Current Status and Prospects of Drugs for Ischemic Stroke Treatment
Ya CAO, Lei SONG, Liying QIU
Southwest Minzu University College of Pharmacy, Chengdu 610025, China
Abstract This paper reviewed the shortcomings of conventional therapies for ischemic stroke and discussed the methods of vascular regeneration, stem cell differentiation, homing and neuronal remodeling after ischemic stroke, to provide potential therapeutic ideas for ischemic stroke.
Key words Ischemic stroke, Vascular endothelial growth factor, Angiogenesis, Neuronal remodeling, Stem cell
1 Introduction
Ischemic stroke is a cerebrovascular disease caused by a blockage of the cerebral artery. It can lead to insufficient blood supply and damage to brain tissue. There are two types of ischemic stroke: thrombotic stroke and embolic stroke[1]. The former is caused by the accumulation of thrombosis in the cerebral artery, and the latter is the appearance of thrombosis in other parts, such as the heart, neck, or chest, and into the artery of the brain, where embolized brain infarction occurs[2-3]. More than 77 million people currently suffer from ischemic strokes, and 7.6 million new ischemic strokes each year around the world[4]. Stroke is the third most common disease worldwide, with high mortality and morbidity[5]. Especially, ischemic stroke causes serious death of nerve cells[6].
Ischemic stroke has complicated pathogenesis. Generally, it results from cerebral ischemia caused by thrombosis of the cerebral artery[7]. It is manifested as atherosclerosis, which can lead to a series of pathological reactions such as brain energy failure[8], excitatory amino acid toxicity[9], inflammatory cytokines[10], free radical damage[11], and apoptosis[12]. Currently, ischemic stroke can be treated with mechanical thrombectomy and medicines. These include thrombolytic drugs, antiplatelet drugs, anticoagulants, hypolipids, neuroprotectants,etc.
However, surgical and traditional medication, are only able to solve thrombosis in order to stop ischemia, and cannot address the regeneration of infarcted tissue[13]. After ischemic stroke, cerebral blood flow decreases, causing oxygen and nutrition to decrease in the tissues and hypoxia[14]. Enhancement of angiogenesis is the most promising method of brain tissue regeneration. The reestablishment of blood supply channels will supply oxygen, nutrients and cytokines to the cerebral infarction area, guide stem cells to homing, promote axonal outgrowth/neurogenesis and then contribute to functional recovery after cerebral ischemia[15]. In brain development, neurogenesis is closely related to angiogenesis.
In this study, we analyzed the pathogenesis of ischemic stroke, the current traditional drug treatment, and the mechanisms of angiogenesis and neuronal remodeling, to provide new ideas for self-repair and functional recovery of injured brain tissue after ischemic stroke.
2 Pathogenesis for ischemic stroke
Ischemic stroke is caused by insufficient blood flow in brain tissue. There are three main causes of inadequate blood supply to brain tissue: cerebral artery blockage due to thrombosis or embolism; cerebral artery stenosis; decreased systemic blood circulation[16-18]. When the blood supply is reduced, nutrients in the brain, such as glucose and oxygen, are reduced. Moreover, the reduction of the blood supply may unbalance the metabolism of nerve cells, leading to acidosis, cell swelling, and ionic imbalance. If ischemia is prolonged, the pH of nerve cells decreases due to increased abnormal lactate levels and proton production, which is likely to lead directly to cerebral edema[19].
Furthermore, ischemic stroke is also related to ionic imbalance. As a result of hypoxia and ATP deficiency, Na+/K+-ATPase pumps fail, resulting in rapid decreases in K+flow through the cytosol to extracellular compartments, leading to cell membrane depolarization[20]. Excitotoxicity is a special neurotoxicity mediated by glutamate. The α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype of ionotropic glutamate receptors (AMPA) and the N-methyl-D-aspartate (NMDA) receptors are glutamate receptors activated by excess glutamate, Na+, and Ca2+influx into cells, which activates protein kinases, neutral proteases, phospholipases, and lipids[21]. The activation of these enzymes, however, leads to the production of NO, arachidonic acid metabolites, and superoxide, which further accelerates excitotoxicity and causes cell death[22]. In addition, a variety of cellular inflammatory factors, such as Tumor necrosis factor-α (TNF-α), Interleukin-1β (IL-1β), Interleukin-6 (IL-6), and Interleukin-8 (IL-18), cause neuronal and glial cell death during brain ischemia by activating cellular production and release of pro-inflammatory factors[23]. The involvement of various molecules in the pathogenesis of ischemic stroke was summarized in Fig.1.
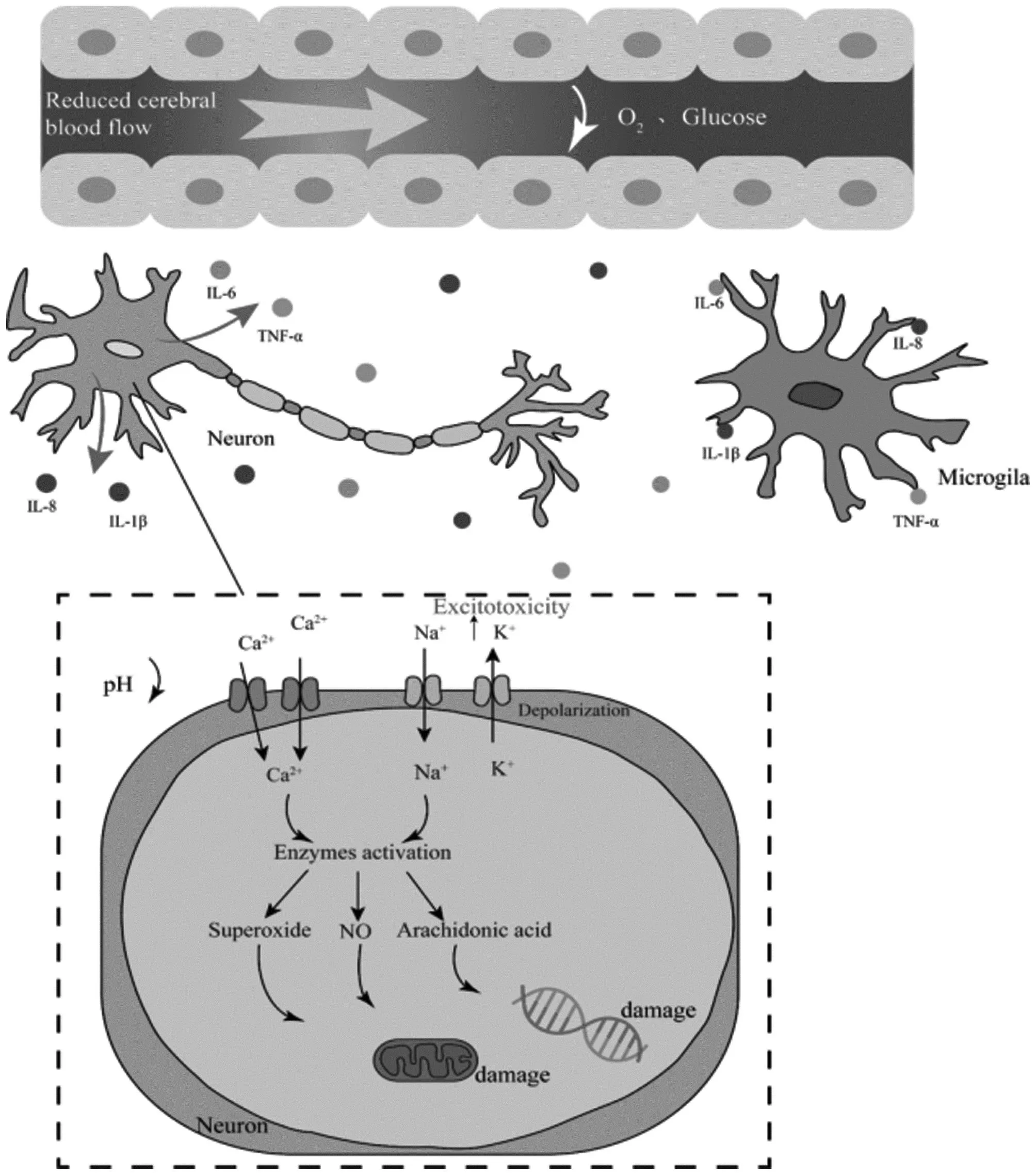
Fig.1 Various molecules in the pathogenesis of ischemic stroke

Fig.2 Copper is involved in the regulation of ischemic regeneration by HIF-1
3 Traditional drug treatment
3.1 Thrombolytic drugsThrombolysis is a common treatment for ischemic stroke. As times have changed, the main thrombolytic drugs used at different times have changed as well. Early thera-peutic drugs include streptokinase (SK), and urokinase (UK). When used to treat ischemic stroke, SK and UK can cause symptomatic intracranial hemorrhage, and the longer duration of administration increases bleeding problems[24-25].
Tissue plasminogen activator (tPA) is the only FDA-approved thrombolytic drug for the treatment of acute ischemic stroke. The thrombolytic mechanism of tPA is the activation of endogenous fibrinogen into fibrinolytic enzymes, leading to thrombus rupture[26]. It is widely used for intravenous or intravascular administration. The study revealed that injections of tPA within 4.5 h after stroke could reduce neuronal death and cerebral infarction area. However, because of the narrow time window of tPA treatment, cerebral hemorrhage may occur during treatment, resulting in secondary injury[27]. Recombinant tissue plasminogen activator (r-tPA) is a molecule in which tPA is genetically modified to activate zymogen plasminogen to produce active plasminase to destroy thrombosis[28]. Nevertheless, since r-tPA poses a risk of bleeding, people are looking for more thrombolytic agents.
Over the last few years, several fibrinolytic agents have been found and applied to treat ischemic stroke, such as alteplase, pamiteplase, ignificantl, monteplase, and lanoteplase. Despite the long half-lives of these thrombolytic agents, their safety and effectiveness have not improved ignificantly[27]. A novel drug named Staphylokinase for acute ischemic stroke has been developed that has a low probability of symptomatic intracranial hemorrhage and serious adverse events compared to alteplase. It has been shown to be safer than alteplase in small trials, but further studies are needed because of inadequate clinical trial results[29]. Although this result shows good promise for application, there are still some studies left to complete.
3.2 Antiplatelets and Anticoagulants drugHemostasis is a complex process in which platelets and clotting factors act to stop bleeding, a process that can lead to thrombosis and obstruction of coronary or cerebral vessels, a predisposing event for myocardial infarction and ischemic stroke[30]. Antiplatelet drugs and anticoagulants play a significant role in treating ischemic stroke. Prevention of ischemic strokes can also be done with antiplatelet and anticoagulant treatment.
Aspirin is an antiplatelet. Aspirin acetylates Cyclooxygenase-1(COX-1), preventing the conversion of arachidonic acid into intraperoxides of prostaglandins, which are intermediates that produce ThromboxaneA2 (TXA2). Platelet aggregation and vasocompression are mediated by TXA2, and aspirin studies reduced hemorrhage[31]. As one of the most commonly used antiplatelet medications in the world, clopidogrel is essential in the secondary prevention of ischemic stroke. It is an ADP receptor inhibitor that controls platelet aggregation without obstructing the metabolism of arachidonic acid[32]. In addition, Ticagrelor can also be used in the prevention of ischemic stroke[33]. These Purinergic Receptor P2Y12 inhibitors combined with aspirin are more effective in preventing recurrent stroke than aspirin alone and have a reduced risk of bleeding[34]. Vorapaxar, a novel antiplatelet agent, does not appear to have a significantly increased risk of hemorrhagic conversion or death in patients treated with first ischemic stroke[35]. Furthermore, either tirofiban alone intravenously or in combination with other drugs intra-arterially reduces mortality in patients with ischemic stroke without the risk of bleeding[36]. Certain antiplatelet drugs are also used to treat ischemic stroke, including Eptifibatide[37]and Abciximab[38].
Traditional anticoagulants include heparin and vitamin K antagonists, and several new anticoagulants have been developed in the last decade or so. Heparins of low molecular weight and regular heparin bind to antithrombin and increase its activity, which indirectly inhibits a number of coagulation factors[39]. Warfarin is typical of vitamin K antagonists. It inhibits the breakdown of vitamin K epoxide, which activates coagulation factors and delays the development of thrombin and fibrin clots, preventing vitamin K regeneration[40]. The use of warfarin before stroke can prevent thrombosis from narrowing and reducing stroke levels[41]. Moreover, Dabigatran is a direct thrombin inhibitor that deactivates enzymes by directly binding to thrombin, preventing fibrin clots from forming. Several Xa-factor inhibitors, including rivaroxaban, apixaban, and edoxaban, can be used to prevent systemic embolism and stroke[39].
3.3 Neuroprotective agentsIschemic stroke can lead to serious consequences, including cerebral infarction, permanent brain injury, and neurological dysfunction[42]. Stroke exacerbates neuronal damage by producing various pro-inflammatory cytokines and Reactive oxygen species (ROS)[43-44]. Therefore, Antioxidant therapy is essential for nerve damage. Neuroprotective agents used alone are ineffective and often used in combination with other drugs. Other categories of neuroprotective agents include gamma-aminobutyric acid (GABA) agonists, calcium channel blockers, calcium chelators, glutamate inhibitors,etc.A list of various novel neuroprotective agents has been presented in the Table 1.

Table1 List of some novel neuroprotective agents
In addition, studies have shown that opioid antagonists such as Naloxone, Naltrexone, and Nalmefene can minimize blood brain barrier (BBB) damage, reduce stroke severity and promote nerve recovery[58]. Some growth factors, such as erythropoietin(EPO)[59], Haematopoietic growth factors (HGFs)[57], and Granulocyte colony-stimulating factor (G-CSF)[60]have good potential in treating ischemic strokes because they can stimulate angiogenesis and neuroregeneration and reduce neuroinflammation. Preclinical studies have shown that neuroprotective agents can significantly improve cerebral infarction area and brain function, but clinical studies are not effective. The prospect of the neuroprotective agent in the treatment of ischemic stroke is not clear.
4 Current challenges in traditional therapy
Until now, thrombolytic therapy and endovascular thrombectomy are effective in the treatment of acute ischemic stroke[61]. Clinical studies and available literature showed that the success rate of single-drug therapy is low and multiple-drug combination treatment can improve the success rate for better treatment and prevention of ischemic stroke. Thrombolytic drugs need to be treated within the time window (4.5 h of the onset of stroke symptoms)[62], and brain hemorrhage can occur over time, causing additional damage to the brain. Thrombolysis can only dissolve thrombosis to stop ischemia, not limit the damage caused by inflammation during reperfusion[63]. Although antiplatelet and anticoagulant drugs help prevent thrombosis to some extent, they may cause intracranial hemorrhage and even gastrointestinal bleeding[31,64]. Preclinical studies have shown that neuroprotective agents can reduce the size of cerebral infarcts and improve functional prognosis, but clinical studies are ineffective and further research is needed[65]. Research data on antihypertensive drugs and cholesterol-lowering drugs in ischemic stroke are not perfect, so some research time is needed in the future. Although these treatments have some efficacy, they do not solve the problem of cerebral ischemia fundamentally and do not regenerate the area of cerebral infarction.
5 Angiogenesis after ischemic stroke
Angiogenesis is essential for recovery from ischemic stroke, which is necessary for wound healing after stimulation by hypoxia. In the human brain, angiogenesis occurs 3-4 d after ischemic injury[66]. It showed that patients with greater cerebral vascular density have better survival times and recovery outcomes than those with less vascular density[67]. The main ways to reverse ischemic injury are: regeneration of blood vessels; establishment of blood supply channels to guide the homing of stem cells[68-89]. Angiogenesis provides nutritional, growth factors to promote maturation of the nervous system[70]. Thus, angiogenesis may improve ischemic cerebral perfusion and play a role in recovery. Angiogenesis is dependent on vascular growth factors, proliferation and differentiation of stem cells, as well as a number of factors and other pathways. Increased expression of some growth factors after cerebral ischemia leads to enhanced proliferation of endogenous neural stem cell (NSCs). It then migrates from the site of birth to the site of ischemia[70]. Microvascular production provides oxygen and nutrients to create the microenvironment needed for nerve cell migration[71]. Second, microvessels provide β1 integrin signals that promote NSCs to form chain-like aggregates and migrate along the vasculature to the lesion site[72]. At the same time, during development and regeneration, blood vessels act as scaffolds in the migration of neurons in the brain, helping them to migrate to the injured area after an ischemic stroke and contributing to neuronal regeneration[73]. After proliferation and migration of NSCs, endothelial cells release brain-derived neurotrophic factor (BDNF) to stimulate NSCs and produce projection neurons and interneuroninvitro[77].
5.1 Angiogenic factors that act directly on the vascular systemAfter ischemic stroke occurs, the body passes through angiogenesis to relieve the injury caused by ischemia. However, vascular development and reconstruction require numerous signaling factors, of which there are two types of regulatory molecules that specifically act on the vascular system: One is the vascular endothelial growth factor (VEGF) and its receptor (VEGFR) structure, VEGF/VEGFR system, another class of angiopoietin (Ang) and its receptors Tie-2 the Ang/Tie-2 of the RTK family.
VEGF is the most potent cytokine known for its pro-angiogenic effect, which increases venous permeability, induces angiogenesis, and plays an important role in wound healing and other processes. VEGF expression increased significantly after cerebral ischemia, peaking at day 4 and decreasing to normal levels by day 7-10[75]. In a cerebral artery occlusion model, delayed VEGF treatment in neonatal rats promotes endothelial proliferation, ameliorates brain injury, and increases total vessel volume after neonatal stroke[76]. Zechariahetal.[77]found that intracerebroventricular injection of VEGF into mice increased brain capillary density and reduced infarct size and inflammation within 10 d and enhanced the integrity of the post-ischemic blood-brain barrier by day 21. In addition, VEGF is an important regulator of neurogenesis. In the ischemic brain, VEGF-A/VEGFR2 promotes post-ischemic neurovascular remodeling in neuroblasts migrating from the ipsilateral subventricular zone along the vasculature to the center of the ischemic lesion[78]. VEGF can also regulate the proliferation of adult hippocampal neural stem cells through MEK/ERK and PI3K/AKT dependent signaling pathways to regulate adult hippocampal neural stem cell proliferation[79]. In the rat MCAO model, 1 or 2 d after MCAO, VEGF reduces infarction volume, improves neurological defects, and stimulates angiogenesis[80]. The above studies show that VEGF can promote angiogenesis and neurogenesis after cerebral ischemia, protect ischemic neurons in the brain, improve axonal plasticity, and participate in vascular repair and functional recovery after cerebral ischemia in many ways. Copper activates vascular endothelial growth factor receptor 1 (VEGFR-1)-dependent signaling channels that inhibit cardiac hypertrophy and inhibits vascular endothelial growth factor receptor 2 (VEGFR-2)-dependent signaling channels that promote myocardial hypertrophy signaling channels[81-82].
There are four main members of the angiopoietin (Ang) family, namely Ang1, Ang2, Ang3 and Ang4. Ang1, through the tyrosine kinase receptor Tie2, can promote cell adhesion protein secretion, increase endothelial interactions, reduce vascular permeability, and inhibit thrombin-induced calcium inward flow and inflammatory responses[83]. Ang1, through the tyrosine kinase receptor Tie2, can promote cell adhesion protein secretion, increase endothelial interactions, reduce vascular permeability, and inhibit thrombin-induced calcium inward flow and inflammatory responses[84]. Linetal.[85]found that Ang-2 expression peaked after 24 h of cerebral ischemia in rats, and Tie-2 expression increased significantly and remained elevated for 2 weeks.
5.2 Related factorsGranulocyte colony-stimulating factor (G-CSF) is the main hematopoietic cytokine that controls the proliferation and differentiation of bone marrow progenitor cells into neutrophils. Sunetal.[86]found that intranasal administration of G-CSF enhances the protective effects of ischemic brain injury in rats, as evidenced by post-ischemic angiogenesis, reduction in infarct size and neurological recovery. In a chronically perfused ischemic stroke model, G-CSF treatment resulted in intracranial collateral artery growth and improved cerebral hemodynamic reserve[87]. In neonatal hypoxic-ischemic encephalopathy, G-CSF is neuroprotective against neonatal brain injury by inhibiting apoptosis and inflammation[88].
Basic fibrobast growth factor (bFGF) is a well-known neuroprotective and angiogenic molecule[89]. Intravenous bFGF injection reduced cortical infarction volume and improved motor function 2 h after occlusion to MCAO rats[90]. bFGF is upregulated in peripheral cells after cerebral ischemia, as well as around early infarction after ischemic stroke. It activates pericellular function through interaction with platelet-derived growth factor (PDGF-BB), which may contribute to nerve protection and angiogenesis[91]. Intranasal delivery of bFGF directly to the brain improves behavior recovery in MCAO rats[92].
Insulin-like growth factor-1 (IGF-1) is considered to be an important neuroprotective factor against cerebrovascular ischemia[93]. Previous studies have shown that, IGF-1 levels increase during brain injury and are involved in neuroinflammation recovery[94]. IGF-1 was injected subcutaneously 30 and 120 min after stroke, and the treatment was found to reduce the infarction area and increase motor function[95].
The self-repair mechanism is initiated immediately after ischemic injury occurs. Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that maintains the oxygen state in hypoxia by participating in angiogenesis, cell proliferation and survival, glucose metabolism[96-97]. HIF-1 is a specific transcription factor activated in the hypoxia state. At the same time, it participates in many important physiological processes such as cardiovascular production, tumor development, anti-cerebral ischemia[98-99]. The expression of HIF-1 is related to the ischemic stroke period. In 1996, researchers first found increased expression of HIF-1α and HIF-1β in the brain when mice or rats were exposed to 60 min of hypoxia[100]. In another brain hypoxia rat model, researchers found that the expression of HIF-1α was upregulated at 4 h, peaked at 8 h, and declined at 24 h after hypoxia[101]. Therefore, considering the different expressions of HIF-1 in the brain at various periods of stroke, may bring a breakthrough in the treatment of ischemic stroke. Copper is an essential element in humans that regulates HIF-1 gene expression[102]. Copper supplementation activates the HIF-1 regulated angiogenesis gene and promotes angiogenesis[102], improving ischemia tissue regeneration. The connection between copper and HIF-1 is shown in Fig. 2.
HIF-1 is composed of HIF-1α and HIF-1β. Under normal oxygen conditions, proline residue Pro402 and Pro564 on HIF-1α subunit can be hydroxylated by proline hydroxylase (PHD) recognition. The hydroxylated HIF-1α binds to the von Hippel-Lindau (VHL) protein, resulting in ubiquitination of HIF-1α and degradation of the proteasome. When the cells are in hypoxic condition, both hydroxylation and degradation of HIF-1α are inhibited and accumulated in the cytoplasm. Copper chaperone for superoxide dismutase-1 (CCS) brings copper into the nucleus. Copper is required for the interaction of HIF-1 with HREs to initiate copper-dependent gene expression.
Sonic hedgehog (SHH) is essential for pattern formation, axon guidance, and proliferation and differentiation of neurons in the developing central nervous system[103]. Huangetal.[104]found that SHH reduced infarct size and stimulated proliferation of nestin(+) neural progenitor cells in a rat MCAO model. Chenetal.[105]found that SHH improved neurological scores and reduced infarct size, improved microvessel density in the ischemic border zone, and promoted angiogenesis and neuronal survival in a rat model of permanent middle cerebral artery occlusion. It also enhanced VEGF expression. The above studies suggest that SHH protects the brain from ischemic damage.
BDNF is one of the most studied neurotrophic factors. In rats after cerebral ischemia, attenuated BDNF levels completely negated the recovery of skilled movements, and increasing BDNF levels improved neural recovery[106]. BDNF is also involved in the expression of VEGF, which can induce vascular regeneration in endothelial cells[107]. Astragaloside IV (AST) promotes neurogenesis in MCAO rats by upregulating the expression of the BNDF/TrkB signaling pathway[108].
EPO is at low levels in normal brain, and EPO can cross the blood-brain barrier under hypoxia, and permeability increases during neurological injury. EPO is well applied to promote angiogenesis and cerebral blood flow recovery in animal models of cerebral ischemia[109-110]. In rats hypoxic for 3 or 21 d prior to permanent MCAO surgery, intracerebral EPO levels increased and infarct size decreased 24 h after ischemia[111]. In a neonatal hypoxic-ischemic rat model, EPO promotes the expression of synaptic proteins Synapsin1 and PSD95, restores axon density, and contributes to the improvement of electrophysiological properties and spatial memory performance of synapses[112]. Currently, recombinant human EPO (rhEPO) has completed Phase II in the treatment of ischemic stroke and may be a promising drug for development despite its shortcomings[113].
5.3 Stem cell populationStem cell therapy is a good way to promote brain tissue regeneration. These methods include intravenous injection of pluripotent stem cells and in situ injection of induced pluripotent stem cells[114-115]. Currently, three main types of stem cells, multifunctional stem cells and neural stem cells, and hematopoietic/endothelial stem cells, are used for ischemic stroke treatment. Stem cell homing needs to be driven by homing factors, the most prominent stem cell homing factor is the chemokine SDF-1α/CXCL12[116]. Stromal cell-derived factor-1(SDF-1), a target gene of HIF-1, is upregulated in animal models of ischemic stroke and in human brains with ischemic injury[117]. It helps to increase and participate in the repair of nerve function after ischemic injury[118]. The mobilization and homing of these stem cells depend on vascular remodeling to function. Copper-induced angiogenesis is expected to trigger natural regeneration of the brain, and improvement of the brain microenvironment through angiogenesis facilitates stem cell homing. Meanwhile, copper (Cu) can upregulate HIF-1α and indirectly increase SDF-1 level to promote stem cell homing[119]. Copper can also direct the differentiation of stem cells into damaged areas for therapeutic effects. Schematic diagram of stem cell therapy for ischemic stroke is shown in Fig.3.
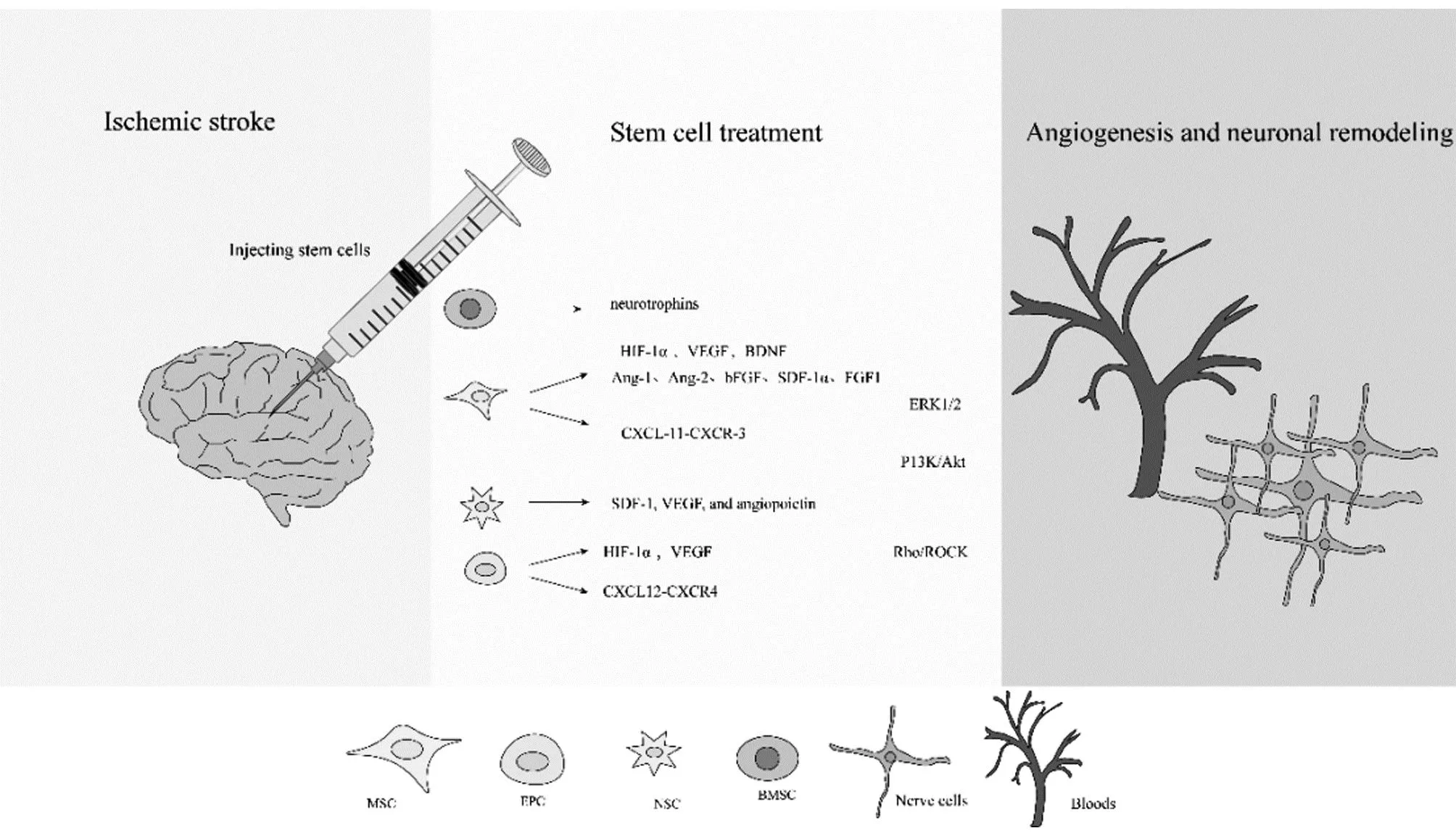
Fig.3 Molecular mechanisms of stem cell therapy in ischemic stroke
By injecting stem cells, they are induced to secrete vascular growth factors, which can open up signaling pathways that ultimately lead to vascular regeneration and neuronal remodeling. Mesenchymal stem cells (MSCs) have differentiation potential with the ability to differentiate into endothelial cells, glial cells and neurons[120]. Animal studies have shown that MSC transplantation reduces the size of cerebral infarcts and improves neurological function[121-122].Invitrostudies have shown that MSC injected into the periphery preferentially migrates to the area of injury and improves recovery of the injury site[123]. VEGF secreted by MSCs promotes vascular maturation. MSCs release CXCL-11, which binds to CXCR-3 of the endothelial cells on the blood-brain barrier, activates ERK1/2 signals and opens tight junctions[124]. Additionally, P13K/Akt pathways and Rho/ROCK signaling pathways are key to MSCs migration[125]. MSCs promote vascular regeneration and restore neural function by expressing VEGF and BDNF[126-128]. In addition, Ang-1, Ang-2, bFGF, SDF-1α, and FGF1 may help promote angiogenesis in the ischemic core and marginal regions[129-133].
After ischemic stroke, endogenous NSCs release chemokine signal SDF-1, VEGF, and angiopoietin through damaged tissue[134]. Hypoxic environments trigger differentiation of NSCs into nerve cell types to support neurogenesis. This process relies on P13k/Akt signaling pathways[135]. Transplantation of NSCs is also a promising approach for the treatment of ischemic stroke as a first step in cell-mediated restoration of homeostasis within the area of injury[136]. NSCs homing to the site of injury are able to generate new neurons and respond to various pathological changes[137-138]. In addition, intra-arterial injections provide a more direct route and are highly effective in strokes[139].
Bone marrow stem cells (BMSCs) also have promising applications in ischemic stroke. In a mouse model of ischemic stroke, BMSC migrates to the site of ischemia, upregulates neurotrophins and produces growth factors, thereby inhibiting inflammation to promote recovery[140]. Delivery of BMSC via the intranasal route allows migration through the nasal mucosa to the site of infarction and provides neuroprotection and repair[141].
In recent years, endothelial progenitor cells (EPCs) have received increasing attention, especially in the field of ischemic brain diseases. EPCs have been reported to promote neurovascular remodeling and functional recovery after brain injury[142]. The mechanism of EPCs to promote vascular regeneration may be associated with increased VEGF in plasma and upregulation of HIF-1α signaling pathways[143]. Hypoxic treated EPCs are more effective in promoting vascular remodeling through CXCL12-CXCR4 axe[144]. Zhangetal.[145]injected bone marrow-derived EPCs into adult mice and found enlarged thin-walled vessels at the border of the ischemic injury or intussusception. This somehow confirms that EPCs promote post-ischemic revascularization. Fanetal.[146]injected EPC into nude mice 1 h after tMCAO and found a significant reduction in ischemic infarct volume and increased angiogenesis in the infarcted region 3 d after tMCAO.
5.4 Other ways to promote vascular regenerationIn recent years, the role of microRNAs (miRs) in mediating angiogenesis has been widely studied. miR-210 upregulated expression of VEGF in endothelial cells promotes angiogenesis[147]. Exosome mediated targeted delivery miR-210 formation of angiogenic agents into the ischemic brain, providing a strategy for ischemic stroke treatment[148]. Upregulation miR-874-3p can activate the Wnt/β-serial protein pathway to inhibit CXCL12 expression and promote angiogenesis[149]. Other miRs that promote vascular regeneration through upregulation are miR-124, miR107, miR-26a[150-152]. However, some miRs upregulation inhibits angiogenesis after ischemia, such as miR-493, miR-27b, miR-191, miR-377[153-156].
In the traditional sense, microglia activation is generally believed to be harmful to ischemic apoplexy, but recent studies have found that microglia activation also plays a role in nerve cell generation and synaptic remodeling, thus promoting recovery after cerebral ischemia[157]. In microglia PPARγ coactivator-1α (PGC-1α) by autophagy and mitochondrial autophagy, neurological dysfunction weakens after ischemic injury[158].
6 Conclusions and prospects
Previous treatment of cerebral ischemia stroke had drawbacks, while some ways have the potential to reverse cerebral infarction and promote blood vessel regeneration. Vascular regeneration is the key to regeneration of brain tissue after ischemic injury. Post-ischemic angiogenesis may regulate axonal growth and NSCs, including the proliferation, migration and maturation of neural stem cells[68]. Microvasculature brings oxygen, nutrients and growth factors to the ischemic injury zone, creating an appropriate microenvironment for cell migration[159]. Angiogenesis may provide a suitable microenvironment to trigger axonal growth and induce neurogenesis. This approach will be of great benefit to patients with ischemic stroke. If we can better promote the regeneration of blood vessels after cerebral ischemia, while enhancing the protection and regeneration of damaged neurons, it will explore a promising path for the treatment of ischemic stroke. However, vascular regeneration also faces the following questions: (i) How can blood vessels grow in infarcted areas rather than elsewhere; (ii) How to restore function of the regenerated blood vessels, and no bleeding. These issues will be the focus of future research, and if they are solved, vascular regeneration will play a significant role in treating ischemic stroke.
- Medicinal Plant的其它文章
- Progress in the Application of Network Pharmacology in Mongolian Medicine Research
- Anti-tumor Effect of Paclitaxel Enhanced by Psoralen at the Cellular Level
- Preparation Process of Plumbagin Nanomicelle In-situ Gel
- Therapeutic Effect of Daphnetin on Mastitis Induced by Staphylococcus aureus in Mice
- Activity Screening Study on the Anti-tumor Effects of Extracts from Mahoniae caulis
- Effects of JAG-1 on the Proliferation and Migration of Gastric Adenocarcinoma Cells after TRAIP Knockout

