesting the preferential loss of nitrogen atoms during the thermal oxidation “etching” process.High-resolution N1s XPS spectra of bulk g-C3N4andPCNSprovide further information for the loss of nitrogen atoms (Fig.1c).Clearly,the N 1s peaks are observed around 398.5,399.6,and 401.1 eV,which are assigned to sp2-bonded N in triazine rings (C=N-C),the bridging N atoms in N(-C)3and terminal NH2groups,respectively[22,31,32].Upon thermal oxidation“etching”,the terminal NH2groups decrease(Fig.1c),indicating preferentially lost of NH2groups over N2cand N3catoms during the thermal oxidation process.The loss of nitrogen atoms may introduce N-vacancy inPCNS,which accelerates photogenerated charge-carrier separation,and thus increasing the photo-catalytic activity of g-C3N4[33,34].Furthermore,the C 1s XPS spectrum ofPCNSis similar to that of bulk g-C3N4,which displays a typical N-C=N peak at 288.1 and the graphitic carbon peak at 284.7 eV (Fig.1d)[22,35].The observations are consistent with the FT-IR results that the featured graphitic structure is remained inPCNSalthough the N-vacancy may be introduced upon thermal oxidation treatment.
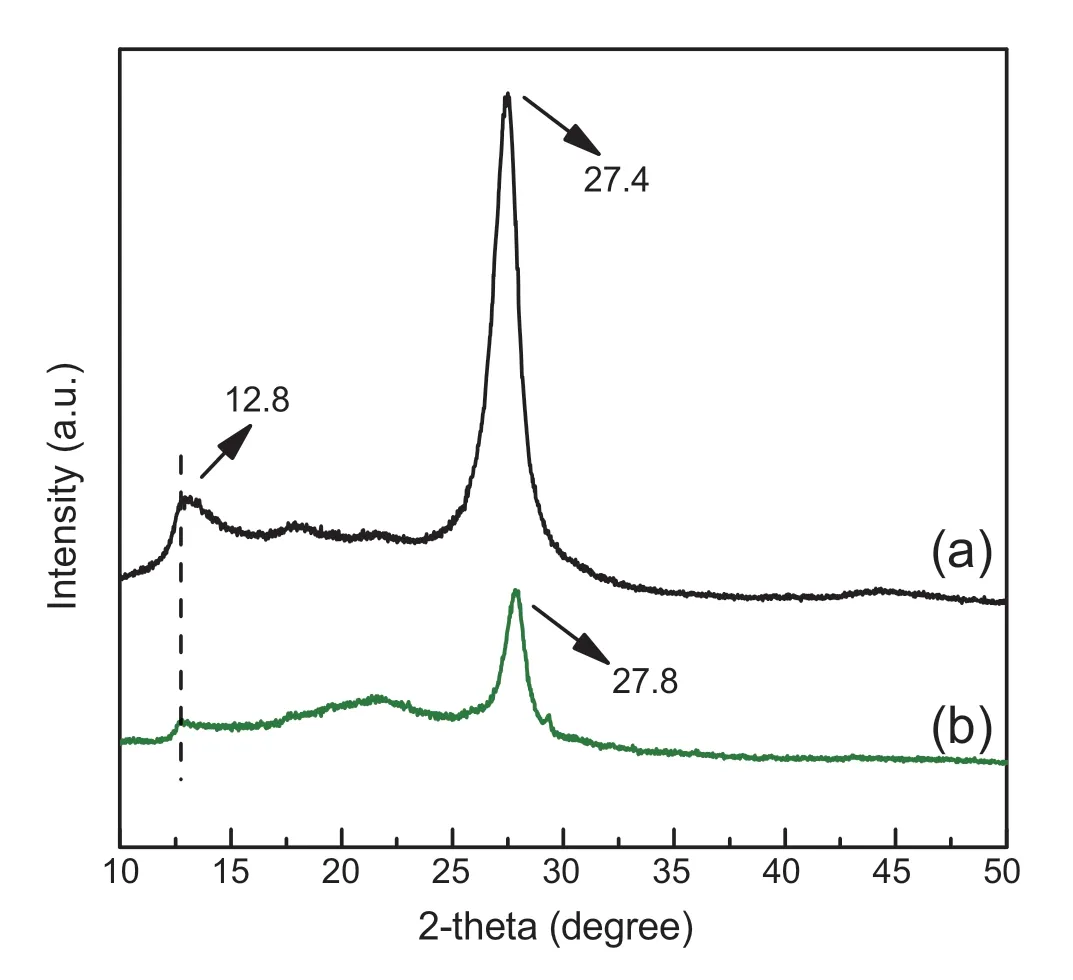
Fig.2.PXRD patterns of bulk g-C3N4 (a) and PCNS (b).
PXRD patterns were used to study the structural change of g-C3N4during thermal oxidation“etching”process.As shown in Fig.2,bulk g-C3N4exhibits two main characteristic diffraction peaks at around 12.8°and 27.4°(Fig.2a),which are assigned to the (100) and (002) diffraction planes of g-C3N4,respectively [20,22].The former peak (100) represents the in-plane packing of tri-s-triazine units,whereas the later(002) is attributed to the interlayer stacking of the conjugated aromatic system [36,37].They are the typical features of graphitic stacking C3N4layers.Notably,the featured graphitic structure is maintained after the thermal oxidation treatment,sincePCNSdisplays similar PXRD patterns to that of bulk g-C3N4(Fig.2b vs.Fig.2a).However,compared to bulk g-C3N4,the (100) and (002) peaks ofPCNSbecome weaker(Fig.2b).The weakened (100) peak (2θ=12.8°) suggests a decreased order degree of the inter-planes due to the pore engineering,while declining (002) peak (2θ=27.4°) originates from a decreased number of stacking layers inPCNS[38,39].These observations demonstrate the formation of porous layer structure ofPCNSupon the thermal oxidation“etching”.Furthermore,the (002) peak associated with interlayer stacking slightly shifts from 27.4 to 27.8°(Fig.2b vs.Fig.2a),when bulk g-C3N4is treated by oxidation “etching”.According to Bragg's law,we can calculate that the interlayer distance is slightly reduced from 0.328 to 0.32 nm.Logically,the single CN layer which is potentially undulated in bulk g-C3N4has been planarized by heating during thermal oxidation process [27,40].It makes a denser stacking of the atomic layers inPCNS.Similar observations have also been made by Niu et al.[27].
TEM images provide further information for the morphology ofPCNS,and bulk g-C3N4for comparison.As shown in Fig.3,bulk g-C3N4exhibits the typical layered platelet-like morphology (Fig.3a).After being thermal oxidation etched,the agglomerate is exfoliated into freestanding nanosheets with the existence of abundant in-plane nanopores (Fig.3b).Nearly transparent feature of nanosheets indicates its ultrathin thickness,which is confirmed by AFM analysis.AFM image ofPCNSreveals a thickness of ca.5.0 nm (Fig.3c),indicating that the exfoliated nanosheets are comprised of about fourteen layers (theoretical thickness of monolayer g-C3N4was about 0.325 nm [41]).The ultrathin layered structure may shorten the transport distance of charge from bulk to the surface,and reduce the possibility of charge recombination,which thus enhance the photocurrent response.Darker part in TEM image should be attributed to the wrinkle of g-C3N4nanosheets or overlap of several nanosheets(Fig.3b).Similar observation has been reported in some other nanosheets [42-44].Notably,the overlapped nanosheets inPCNScan be re-exfoliated into ultrathin nanosheets in water,benefiting from the highly negative surface charge[16].Welldefined Tyndall effect ofPCNSsolution indicates the presence of highly monodisperse ultrathin nanosheets in water (in the inset of Fig.3c).The high dispersion ofPCNSin water makes all active sites accessible,which thus allows the heterogeneous catalyst to behave as a pseudo-homogeneous catalyst in the aqueous system.

Fig.3.TEM images of bulk g-C3N4 (a) and PCNS (b),and AFM image of PCNS (c).Inset of (c) is the Tyndall effect of PCNS dispersed in water.

Fig.4.Nitrogen adsorption-desorption isotherm (a) and cor111responding pore size distribution (b) of bulk g-C3N4 and PCNS.
Nitrogen adsorption-desorption isotherms were used to evaluate the specific surface area and pore size distribution ofPCNS,as shown in Fig.4.Clearly,different from the nonporous bulk g-C3N4,PCNSexhibits a type IV adsorptiondesorption isotherm with an obvious hysteresis loop,which is characteristic of mesoporous material (Fig.4a).Probably,the thermal etching treatment generates gases,such as H2O and CO2,which act as bubble-soft-template to form the mesopore[29].Pore size distribution ofPCNSis determined from the desorption branch of isotherms using Barrett-Joyner-Halenda(BJH) method.It indicates that most of the pores are smaller than 50 nm,and the BJH desorption average pore diameter is around 24.3 nm,which is in good agreement with the mesoporous structure (Fig.4b).The mesoporous structure together with ultrathin 2D nanosheet endows thePCNSwith large surface area.Indeed,the specific surface area ofPCNSis found to be up to 182.4 m2g-1,which is almost 82 times higher than that of bulk g-C3N4(2.22 m2g-1).The larger surface area should provide more potential reaction sites and,especially,afford more illumination area for visible light harvesting [26,45],which are desirable for the visible lightdriven chemical transformation.
3.3.Optical and electrochemical properties of PCNS

Fig.5.UV-vis DRS (A),the cor111responding Tauc plots (B) and PL spectra (C) of bulk g-C3N4 (a) and PCNS (b).
The optical properties of g-C3N4samples were investigated by UV-vis DRS.As shown in Fig.5,the absorption edge ofPCNSshows a remarkable blue shift as compared with bulk g-C3N4(Fig.5A).It should be attributed to two reasons: (i)Monolayer deformation of g-C3N4is partially eliminated during the stacking process ofPCNS,the energy difference of n-π*thus becomes larger and the adsorption wavelength shifts to the shortwave;(ii) the decreased size and thickness forPCNSenhanced the quantum confinement effect [22,41].Band gaps ofPCNSand bulk g-C3N4are thus estimated to be 2.67 and 2.75 eV,respectively,according to the Tauc approach(Fig.5B) [46].The enlarged band gap suggests the improved redox activity ofPCNSfor photo-catalytic reactions [34].Furthermore,the valence band (VB) and conduction band(CB) potentials can be determined using empirical equations[33]according to the band gap energy.The calculated VBs and CBs were 1.565 and -1.105 eV for bulk g-C3N4,1.605 and-1.145 eV forPCNS,respectively.Clearly,PCNSsimultaneously shifts the conduction and valence band edges in opposite direction,which implies the enhanced photo-catalytic redox capability [41].
PL spectra analysis was used to further explore the separation efficiency of the photogenerated electron-hole pairs.Fig.5C shows PL spectra of bulk g-C3N4andPCNSunder an excitation of 318 nm.Clearly,bulk g-C3N4gives a strong fluorescent emission peak due to the fast recombination of electrons and holes in g-C3N4.The PL intensity ofPCNSwas dramatically lower than that of bulk g-C3N4.It indicates that the charge recombination is greatly suppressed and thus the separation between photogenerated electrons and holes is enhanced [25,33].The enhanced separation efficiency can be attributed to the decreased thickness and porous structure[25],which could effectively expedite the transfer and separation of electron-hole pairs on catalyst interfaces,and then improve the photocatalytic activity.
Structural change also influences the photoelectrochemical properties of g-C3N4,which is determined by the photocurrent and EIS measurements (Fig.6).Fig.6A shows a comparison of the photocurrent-time curves ofPCNSand bulk g-C3N4with periodic irradiation of visible light.Obviously,the photocurrent response ofPCNSis nearly 4 times higher than that of bulk g-C3N4.It illustrates thatPCNSpromotes the mobility of photogenerated charges,which improves separation efficiency of photogenerated electro-hole pairs upon visible light illumination [20,22,34].EIS irradiation was performed to further study the separation and transport of photogenerated carriers.Fig.6B shows the EIS Nyquist plots ofPCNSand bulk g-C3N4electrodes under visible light irradiation.The length of arc radius indicated the charge transfer resistance at the electrode/electrolyte interface.As expected,PCNSexhibits the much smaller radius of Nyquist circle than bulk g-C3N4,which suggests more efficient charge transfer across the interface [26,28,35].The rapid electronhole pair migration potentially reduces the possibility of charge recombination,which enhances the photo-catalytic efficiency [41,47].
3.4.Catalytic performances
The superiority ofPCNSfor photo-catalysis application was evaluated by oxidant-free dehydrogenation of benzyl alcohol under visible light irradiation,which is a sustainable way to give the important carbonyl compound of benzaldehyde.Bulk g-C3N4was also employed as the control catalyst to further demonstrate the superiority of porous 2D-structure ofPCNSin photo-catalysis.The results are summarized in Table 1.

Table 1 Results of the oxidant-free dehydrogenation of benzyl alcohol to benzaldehyde under visible light irradiation over various g-C3N4 based catalystsa.

Fig.6.Transient photocurrent response curves (A) and EIS Nyquist plots (B) of bulk g-C3N4 (a) and PCNS (b) samples.

Table 2 Results of the oxidant-free dehydrogenation of alcohols in water under visible light irradiation over PCNSa.
As expected,only trace benzaldehyde was acquired without light or photo-catalyst (Table 1,entries 1 and 2).Upon visible light irradiation,PCNSsignificantly accelerated the oxidantfree dehydrogenation of benzyl alcohol,giving almost quantitative yield (99%) of benzaldehyde within 12 h (Table 1,entry 4).In contrast,lower conversion(50%)of benzyl alcohol was obtained when bulk g-C3N4was used as the photo-catalyst,although the selectivity is encouraging (99%) (Table 1,entry 3).The great difference in photo-catalytic activity demonstrated the advantage ofPCNSin the visible lightdriven dehydrogenation.Apart from the increased surface area ofPCNSwhich ensures more active sites and more illumination area for visible light harvesting [15,34],the ultrathinPCNSreduced the transfer distance for charge carriers from the generated place to solid-liquid interface,and improved the separation efficiency of photoexcited charge carriers [22,26].In addition,the unique quantum confinement effect enhanced photo-catalytic redox capability ofPCNS[22,28],which further enhanced its photo-catalytic efficiency.
Benefiting from the larger surface area,as well as efficient separation of photogenerated charge carriers,PCNSas the photo-catalyst is universal for a wide range of alcohols,either primary or secondary.As shown in Table 2,various substituted benzyl alcohols underwent the visible light-driven dehydrogenation smoothly,forming the cor111responding aldehydes with almost quantitative yields (>99%) (Table 2,entries 1-6).Electron-donating substituents on the benzene ring,such as-OCH3or -CH3group,seem to accelerate the dehydrogenation (Table 2,entries 1-4 vs.entries 5 and 6).It is probably due to that the electron-donating substitution which increases the charge density on the benzene ring may enhance the preadsorption of substrates on the surface ofPCNS,and thus accelerating the catalytic efficiency [34,48,49].Furthermore,the different locations of methyl group on benzene ring didn't affect the product yield and reaction rate (Table 1,entries 1–3).Similar phenomenon has been reported in the literatures[34,48,49].For this reason,it is not surprise that primary aliphatic alcohol,such as cyclohexylmethanol and n-heptanol,are less reactive in the visible light-driven dehydrogenation overPCNS(Table 2,entries 7 and 8).Heterocyclic alcohol,such as furan-2-ylmethanol,was also readily dehydrogenated over thePCNSunder visible light irradiation,giving excellent yield (97%) of furfural which is a very important carbonyl compound for widespread applications in synthetic rubber,medicine,pesticide,and so on (Table 2,entry 9).Secondary alcohols underwent a slower dehydrogenation overPCNSprobably due to the unfavorable accessibility of the bulky substrate to active sites onPCNSsurface (Table 2,entries 10 and 11).It must be noted that all of the dehydrogenation systems produced cor111responding aldehydes or ketones as the sole product without any peroxidation byproducts.It simplifies the separation of product and benefits for industrial applications.The results confirm the general applicability ofPCNSin the visible light-driven oxidant-free dehydrogenation of alcohols.
Chemiluminescence method was employed to detect the key radicals formed in the photochemical reactions overPCNS.It is well known that TA can react with·OH to yield chemiluminescent product of TAOH.To check if hydroxyl radical was formed in our system,TA-based chemiluminescence method was conducted in the aqueous solutions under the visible-light illumination in argon atmosphere.Fig.7a shows FL spectra observed for the supernatant solution of thePCNSsuspension containing 3 mmol L-1TA irradiated for various duration times.Obviously,the fluorescence intensity at around 445 nm increased steadily with time in the presence of TA.It is concluded that TAOH was generated from TA by the reaction with·OH.Considering that the measurement is under oxidant-free condition,the·OH may be generated from water.Based on the band structure ofPCNS(+1.605 eV vs NHE),PCNSdoes not possess enough energy to proceed the oxidization from H2O to·OH (2.38 eV for H2O/·OH),but O2can be generated from H2O (+0.83 eV for H2O/O2),and further provide theradical (-0.056 eV for O2/) on the surface ofPCNSunder the visible light irradiation.Therefore,the·OH radical should be derived from the transformation of H2O following the process: H2O+2 h+→

Fig.7.(a)Fluorescence spectra obtained for the supernatant liquid of the irradiated PCNS suspension containing 3 mmol L-1 TA at various irradiation periods,and(b) dependence of the yield of benzaldehyde on the added amount of the ·OH inhibitor of DMSO.
To evaluate the effect of reactive radical species of·OH,and h+involved in the oxidant-free dehydrogenation of benzyl alcohol,a radical trapping experiment was performed by using radical scavengers to trap the cor111responding specific reactive specie.Three radical scavengers including DMSO(2 mmol L-1),benzoquinone (1 mmol L-1) and disodium ethylenediaminetetraacetate (EDTA-2Na,10 mmol L-1) were added into the reaction system for trapping the specific reactive specie·OH [12],[50] and h+[50],respectively.As shown in Fig.7b,a significant decrease in the photodegradation efficiency is observed by the addition of the radical scavengers.In particular,the reaction was almost inactive with the presence of EDTA-2Na scavenger for h+.This result demonstrates that·OH,and h+are the active species in the dehydrogenation process.ThePCNSenhanced the separation between photogenerated electrons and holes in the photocatalytic reaction,hence leading to the transformation of H2O to·OH via the H2O →O2→→H2O2→·OH.Indeed,we found that benzyl alcohol was difficult to transfer to benzaldehyde(conversion <1%)when toluene was used instead of water (Table 1,entry 7).Furthermore,acidtreatedPCNSwas also found to give low conversion(21%)of benzyl alcohol,although the selectivity was encouraging(>99%) (Table 1,entry 8).It appears that the electronic properties of the nitrogen atoms inPCNSmay play a crucial role in the photo-efficiency.
Table 3 Representative photo-catalysts for the transformation of benzyl alcohol to benzaldehyde.
Considering the above results,we proposed the possible mechanism for the photo-catalytic dehydrogenation of alcohols to cor111responding aldehydes.It should involve four steps,as shown in Scheme 2.Upon visible light excitation,PCNScreates the e--h+charge-separated state,where the holes (h+) are located at the N2 and N6 positions,and photogenerated electrons(e-) are located at C1 and N4 positions (Step a) [51].The generated holes(h+)remove H of alcohol,forming and alkoxide radical intermediate.Simultaneously,the ·OH radicals derived from the partial transformation of H2O via the H2O →O2→→H2O2→·OH (Step b).After this step,the hydroxyl radical abstracts α-H from the free alkoxide radical intermediate of alcohols,producing cor111responding aldehydes or ketones (c).The photogenerated electrons (e-) on either C1 or N4 position reduced H+to yield H2and complete the photo-catalytic cycle(d).Indeed,almost quantitative yield of hydrogen (98.5 μmol)was detected during the oxidant-free dehydrogenation of benzyl alcohol,by using gas chromatography(GC,Shimadzu GC-2014 with TCD detector and MS-5A column,argon carrier gas).PCNScan thus be recovered from the photo-catalytic system.After being centrifuged,thePCNScould be recovered and reused for the recyclingexperiment.Table 1 shows the reusability ofPCNSin the typical oxidant-free dehydrogenation of benzyl alcohol to benzaldehyde in water under visible light irradiation(Table 1,entries 5 and 6).Obviously,PCNScan be reused for up to seven times with no appreciable decrease in photo-catalytic efficiency (Table 1,entry 4-6).The results demonstrated the excellent stability and reusability ofPCNS,which is a prerequisite for practical application.
To our delight,thePCNSis even superior to the other reported state-of-the-art carbon nitride-based photo-catalysts either porous or layered,such as mesoporous g-C3N4(mpg-CN) [19],N-hydroxyphthalimide-modified mesoporous g-C3N4(MCN/NHPI) [52],and self-supported crooked g-C3N4nanolayer (g-C3N4aerogel) [34].As shown in Table 3,under visible light irradiation,benzyl alcohol could be quantitatively transformed to benzaldehyde in water with the employment ofPCNSat room temperature(Table 3,entry 1).Despite possessing similar a mesoporous structure,mpg-CN is less efficient.Relatively harsh conditions (100°C,8 bar O2and high-intensity visible light) were required to give only 57% conversion of benzyl alcohol over the mpg-CN (Table 3,entry 2).Combining a cocatalyst of NHPI with mpg-CN made the photo-catalytic system more active (85% of conversion)under mild conditions (Table 3,entry 3).But it suffered from low selectivity (82%) due to unavoidable further oxidization of benzaldehyde into benzoic acid.Furthermore,a large amount of acetonitrile was required in the MCN/NHPI system to ensure the conversion to benzyl alcohol,which was undesirable for industrial applications.Layered g-C3N4photocatalyst,such as g-C3N4aerogel,increased the availability of active sites for adsorption and reaction,enabling the selective photooxidation of benzyl alcohol in water.But the conversion of benzyl alcohol(68%)was significantly lower than that overPCNSunder identical conditions (Table 3,entry 5 vs.4).Furthermore,thePCNSin water was even more efficient than the reported metal-based photo-catalyst of Ni-modified CdS[53] in organic solvent (Table 3,entry 6 vs.1).The Nimodified CdS catalyst gave only 92%yield of benzaldehyde in acetonitrile even though the visible light-driven oxidant-free dehydrogenation was performed for 20 h (Table 3,entry 6).The results demonstrate the advantages ofPCNSused for visible light-driven oxidant-free dehydrogenation of alcohols in water.
4.Conclusions
In summary,porous g-C3N4nanosheets have been prepared through a simple two-step approach including thermal polymorization of dicyandiamide and followed thermal oxidation etching process.Compared with bulk g-C3N4,the obtainedPCNSpossesses larger surface area and improved separation efficiency of photoexcited charge carriers.As a consequence,it is far more efficient than bulk g-C3N4in visible light-driven oxidant-free dehydrogenation of alcohols in water,giving a wide range of carbonyl compounds with almost quantitative yields(99%).Furthermore,the heterogeneousPCNScould be facilely recovered from the aqueous system for efficient reuse.The high efficiency and outstanding reusability made thePCNShighly promising for photo-catalysis of various organic reactions in water.The nanostructure engineering approach also inspired researchers to perfect other carbon nitride-based photo-catalysts,and to tailor their functionality for a much wider range of applications in the field of solar energy utilization.
Conflict of interest
The authors declare no competing financial interest.
Acknowledgements
The authors are grateful for the financial support provided by the National Natural Science Foundation of China(21676078),the Natural Science Foundation of Hunan Province for Distinguished Young Scholar(2016JJ1013),Scientific Research Fund of Hunan Provincial Education Department(19A323),and Science and Technology Planning Project of Hunan Province (2018TP1017).We also thank Dr.Chaoping Li and Shiye Li for their helpful discussions.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
