Current Situation and Prospect of the Application of Real-World Evidence in Health Care
Xiao Feiyi,Zhang Fang,Li Xue,Dong Li*
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China;2.ZTE Foundation,Shenzhen 518055,China;3.Health Development Research Center of the National Health Commission,Beijing 100044,China)
Abstract Objective To summarize the application of real-world evidence (RWE) in the medical and healthcare field of various countries,including relevant policies,application scenarios and application methods.Methods Relevant policies and application scenarios were obtained by consulting the official websites and public documents of various countries’ healthcare institutions.Systematic literature retrieval was adopted to search PubMed,EMBASE,Cochrane Library,CNKI,CBM and Wanfang databases,and all papers related to real-world study and application were included.Then,these papers were classified and analyzed by country and application method.Results and Conclusion The RWE was mainly applied to supporting the preliminary approval of a new drug,expanding drug indications,accelerating approval or supporting conditional marketing authorizations and drug safety evaluation,etc.The United Kingdom,the United States,Germany,the Netherlands,Italy,Sweden,and France admitted RWE,but they treated the data obtained from RWE with caution.After systematic literature retrieval,a total of 701 articles were obtained,including relevant studies from 36 countries,among which the United States published 264 in total.The most common study was about using real-world data (RWD) to calculate treatment-related costs,which had a total of 259 studies.Secondly,158 articles were used for epidemiological analysis.Then,138 articles were about establishing risk models to analyze disease risk factors.A total of 70 articles were real-world efficacy evaluation of the drug treatment schemes,54 articles were about pharmacoeconomic evaluation with RWD as parameters.A total of 29 articles used RWD to build predictive models,and 15 articles used RWD to evaluate the health-related quality of life in patients.The application of RWE has been used widely in the medical and healthcare field of various countries.The application scenarios are gradually diversified,the application methods of RWD become mature,and the evidence quality of RWE is also improved greatly.
Keywords: real-world data;real-world evidence;real-world study;medical and health care
Randomized controlled trial (RCT) is an ideal trial design that demonstrates the expected or unintended effects of a specific drug under ideal conditions.Patients usually have strict inclusion and exclusion criteria in most phase III clinical trials.Randomization is then performed to counteract the effects of known or unknown confounding factors.In addition,monitoring and follow-up procedures for trial subjects are strictly controlled.However,this is not the ideal situation in the real world.In clinical practice,patients with different complications who have received drugs for other diseases,or patients with different genotypes are treated with drugs may not achieve the expected therapeutic effect after strict screening controls in clinical trials due to the above factors.Therefore,although the data provided by the clinical trials on which new drugs are based have high internal effectiveness before marketing,the external effectiveness is poor,and the results may be difficult to generalize to a wider and heterogeneous real-world population.Eichler,et al.[1]previously demonstrated the difference in efficacy between drugs in strictly controlled RCTs and real world,and referred to the difference as “efficacy-effectiveness gap”.Therefore,many health regulators,health technology assessment(HTA) agencies,and the pharmaceutical industry have begun to explore the option of using real-world data(RWD) as a complementary source of RCT data to accumulate more reliable evidence.
1 Definition of RWD and real-world evidence(RWE)
There are some differences in definitions given by various international health care organizations,and Table 1 shows the definitions of RWD by national health care organizations.

Table 1 Definition of RWD by international health care organizations
In 2019 the FDA provided new definitions of RWE and RED in the “Framework for FDA’s Real-World Evidence Program”: RWD are data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources.RWE is the clinical evidence about the usage and potential benefits,or risks of a medical product derived from analysis of RWD.
RWD includes data derived from electronic health records (EHRs);medical insurance payment data,data from medical product and disease registries,and patient-generated data.For instance,these data are from in-home-use settings,data about patients’health status gathered from other sources,such as mobile devices.Most RWE studies to date have relied on medical databases that aggregate data on a large number of patients’ health information in real practice.However,the retrospective studies generated by these databases consistently lack randomization because the treatments provided to the registered patients are simply observed rather than randomly assigned,and the treatments received by patients are chosen in consultation with their physicians without researcher intervention.Therefore,these factors,as well as bias,become significant when comparing patients who receive such selective treatments.This is the main reason for reducing the validity of such studies[6].
2 Scenarios of RWE in medical decision making
RWE is currently used to support health care decisions in four scenarios[7].
Firstly,RWE is used to support primary approval of a new drug.In this scenario,there is no RWE for the new drug because the new drug has not been admitted to the market and has not been applied in clinical practice.Only efficacy data from trial data collected from patients receiving the new drug can be used.However,efficacy data of the control group patient can be obtained from RWD sources that is taken as an external control to the trial data.This situation arises when the disease under study is rare,such as some rare diseases or highly targeted therapeutic technologies where the molecular subtypes of the target are less frequent.So it is difficult to successfully implement a large scale,high quality RCT due to the lack of patients in the trial.Besides,there is another situation where,if it can be judged that the indication for the new drug is not preceded by a therapeutic regimen with significant efficacy,and the preliminary results of an uncontrolled clinical trial of the new drug proves that the new drug is more effective than conventional therapies,then this scheme using clinical trial data as the experimental group and RWD as the control group will be more likely to succeed if it can be clearly judged that not using the new drug would result in a significant reduction in health benefits for the patient.In 2014,FDA adopted an accelerated review of blinatumomab,a drug used to treat Philadelphia chromosome-negative relapsed/refractory precursor B-cell acute lymphoblastic leukemia.To prove its effectiveness and better understand the heterogeneity of patient population in terms of prognostic factors,a phase II,fixed dose,open-label single-arm trial design was used.RWD of 694 patients drawn from more than 2 000 patient registries from clinical research and treatment centers in the EU and US was used as historical controls,and the weighted analysis showed that the expected complete remission/complete remission with partial blood cell recovery (CR+CRh*) rate was 24% (95%CI,20% to 27%),which was statistically significant[8].
Secondly,RWE is used to support a supplemental indication.For example,the clinical trial outcome indicators provided by many drugs at the time of marketing approval are intermediate outcome indicators,rather than endpoint outcome indicators,such as antihypertensive drugs or hypoglycemic drugs,where the clinical trial outcome provided is for the amount of reduction in glycated hemoglobin and the rate of reduction.After such drugs are marketed,RWE can be used to assess the effects of the drug on clinical endpoint outcomes,such as the occurrence of events like myocardial infarction or stroke,so as to achieve further validation of clinical efficacy and possibly support its expansion of indications.For example,if it is initially approved for adults,RWD can be used to demonstrate that it can also be used in other disease stages or pediatrics.
Thirdly,RWE is used to support adaptive or accelerated conditional marketing approval.Conditional approval is a special new drug approval pathway established by drug regulators to treat major diseases or to meet urgent clinical needs,such as“breakthrough therapy recognition and fast track” in the United States.In addition,the European Medicines Agency (EMA) conducted a pilot project to explore the use of adaptive pathways with medicines under development.In the adaptive pathway,an initial conditional marketing authorization is made for a limited population with high unmet medical need based on biomarker data or small clinical trials.The sponsor is then required to collect additional data to substantiate therapeutic effectiveness and safety to receive full marketing authorization.In 2015,the FDA accelerated the approval of osimertinib as a secondline therapy for advanced EGFR T790M+non-small cell lung cancer under the breakthrough therapy pathway.After marketing approval,the FDA required AstraZeneca to conduct a post-marketing study to provide “overall response rate data from one or more real-world cohort studies in at least 100 patients(patients with EGFR T790M mutation-positive outcomes who were selected to receive treatment)”.The FDA explicitly required RWE of osimertinib to support its indication effectiveness.AstraZeneca further adopted a comprehensive RWD approach to track the efficacy and safety of osimertinib in near real-time using electronic health record (EHR) data.In 2018,the FDA approved osimertinib for the first-line treatment of patients with EGFR-mutated metastatic non-small cell lung cancer and updated overall survival data[9].
The first three scenarios use RWE to obtain evidence of drug effectiveness to support approval,while the fourth scenario addresses the assessment of drug safety.The FDA and many other regulatory agencies have used RWE to assess the safety of marketed drugs for a long time.RWE has played an important role in many cases.For example,RWE warned that rofecoxib could put patients at increased risk of cardiovascular disease[10].Besides,it also demonstrated that dabigatran did not cause bleeding in patients[11,12].Sentinel initial,officially implemented in the United States in 2008,is the FDA’s response to the requirement of “Food and Drug Administration Amendments Act” to work with the public,associations,and private entities for obtaining multisource electronic health information to assess the safety of medical products.The monitoring objects of the program are drugs,vaccines,and other biologics(such as blood products).
In addition,RWD can also be used for disease cost calculation,epidemiological analysis,pharmacoeconomic analysis,and disease risk factor analysis.RWD is of great significance in the drug development process,from which relevant natural history can be learned,clinical pathways can be identified,and the cost and resource use associated with therapeutic interventions can be determined.
3 States’ perceptions of RWE
Although there is currently a growing emphasis on real-world study in various countries,health care institutions differ in their perception and related polices.Some studies have examined the views of health care institutions including Tandv?rds -och l?kemedelsf?rm?nsverket (TLV),the National Institute for Health and Care Excellence (NICE),Institut fuer Qualitaet und Wirtschaftlichkeit im Gesundheitswesen(IQWiG),Haute Autorité de Santé (HAS),Agenzia Italiana del farmaco (AIFA),and Zorginstituut Nederland (ZIN).Semi-structured interviews were conducted with internal managers of six major global HTA agencies[13].The application of RWE in the initial access and pharmacoeconomic evaluation of new drugs in each country was sorted out,and the main contents were shown in Table 2 and Table 3.

Table 2 Summary of policies on RWD accepted or requested and the appraisal of RWD in the context of initial reimbursement discussions
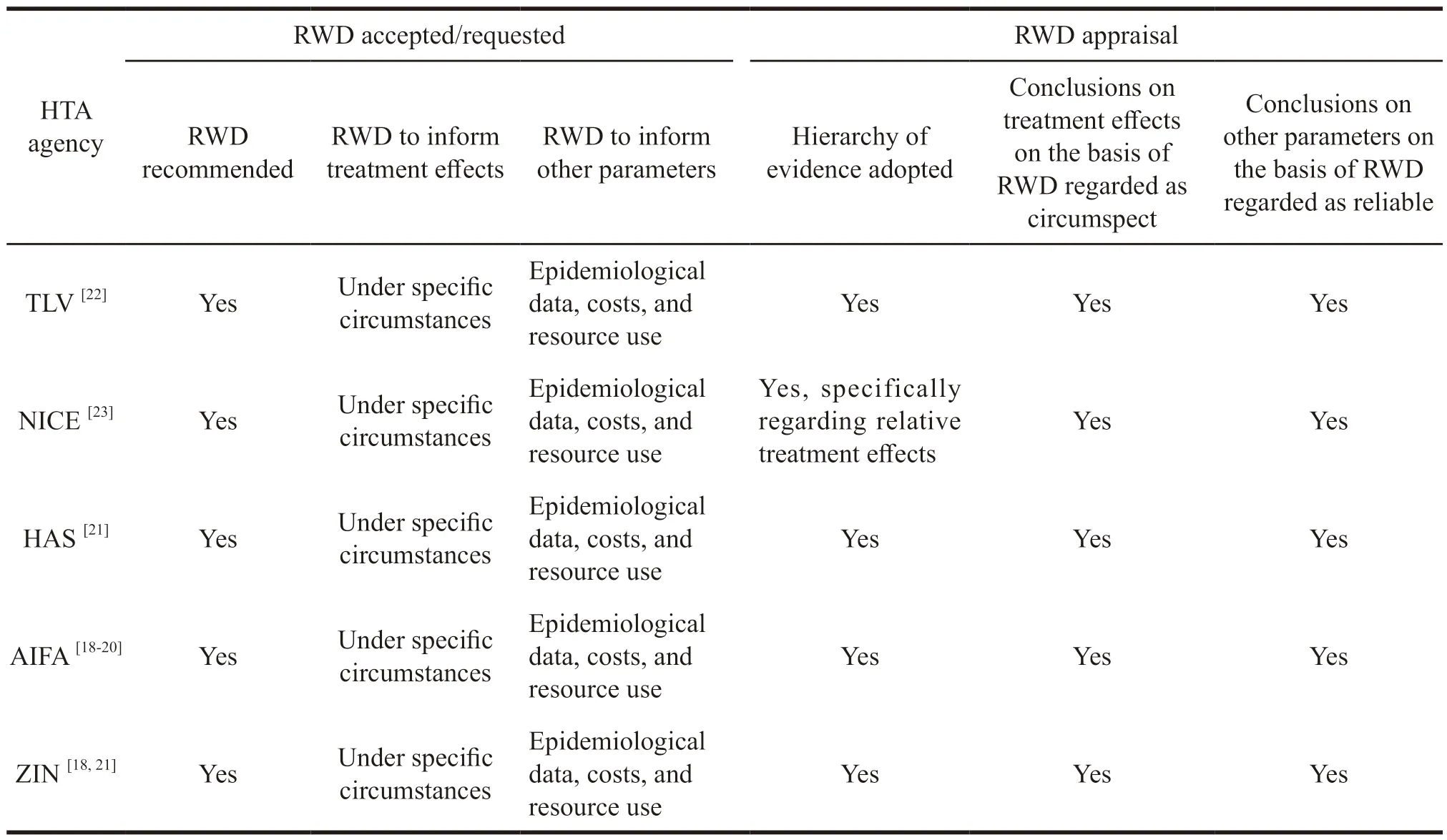
Table 3 Summary of policies on RWD accepted or requested and the appraisal of RWD in the context of PEA per agency
All institutions indicated that they were able to accept RWD for initial access to new drugs.When the required head-to-head control group was missing due to evidence from RCTs,the treatment effects from RWD could be used.But studies had to be analyzed and biases associated with RWD should be documented.All institutions were able to accept data from RWD on epidemiology,natural history of disease,or resource use.None of them gave recommended types of RWDs nor guidelines on methods for collecting RWDs.All institutions would use evidence-based medicine to specify levels of evidence,and RWDs were consistently of lower quality than RCT data.Causal inferences based on RWDs were treated with caution.RWDs were considered to complement or extend the effects of RCT-based treatments.As to some institutions,RWDs were more likely to be used in situations where RCTs were difficult to perform (e.g.,rare diseases).
When RWD is used in pharmacoeconomic evaluations,RWD is the preferred source of cost data (direct and indirect) and resource usage data.Parameters other than treatment effect derived from RWD have been widely accepted,including quality of life,adherence,epidemiological data,and data on transition probabilities of the model,etc.RWD can be used as treatment effect data only when there is a lack of RCT evidence in a specific head-to-head comparison.Besides,bias associated with RWD must be explored and documented.However,the hierarchy of evidence used by the HTA agencies consistently ranks RWD at a lower level of quality than that of RCT data.
4 Application state of RWE in the field of oncology
The field of oncology has always been the focus of the medical and health industry.In recent years,the global investment in new drug research and development has been increasing,especially in the field of anti-tumor drugs.New drug targets have been discovered continuously and new therapeutic drugs have entered clinical application one after another.Several domestic documents have been issued to encourage drug innovation.The development and application of anti-tumor drugs is one of the focuses of pharmaceutical companies and clinical research institutions.In terms of disease burden,cancer has become one of the leading causes of death in China’s population.The loss of healthy life due to cancer is nearly twice that of the global average and is increasing at a fast rate.The cost of cancer treatment is also rising rapidly,imposing a huge economic and social burden on society and families.Therefore,this paper focuses exploring the application of RWD in oncology.
To understand the status and methods of realworld study in the field of oncology at home and abroad,we searched literature review in Chinese databases CNKI,CBM,Wanfang and English databases PubMed,EMBASE,Cochrane Library,etc.The literature about RWD was categorized statistically.Taking PubMed as an example,the search strategy was based on “RWD” or “RWE” or “registry study”and “Neoplasms” [Mesh].The search time was limited to 2010 to present,and the results included a total of 2 770 relevant literature.All 2 770 literature were read in abstract,and after excluding duplicate literature,review literature,non-oncology field diseases,and non-pharmacology field studies,the remaining include literature were classified and counted according to the abstract information to determine their types of studies for RWD as well as their national sources.If multiple types of studies were involved in the same literature,they would be counted in each classification.A total of 701 oncology-related real-world studies were finally included,containing relevant studies from 36 countries,with the most published studies from the United States which had a total of 264 papers,followed by 78 papers from the Netherlands,51 papers from Canada,44 papers from Italy,32 papers from the United Kingdom,34 papers from China,31 papers from Germany,and 19 papers from Sweden.The investigators initially classified the types of studies into 31 different categories,with the largest number of studies using RWD to calculate the costs associated with cancer treatment,totaling 259,followed by 158 for epidemiological analysis,138 for risk modeling to analyze disease-related risk factors,70 for real-world efficacy evaluation of oncology drugs or treatment regimens,54 for pharmacoeconomic evaluation with all parameters derived from RWD,29 predictive models using RWD,and 15 assessments of patient health-related quality of life using RWD,see the details in Table 4.

Among the 259 papers that used RWD to calculate costs associated with cancer treatment,136 were from the United States,20 from Canada,17 from Finland,15 from Italy,9 from the Netherlands,and 9 from the United Kingdom.The content of this sort of literature included the use of health insurance databases,patient medical record databases,cancer registry databases to measure the costs of various aspects of cancer.The most frequently used database was SEER-Medicare in the United States,which included the measurement of the average life-cycle cost of cancer treatment,the real-world treatment cost of a particular treatment or comparison of multiple treatments,the cost attribution analysis of cancer treatment,the total cost of a particular or comparison of multiple cancer drugs,the measurement of the cost of cancer adverse effects,the cost of hospice and supportive care,the cost of cancer screening,the impact of various types of cancer on health insurance expenditures,and the measurement of lifetime costs of cancer from the patient’s perspective.Most of the studies on cost measurement were aimed at measuring the real-world economic burden of disease,conducting budget impact analysis,or serving as cost data for cost effectiveness analysis.
Among the 158 papers using RWD for epidemiological analysis,53 were from the United States,19 from the Netherlands,12 from Germany,12 from Italy,7 from Canada,6 from Sweden,5 from Australia,5 from France,5 from Denmark,and 5 from Iran.The most common purpose of these studies was to analyze the prevalence,incidence,mortality rates and trends of various types of cancer based on RWD statistics from various cancer patient registries and health insurance databases.Analysis of disease distribution and incidence factors and natural history of the disease was in the second position.The main purpose of these studies was to assess the epidemiological burden of cancer diseases.At the same time,a large part of these studies also analyzed the economic burden of the disease.
Risk models were developed to analyze risk factors associated with the disease in 138 studies,including 60 studies from the United States,12 from Germany,10 from China,9 from the United Kingdom,7 from the Netherlands,7 from Canada,5 from Italy,3 from Sweden,3 from France,3 from Denmark,3 from Ireland,and 3 from Belgium.All the studies were on the impact of various internal or external characteristics of the disease on survival,treatment modalities or other outcome indicators by using data from cancer patient registries,health insurance databases,and the correlation between different treatment schemes and outcome indicators such as survival rates as well.The main modeling approaches included the use of multiple regression models,multivariate Cox proportional risk regression models to explore the correlation between patient characteristics and the application of treatment options,and logistic regression models to compare potential confounding factors influencing the use of different drugs for patient treatment.
A total of 70 real-world efficacy assessments of oncology drugs or treatment regimens were conducted,including 36 from the United States,5 from the Netherlands,5 from Italy,5 from Poland,4 from Germany,3 from Canada,3 from the United Kingdom,and 3 from China.These studies mainly used hospital case data,prospective trial data,cancer registry to conduct statistical analysis on the realworld efficacy of cancer patients after receiving some intervention programs.The most frequently assessed outcome indicators were real-world overall survival(OS) and progression-free survival (PFS).Most of the studies analyzed survival data by plotting Kaplan-Meier curves to assess overall survival or stage specific survival,while other studies calculated only PFS or OS at 3 or 5 years,and a few studies analyzed intermediate indicators as endpoint outcomes,assessed time to discontinuation,the incidence and severity of adverse events.Most of these studies evaluated effectiveness while also performing risk analysis or costing of relevant treatments for factors affecting outcome indicators.When comparing the efficacy of different interventions,propensity matching scores were commonly used to group patients to reduce confounding factors.
There were 54 pharmacoeconomic evaluation studies that used RWD to construct cost effectiveness analysis models and derived all parameters from RWD,including 14 from the United States,10 from the Netherlands,7 from Canada,5 from Germany,5 from Sweden,5 from China,and 5 from Japan.No matter the cost,effect or other data in pharmacoeconomic evaluations conducted by such studies were derived from RWD,and no RCT data was selected to assess the economics of a certain intervention in the real world.Unlike the use of data from RCTs to construct carcinoembryonic antigens(CEAs),most studies on cost-effectiveness analysis using RWDs did not construct models because,with sufficiently complete data and large samples,the parts that would otherwise be simulated using models were known in the real world,there was no need to use models to calculate cost effects.However,there were some studies that still constructed models for analysis because of limited availability of real-world patient samples or missing values,and they wanted to simulate and calculate cost effects for the full patient population.The models used included multivariate regression analysis to build predictive models for individual patient-specific simulations to obtain their complete disease course results,which could achieve the effect of filling the missing data.The other most used models were multi-state transfer models,which included Markov models,discrete-event simulation models,and microsimulation models.The model structure and sub-states were constructed using known clinical pathways of the patient’s disease.Transfer probabilities were calculated using the transfer data between each state.When removing confounding factors between experimental and control groups,the most used methods were propensity matching scores and multiple regression modeling.As to the case of random missing data,methods such as multiple interpolation should be used to deal with them.
A total of 24 studies used RWD to construct clinical prediction models for disease,including 9 from the United States,3 from the United Kingdom,2 from the Netherlands,and 2 each from Sweden,Finland,Australia,Japan,and Belgium.The categories of models built in the studies included using patient count models to predict and estimate the number of patients with a particular cancer,constructing segmented linear regression models to predict survival rate at different ages or time periods.Besides,Bayesian network models were used to predict each stage and overall survival rate,and machine learning logistic regression models were used to predict drug effectiveness.Cox multivariate models were used to plot column line graphs to make disease survival prediction,and decision trees were combined with multiple Markov models to predict long-term medical consumption and survival time of patients.Such studies had many similarities with risk prediction models,and researchers distinguished them by the purpose of the article.If the purpose of the study was to build a model to explore factors that affected outcome indicators,rather than to predict patient outcome indicators by using known patient risk factor data into the model,such study was classified as a risk prediction model only.If the purpose of the study was to build a model to predict some unknown clinical outcome indicators for that patient from existing patient data,this type of study was classified as a clinical prediction model.
A total of 15 studies assessed patient healthrelated quality of life using RWD,including 7 from the Netherlands,3 from Germany,2 from France,and 2 from Ireland.Measures of patient health-related quality of life included the following two types of studies: The first was a prospective survey study in which one or more questionnaires or long-term followup surveys were administered to patients who met the inclusion criteria and had been treated in a singlecenter or multi-center hospital during a certain time period;the other was to administer questionnaires to patients with related diseases who had been enrolled in a center or multiple centers to investigate their quality of life during their illness.The scales used in the study to measure health-related quality of life in cancer patients included: EORTC QLU-C10D,EORTC QLQ-C30,EORTC QLQ-C15-PAL,FACT-G,EQ-5D,EQ-VAS,and FACT-HN.
There were 15 studies using RWD to assess screening technology,including 5 from the United States,3 from Canada,2 from Finland,2 from Australia,and 2 from Denmark.RWD was used to assess the value of a particular cancer screening technology,including cost or cost-effectiveness.Most studies constructed CEAs to assess the costeffectiveness of screening technologies,and CEA models for cancer screening were used for microsimulation.The types of cancer screening included breast cancer,cervical cancer,bowel cancer,lung cancer,and prostate cancer.
A total of 12 studies used RWD in cost and resource use data for CEA only,including 7 from the United States,3 from Italy,and 2 from Canada.In such studies,RWD was used only as cost and medical resource consumption data for CEA,while the effect data still used RCT data.
10 studies constructed CEA models to compare the differences in outcomes between RCT data and RWD,9 from the Netherlands and 1 from the Czech Republic.Most of the health technology assessments done in the Netherlands were based exclusively on RWD to assess real-world cost effectiveness,and some of them also compared the results of real-world assessments with those of RCT-based trials to assess the differences between them.In the above 10 studies,although there were some numerical differences in the results obtained by using RWD and RCT data,the differences did not affect the same conclusion as to whether they were cost effective.The purpose of such studies was to use RWD to validate and supplement additional RCT evidence submitted for marketing to demonstrate external validity,or to improve the quality of evidence using dual evidence from RWD and RCT.
A total of 14 studies analyzed the use of realworld clinical regimens through RWD,7 from the United States,4 from the Netherlands,1 from Canada,1 from the United Kingdom,and 1 from Japan.These studies analyzed the real-world treatment patterns,choices in clinical practice for a particular cancer type,and the corresponding clinical pathways.Besides,they compared the efficacy of various treatment regimens by RWD statistical analysis.Some of these studies compared the results with the guidelines,and most of them showed that the real-world treatment options were consistent with the guidelines.However,some studies found that the real-world clinical treatment options were not consistent with the guidelines,mostly due to the complexity of the real-world patients who did not have the inclusion criteria in the RCT trials and often had different comorbidities or other medication histories.Therefore,the recommended regimens in the guidelines could not be achieved or the effect was poor.
The studies that compared real-world efficacy to efficacy in RCTs included 3 from the United States and 1 from Poland.These studies analyzed the realworld efficacy of an oncology drug by RWD and then compared it to the test reports of the RCT submitted at the time of the drug’s launch.Three of the comparisons showed that real-world outcomes were generally comparable to those of RCTs,and 1 study from the United States comparing real-world outcomes with RCTs using idelalisib for relapsed follicular lymphoma and chronic lymphocytic leukemia concluded that there was a significant imbalance in baseline comorbidities and treatment outcomes between Medicare recipients and RCT trial participants.In clinical trials,the risk of hospital-acquired infections in the first 6 months of treatment with idelalisib in the real world was 2.11 times higher than that of the RCT,possibly due to frequent dose reductions or draining of patients who had developed infections,which resulted in somewhat lower immunosuppression-related toxicities with idelalisib[24].
The studies that used RWD to calculate relative efficiency compared with RCT trials included 1 from the UK and 1 from the Czech Republic.The study from the Czech compared rituximab monotherapy in patients with highly refractory multiple myeloma with historical data on real-world treatment,first adjusting for imbalances in patient characteristics between cohorts and then using multivariate proportional risk regression models to estimate the relative treatment effect of up to rituximab versus historical controls.It obtained heart rates of PFS and OS for real-world patients on rituximab versus other treatment options values[25].It showed that in the absence of head-tohead comparative studies,comparisons between trial data and historical cohorts could provide clinicians and health care decision makers with valid information about relative treatment effects among other things.
The control group used RWD data sources,while the experimental group used RCT test data for health technology assessment.One study was from the United States and another from Canada.In both studies,the efficacy data for the trial group were from single-arm clinical trials and the control group from RWD.In the study from Canada,for example,to assess the costeffectiveness of the BREN+AVD regimen versus the ABVD (doxorubicin,bleomycin,vinblastine and dacarbazine) regimen for advanced Hodgkin’s lymphoma,a probabilistic Markov model with six mutually exclusive health states was constructed,in which the probability of metastasis component of the BREN+AVD regimen for the trial group was derived from the ECHELON-1 trial,whereas the ABVD regimen for the control group was derived from data from the Lymphoma Patient Center[26].
5 Prospects of real-world study
RWE is an important part of the evidence chain for the evaluation of the effectiveness,safety,and economy of clinical treatment measures.As medical technologies continue to emerge,more and more treatment technologies have uncertain safety and efficacy in clinical applications.Countries such as the United States,the European Union,and Japan have been conducting real-world studies at the government level since 2016 and using RWE to support regulatory decisions on drugs and other medical products,which can accelerate the development of pharmaceutical products.With the promulgation and implementation of real-world study guidelines in various countries,real-world study has become a hotspot in the healthcare field.Although the importance of realworld study is gradually increasing,countries still treat RWE cautiously.When it comes to obtaining treatment effect data,they still give priority to using the results of RCTs with higher quality levels and accept RWE only in a small number of special cases.
From the current real-world studies in the field of oncology,they are also basically consistent with the above conclusions.The most frequent types of studies use RWD to calculate the costs associated with cancer treatment,epidemiological analysis,and building risk models to analyze disease-related risk factors.Meanwhile,few studies try to use RWE to assess efficacy or use real-world efficacy data for pharmacoeconomic evaluation.Although the number of studies is gradually increasing,most of these studies on real-world efficacy assessment are not used for drug marketing and access,but only as a reference for real-world efficacy.In addition,there are studies that construct CEA to compare RWD with RCT data,compare real-world efficacy with RCT efficacy,use propensity matching to assess treatment options to fill RCT gaps,extrapolate OS data with RWD,and use RWD as a supplement to RCT.They all indicate that the enthusiasm for using RWE to replace or supplement RCT trial data is growing,and related methodological studies are gradually being developed.
The quality of evidence from RWD was for a long time considered to be lower than that from systematic evaluations,Meta-analyses,and randomized double-blind controlled trials.The main reasons are that its lacks strict inclusion and exclusion criteria,it is unable to ensure consistency at the baseline patient level,and it tends to produce many undetected results or biases in non-RCT methods.However,with the growth of real-world studies,more and more relevant statistical methods have been generated,and methods to deal with RWD confounders as well as bias have gradually matured,such as propensity matching scores and inverse probability weighting to deal with between-group differences and balance patient baselines,multiple interpolation,central tendency value filling,and K-nearest neighbor methods can be used to deal with missing data.Even more,machine learning can be used to deal with confounding and bias.So the processed RWD can achieve similar results to those of RCTs,resulting in more credible conclusions.In addition,because the data volume is large enough,researchers can select patient populations with specific characteristics based on their needs,without being limited to the enrollment requirements of a RCT,which offers greater flexibility.The biggest advantage of RWD over RCTs is that it has strong external validity,better reflecting the effect of the intervention on patients in real-world situations.The application of RWE is still being explored in various countries,but it can be inferred from existing trends and research findings that the status of realworld study in health care will gradually increase,and the quality of evidence from RWD will also be improved greatly.
- 亞洲社會藥學雜志的其它文章
- Research on Incentive Policies for Chinese Innovative Drug R&D -Taking Innovative Anti-tumor Drugs as an Example
- Research on the Impact of Independent R&D and Collaborative Innovation on Economic Performance in China’s pharmaceutical Industry
- Research on the Effect of R&D lnvestment lntensity and Sales Expense on the Performance of Biomedical Enterprises
- The Status Quo and Enlightenment of the Foreign Extended Clinical Trial System
- Foreign Experience and Enlightenment of Reimbursement Management of Multi-indication Drugs
- Study on Public Health Behavior against the Background of COVID-19 Pandemic -Based on Bandura Reciprocal Determinism

