Effects of Remifentanil Pretreatment on Inflammatory Response in Rats with Acute Cerebral Ischemia Injury
Jingfeng ZHOU, Xianjing ZENG, Shu LIU, Jinghua YUAN, Fan XIAO
1. Department of Anesthesiology, Jinggangshan University, Ji’an 343009, China; 2. Department of General Practice, Affiliated Hospital of Jinggangshan University, Ji’an 343000, China; 3. Jinggangshan University, Ji’an 343000, China
Abstract [Objectives] This study was conducted to investigate the effects of remifentanil pretreatment on inflammatory factors in rats with acute cerebral ischemia. [Methods] Sixty SD rats were randomly divided into the normal control group, sham operation group, ischemic brain injury group, and remifentanil pretreatment group. Except the normal control group, each group was divided into three subgroups (six in each group) according to the sampling time points of 6, 12 and 24 h after execution. After modeling, the rats were scored for neurological deficit, and observed for pathological changes of neurons in the brain tissue by HE staining and the brain infarct volume by TTC staining, and the expression levels of TNF-α, IL-6 and IL-8 were detected by RT-PCR. [Results] HE staining: No significant changes were observed in the pathological morphology of the brain tissue in the blank group and sham operation group; and the neuronal structure of rats in the acute cerebral ischemia group was obviously damaged, and the neuronal damage in the remifentanil pretreatment group was less than that in the acute cerebral ischemia group at each time point. TTC staining: The gray brain infarct area in the remifentanil pretreatment group was significantly smaller than that in the cerebral ischemia group (P<0.05). RT-PCR detection results: The expression levels of TNF-α, IL-6 and IL-8 in the blank group and sham surgery group did not show significant changes at different times (P>0.05); and compared with the cerebral ischemia group, the expression levels of TNF-α, IL-6, and IL-8 in the remifentanil pretreatment group were significantly reduced at all time points (P<0.05). [Conclusions] Remifentanil pretreatment could protect the brain by reducing the expression of inflammatory factors after cerebral ischemia injury.
Key words Remifentanil, Acute ischemic brain injury, Tumor necrosis factor, Interleukin-6, Interleukin-8
1 Introduction
Ischemic cerebrovascular disease (ICD) is a kind of neurological dysfunction disease caused by the interruption of local blood circulation in the brain and characterized by high incidence rate, high mortality and high disability rate. However, with the accelerated aging of China’s population, cerebrovascular diseases have become one of the main causes of death and disability in China, with ischemic stroke being the most prominent, accounting for about 70% of cerebrovascular diseases[1]. At present, there is a lack of effective prevention and treatment measures for ICD, which brings a heavy medical burden to affected families. Studies have shown that after the occurrence of cerebral ischemia and hypoxia, cytokines are involved in initiating the inflammatory response process of cerebral ischemia injury, and inflammatory response plays an important role in the process of acute cerebral ischemia injury and can affect the prognosis of patients[2-3]. Therefore, early active and effective intervention in the initiation of inflammatory cascade reaction caused by ischemic injury is an important link to preventing the aggravation of brain injury, and finding the key inflammatory mediators and clarifying their role in the pathophysiological process of ICD is of great significance. We found that remifentanil pretreatment can inhibit neuronal necrosis and apoptosis in previous experiments[4]. In this study, the effects of remifentanil pretreatment on the expression of inflammatory factors in rats with acute cerebral ischemia injury were further explored, and the mechanism of remifentanil pretreatment on brain injury was further explored to provide a theoretical basis for clinical application.
2 Materials and methods
2.1 Experimental animals and modeling methodsSixty healthy SPF SD rats (aged 12-15 weeks), weighing (250±50) g, purchased from Hunan SJA Laboratory Animal Co., Ltd., were divided into a blank group with 6 rats, and a sham surgery group, an acute cerebral ischemia group and a remifentanil pretreatment group, 18 rats for each group. The experimental unit used the license number: SYXK 2012-0001, and the production license number: SCXK 2016-0002. After one week of adaptive feeding in the animal laboratory of Jinggangshan University, a rat model of middle cerebral artery occlusion (MCAO) was prepared using the longa method[5]. After successful modeling, each group was further divided into three subgroups at different execution time points of 6, 12 and 24 h, with 6 animals in each subgroup. Sham surgery group: Only the left common carotid artery was separated and sutured, without vascular ligation or hypoxic ischemic treatment. Remifentanil pretreatment group: Rats were fixed in a rat box 2 h before surgery, followed by tail vein puncture and indwelling a venous trocar needle, which was collected to a micropump (Humanwell Healthcare Company, National Medical Products Administration (NMPA) approval number: H20030197), which was used to infuse remifentanil with a dosage calculated based on the equivalent dosage coefficient conversion algorithm of human and animal body surface area[6], at an infusion rate of 0.8 μg/kg·min, for three consecutive times, 5 min each time. A 5 min interval was given after each infusion, and the entire pretreatment process was about 30 min. Acute cerebral ischemia group: The procedure was the same as that of the remifentanil pretreatment group, and an equal amount of physiological saline was infused instead of remifentanil. The criteria for successful MCAO modeling of the brain included: (i) partial or complete flexion of the affected right anterior paw, (ii) rotation of the body towards the hemiplegic side while walking; (iii) falling of the body towards the hemiplegic side while walking.
2.2 Sampling and section preparationRats were anesthetized with 10% chloral hydrate and fixed. The chest and abdominal cavities were cut open, and the heart was exposed. Approximately 100 mL of physiological saline was rapidly infused through the heart until the liver turned white and clear liquid flowed from the right ear. Next, approximately 150 mL of 4% PBS solution was infused for fixation. After the tail and limbs of the rats became stiff, the skull was pried open. The complete brain was taken out and rinsed in physiological saline to remove residual blood, and then fixed in 4% PBS. After 24 h, the brain tissue was cut into sections, which were dehydrated with different gradients of ethanol, immersed in wax, and embedded in paraffin. Coronal sections with a thickness of about 4 μm were made using a microtome. After routine HE staining, the morphological and pathological changes of neural cells in the ischemic side of the brain tissue were observed under light microscopy.
2.3 RT-PCR detection of TNF-α, IL-6 and IL-8 expression levelsThe brain tissue at the ischemic side of rats was cut, frozen and ground, and homogenized until the tissue was completely lysed. mRNA was extracted to perform reverse transcription. Next, the obtained QPCR primers and templates were used for amplification. Primers for TNF-α, IL-6, IL-8 mRNA and β-actin were designed with Primer 5.0 software and synthesized by Sangon Biotech (Shanghai) Co., Ltd., and the synthesized primers were freeze-dried powder. Appropriate amount of enzyme-free water was used to dissolve each primer to obtain a 100 μM primer mother liquor, which was diluted to a primer concentration of 2 μM for PCR amplification. The sequences are shown in Table 1.

Table 1 PCR primer sequences
Result calculation: The reference gene used was β-actin, and theCTvalue detected byLC480 was the number of cycles when the fluorescence intensity of cDNA reached the set threshold, andΔCT=CTvalue of target gene-CTvalue of reference gene. The relative expression level of mRNA in each sample could be calculated according to following equation: Relative expression level of mRNA= 2-ΔCT×100%.

3 Results and analysis
3.1 Neurological deficit scoreThe neurological function of rats in the blank group and sham operation group showed no deficit, and the scores did not change significantly at each time point. Rats in both the acute cerebral ischemia group and the remifentanil pretreatment group had different degrees of neurological deficit symptoms after operation, and compared with the cerebral ischemia group, the neurological deficit score of rats in the remifentanil pretreatment group was reduced, and the difference was statistically significant (P<0.05), as shown in Table 2.

Table 2 Neurological deficit scores of rats in various groups at different time points (points,
3.2 TTC staining results of ischemic brain tissueA piece of brain tissue was randomly selected from each group, trimmed, and rinsed to remove residual blood. The cleaned brain tissue was fixed in 4% PBS, frozen, sliced, and finally dyed evenly in 2% TTC staining solution. The normal brain tissue was stained red, and the infarct area was grayish white. The results showed that there was no obvious abnormality in the staining of brain slices in the sham operation group, while pale infarct areas with different sizes and clear boundaries could be observed in brain tissue samples of rats in the cerebral ischemia group and remifentanil pretreatment group; and compared with the cerebral ischemia group, the infarct volume of brain in the remifentanil pretreatment group was significantly reduced (P﹤0.05). The results are shown in Table 3 and Fig.1.

Fig.1 Infarct volumes of ischemic brain tissue stained by TTC in rats of various groups

Table 3 Comparison of infarct volumes of brain tissue in rats of various groups
3.3 HE staining resultsBlank group and sham operation group: Fig.2A and Fig.2B show the staining conditions of neurons in the blank group and sham operation group. The sections showed that the neurons in rats were complete in shape, round or oval, neatly arranged, and obvious nucleoli and nuclei stained clearly and uniformly could be observed.

Fig.2 HE staining of brain tissue sections from the ischemic side of rats in various groups (10×40)
Cerebral ischemia group: Fig.2C and Fig.2D show the staining conditions of the cerebral ischemia group at 6 and 24 h after execution. At 6 h under the microscope, the cell body was swollen, and the edema in the ischemic area was obvious, and the neuron structures in the infarct area were fuzzy and arranged irregularly. At 24 h, necrosis and apoptosis of neurons were obvious, and edema and swelling were reduced, and the cell body was reduced. Furthermore, a large number of pyknotic necrotic nerve cells were observed, and nuclei disappeared. And the nuclei in some areas were pyknotic and deeply stained, and inflammatory cells were infiltrated in the marginal areas, and showed microvesicles formed in the cytoplasm.
Remifentanil pretreatment group: Fig.2E and Fig.2F show the staining results of the remifentanil pretreatment group at 6 and 24 h after execution. At 6 h, the cells were densely arranged in multiple layers, and exhibited relatively intact cell membrane, and edema was not obvious. At 24 h, some neuronal bodies shrank and were deeply stained, and a small number of pyknotic and necrotic neurons were observed around them. Furthermore, inflammatory cell infiltration and a small amount of proliferated glial cells were observed, and pathological changes were overall lighter than those in the cerebral ischemia group.
3.4 Results of TNF-α, IL-6 and IL-8 mRNA expression levels in brain tissue of rats in various groupsAfter the rat model was established, there were no significant differences in the mRNA expression levels of TNF-α, IL-6 and IL-8 between the blank group and the sham operation group (P>0.05). Compared with the blank group and sham operation group, the mRNA expression levels of TNF-α, IL-6 and IL-8 in the acute cerebral ischemia group and remifentanil pretreatment group significantly increased at each time point, with statistical significance (P<0.01). Compared with the acute cerebral ischemia group at the same time point, the mRNA expression levels of TNF-α, IL-6 and IL-8 in the remifentanil pretreatment group were significantly lower, with statistical significance (P<0.05). The results are shown in Table 4.
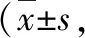
Table 4 Effects on expression levels of TNF-α, IL-6 and IL-8 mRNA in brain tissue of rats n=5)
4 Discussion
After cerebral ischemia injury, the release and aggregation of a large number of inflammatory factors will cause damage or apoptosis to neurons, and then induce brain tissue damage[7]. One of the mechanisms is starting the inflammatory cascade reaction program. With the increase of the release and aggregation of inflammatory factors, the neuronal injury become more serious, which makes the ischemic injury change to inflammatory injury, and then promotes the amplification of the inflammatory cascade reaction. The process involves a variety of inflammatory cytokines, and the "waterfall effect" formed by the uncontrolled release of inflammatory mediators such as TNF-α, IL-6 and IL-8 is the basis of immune inflammatory response to cerebral ischemia injury[8]. TNF-α is an active component of TNF, which can not only regulate the body’s immunity and control the production and secretion of many inflammatory mediators such as IL-8 and IL-6, but also participate in vascular endothelial injury and coagulation process, so it plays an important role in cerebral ischemia injury[9]. The increase of IL-6 can cause the chemotaxis of neutrophils to the local area of inflammatory injury, so as to secrete a large number of inflammatory mediators, promote the formation of inflammatory waterfall, aggravate inflammatory injury and affect the repair of nerve cells[10]. IL-8 is an inflammatory or inducible chemotactic cytokine, which has all the characteristics of neutrophil chemotactic agents. It can induce morphological changes and chemotaxis, transient increase in the concentration of intracellular free Ca2+, release of granular inclusions and up-regulation of adhesion proteins, thus promoting the inflammatory reaction[11]. The severer the brain injury, the more obvious the release of inflammatory mediators. TNF-α can promote the mRNA transcription and expression of factors such as IL-8 and IL-6 in brain injury, while IL-8 and IL-6 further stimulate the activation of TNF-α. That is to say, they play a synergistic role in promoting inflammation.
The pathophysiological mechanism of ischemic brain injury is complex, and there is still a lack of effective intervention methods. In recent years, animal experiments have shown that drug pretreatment may be an effective means to prevent and treat ischemic injury. After one or more short-term ischemia, some organs have good tolerance to subsequent long-term ischemia, which can obviously alleviate the injury caused by ischemia. In the process of drug pretreatment, narcotic analgesic drugs are one of the hot spots in the selection of pretreatment drugs in recent years. The research results of opioid drugs on cerebral ischemia-reperfusion injury show that δ, μ and κ receptors are all involved in the protective mechanism of alleviating cerebral ischemia-reperfusion injury[12-13]. Studies have shown that remifentanil can regulate the function of mitochondria, and μ receptor and δ receptor are both coupled with K+channel through G protein, and both can cause the opening of K+channel after being excited[14], while the opening of ATP-sensitive potassium channel in mitochondria can alleviate the damage of mitochondrial peroxidation and may have the effect of alleviating cerebral ischemia-reperfusion injury, and the activation of δ receptor also can reduce the death of neurons caused by glutamate and tissue hypoxia[15]. In addition, opioid receptors can alleviate the activation of protein kinase A (PKA) by reducing the production of cyclic adenosine monophosphate (cAMP), so that the inhibition of PKA on potassium channels activated by Ca2+is weakened, which leads to weakened depolarization of cell membrane, decreases in the release of Glu, the activation of NMDA receptors and the influx of Ca2+and alleviated "calcium overload", and they thus play a protective role in the brain[16]. In this study, the expression levels of TNF-α, IL-6 and IL-8 in the remifentanil pretreatment group were significantly lower than those in the acute cerebral ischemia group at each time point, and the pathological reaction of nerve cells was alleviated, suggesting that remifentanil pretreatment could alleviate inflammatory factors, which is consistent with clinical reports[17].
To sum up, after remifentanil pretreatment, the brain infarct volume in rats was reduced, and the pathological reaction of neurons was alleviated, and the expression of inflammatory factors in brain tissue was reduced, indicating that remifentanil pretreatment had certain protective effect on acute cerebral ischemia injury.
- Medicinal Plant的其它文章
- Progress in the Application of Network Pharmacology in Mongolian Medicine Research
- Anti-tumor Effect of Paclitaxel Enhanced by Psoralen at the Cellular Level
- Preparation Process of Plumbagin Nanomicelle In-situ Gel
- Therapeutic Effect of Daphnetin on Mastitis Induced by Staphylococcus aureus in Mice
- Current Status and Prospects of Drugs for Ischemic Stroke Treatment
- Activity Screening Study on the Anti-tumor Effects of Extracts from Mahoniae caulis

