Study on the corrosion behavior of 316H stainless steel in molten NaCl–KCl–MgCl2 salts with and without purification
Hua Ai · Xin-Mei Yang · Hua-Jian Liu · Bing-Chuan Chen · Ling Han,3 · Hua Sun · Yan-Jun Chen,3 ·Yuan Qian · Jian-Qiang Wang
Abstract The corrosion behavior of 316H stainless steel (SS) in the impure and purified NaCl–KCl–MgCl2 salt was investigated at 700 °C.Results indicate that the main deleterious impurity induced corrosion in the impure salt was the absorbed moisture,present in the form of MgCl2·6H2O.316H SS occurred severe intergranular corrosion with a corrosion depth of 130 μm for 1000 h in the impure NaCl–KCl–MgCl2 salt.In contrast, the purification treatment of molten chloride salt by the dissolved Mg metal can remove the absorbed moisture, and the corresponding reactions were also discussed.As a result, the corrosiveness of NaCl–KCl–MgCl2 salt is reduced significantly.316H SS occurred slight uniform corrosion with a depth of less than 5 μm for 3000 h in the purified NaCl–KCl–MgCl2 salt.
Keywords Stainless steel · Alloy · Chloride salt · Molten salt corrosion · High-temperature corrosion
1 Introduction
The sustainable growth of research interests in molten chloride salts are attributed to their superior physical and chemical properties; these salts act as heat transfer mediums and coolants at elevated temperature [1, 2].Currently, they have been utilized in molten chloride salt fast reactors (MCFRs)[1, 3, 4], concentrated solar power (CSP) plants [5–9], and hydrogen manufacturing equipment.Meanwhile, it poses a rigorous challenge due to the strong corrosiveness of molten chloride salts, resulting in the poor corrosion resistance of structural materials.Gregoire et al.[10, 11] verified that the corrosion rate of Inconel 600 in molten NaCl–KCl and NaCl–KCl–MgCl2was 10 and 5 mm/year, respectively.Vignarooban et al.[12] revealed a considerably greater corrosion rate of Hastelloy N alloy (> 150 μm/year) in NaCl–KCl–ZnCl2chloride salt than in LiF–NaF–KF fluoride salt.Ding et al.[13] evaluated the corrosion rates of 310 SS(1581 μm/year), Incoloy 800H (364 μm/year), and Hastelloy C276 (79 μm/year) in NaCl–KCl–MgCl2salt, pointing out that these alloys cannot meet the corrosion resistance requirements for commercial applications (< 30 μm/year)[9, 11, 14, 15].Thus, the strong corrosiveness of molten chloride salts limits their widespread application in industrial fields.
The corrosion of chloride salts is attributed mainly to significant oxidizing impurities [16–19], such as moisture, oxygen, hydroxide ions, metallic ions, and acid radicals.Removing these impurities by the purification treatment effectively reduces the corrosiveness of molten chloride salts.After the purification treatment, the Fe-based alloys have been verified to possess excellent corrosion resistance at high temperatures [20, 21].Thus, low-cost alloys have the potential to become structural materials for industrial applications.To date, researchers have attempted several techniques to purify molten salts [9, 22–24], such as:
(1) Through the drying step upon heating the salt in an inert (Ar/N2gas) atmosphere.
(2) By a chlorinating process with CCl4, HCl, or others.
(3) By adding active Mg, Al, and Li metals into salts.
(4) By vacuum distillation to remove oxygen-containing impurities from salt.
Adding reactive metals (such as Mg and Al) to chloride molten salt is the feasible method among these purification techniques.The Al powder could diffuse inward to the alloy substrate to form an aluminum-rich layer to reduce the corrosion of structural alloys [2].However, adding Al would seriously affect the thermal-physics properties of the molten chloride salt.Ambrosek et al.[25] compared several purification methods (including Ar, Ar + CCl4, Ar + HCl, and Ar + Mg), and results showed that Mg was the preferable method to purify the MgCl2-based salts.The Mg addition could significantly affect the redox potential of molten chloride salts [26–28].Raman and infrared spectra demonstrated that Mg treatment resulted in the formation of multiple complex chloride structures with low corrosiveness of molten salt [22].Thus, the active Mg metal reduces molten salts’corrosiveness [29].However, limited studies are comparing the corrosion behavior of iron-based alloys in purified and impure salts, and these results are important for the engineering application of chloride salts.
NaCl–KCl–MgCl2(33–21.6–45.4 mol.%) ternary eutectic salt is one of the most promising chloride salts with a relatively low melting point (385 °C) and a wide range of operating temperatures.316H SS alloy is the candidate structural material for MCFR and CSP systems owing to its low cost and applicable mechanical properties at high temperatures[30, 31].The approved usage temperature of 316H SS is up to 816 °C in ASME Section III, Subsection NH.The higher the molten salt temperature, the higher the energy conversion efficiency; simultaneously, the increased corrosiveness of molten chloride salts at higher temperatures results in severe corrosion damage to structural alloys [20].The molten NaCl–KCl–MgCl2salt should be purified to solve the rigorous corrosion and reduce its corrosiveness before use.
Considering the ideas above, the corrosion behavior of 316H SS alloy in the impure and purified salt was compared to evaluate the purification effect of Mg metal.The corrosion mechanism and corresponding reactions were discussed by analysis of molten salts and alloy samples.
2 Materials and methods
2.1 Materials
The test samples used in this work was 316H SS, which is a kind of Fe-based alloy composed of 17.31 wt% Cr, 13.04 wt% Ni, 2.56 wt% Mo, 1.98 wt% Mn, 0.4 wt% Si, and 0.06 wt% C.The bulk metal was cut into samples with a dimension of 15 mm × 10 mm × 5 mm by electrical discharge machining (EDM).Before corrosion tests, 316H samples were polished to 2000 grit sequentially with emery paper,then ultrasonically cleaned by deionized water and ethyl alcohol, respectively.All alloy samples were stored in the glove box, filled with high-purity Ar gas, when the preparations were completed.
The experimental ternary eutectic NaCl–KCl–MgCl2salt was composed of 33 mol% NaCl (99.5 wt% purity, analytical grade), 21.6 mol% KCl (99.5 wt% purity, analytical grade),and 45.4 mol% MgCl2(99.9 wt% purity, analytical grade).There were two types of NaCl–KCl–MgCl2salts.A portion of these component salts was mixed at room temperature,dried at 100 oC for 24 h in a stainless steel crucible, and named the impure NaCl–KCl–MgCl2salt.The other was mixed at room temperature in a stainless steel crucible and heated at 600 oC for 24 h by inserting a Mg metal rod into the salt in a glove box.During the heating process, some metallic Mg was dissolved into the liquid salt, and the concentration of the soluble Mg was measured to be approximately 250 ± 50 mg/Kg (~ 0.025 wt%).This concentration is far below the saturation solubility [26] of Mg metal in molten NaCl–KCl–MgCl2salt.This part was named as the purified NaCl–KCl–MgCl2salt.Before the corrosion experiments, the concentrations of major impurities in these two salts were analyzed by inductively coupled plasma optical emission spectrometer (ICP-OES) and shown in Table 1.

Table 1 The major impurities in the impure and purified NaCl–KCl–MgCl2 salts before corrosion by ICP-OES (unit: ppm by weight, mg/kg)

Fig.1 (Color online) The schematic diagram of the experimental setup for the corrosion test
2.2 Corrosion experiments and characterizations
Given that the service temperature of molten chloride salts is 500–700 °C [11, 22, 27], the experimental temperature for immersion corrosion tests is set at 700 °C in this study.The experimental setup consisted of two-layer crucibles in Fig.1.The inner layer was a 316H SS crucible, which was the same material as the 316H SS samples to avoid dissimilar material interaction.The outer layer is the 316 SS crucible to ensure experimental safety.For each inner crucible, 200 g of the chloride salt (the impure or purified NaCl–KCl–MgCl2salt)was loaded with three pieces of parallel 316H SS samples to obtain the average corrosion data.Then, the outer crucible was welded and sealed to insulate it from air.All these preparations were conducted in the glove box.Then, the sealed crucibles were put in the heating furnace for different periods.The immersion times of 316H SS samples in the impure and purified NaCl–KCl–MgCl2salt were performed from 100 to 3000 h to investigate the microstructure evolution of corrosion.
After experiments, 316H SS samples were removed from the inner crucibles, cleaned with deionized water to remove residual solid salts, and dried in the air.The mass and dimension of each 316H SS sample were measured by an electronic balance and a Vernier caliper before and after corrosion.The weight change per area (Δm) for each sample was obtained through Eq.1.
wherem0is the weight of a sample before corrosion, mg;m1is the weight after corrosion, mg; S is the surface area of the sample before corrosion, cm2.After corrosion, the microstructure and elemental distribution of 316H SS samples were characterized using a scanning electron microscope (SEM, Carl Zeiss Merlin Compact) equipped with an energy-dispersive X-ray spectrometer (EDS, Oxford Instruments X-Max).The X-ray diffraction (XRD: Bruker D8 Advance) analyzed the crystalline phases of the NaCl–KCl–MgCl2salt and 316H SS samples with a Cu Kα1radiation source (λ= 1.5406 ?) at 40 kV and 40 mA.The samples were scanned in a continuousθ–2θpattern in a range of 10o ≤ 2θ≤ 90o in steps of 0.02o (2θ) and 0.15 s per step.
3 Results and discussion
3.1 Corrosion behavior of 316H SS in the impure NaCl–KCl–MgCl2 salt
The major metallic impurities in the impure NaCl–KCl–MgCl2salt before corrosion were Fe: 45 mg/Kg, Cr: 2.1 mg/Kg, Ni: 2.6 mg/Kg, and Mo: 1.5 mg/Kg.The concentrations of metallic impurities were relatively low and could not drive the severe corrosion of 316H SS.Figure 2 shows the XRD patterns of the analytical grade NaCl,KCl, and MgCl2used in this work before corrosion.The characteristic peaks of pure species NaCl, KCl, and MgCl2phases are observed clearly in Fig.2.Notably, the peaks corresponding to the MgCl2·6H2O were identified in the XRD pattern of MgCl2, suggesting that MgCl2could absorb H2O to form hydrated compounds MgCl2·xH2O (x= 6), which has been reported to be stable at room temperature [32].
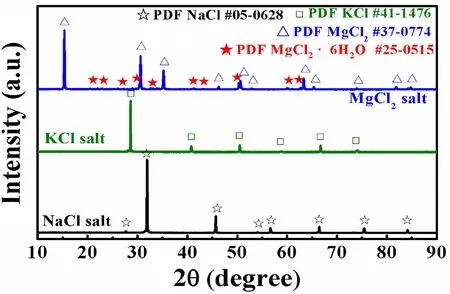
Fig.2 XRD patterns of the analytical grade NaCl, KCl, and MgCl2 salt at room temperature

Fig.3 Weight changes per area of 316H SS samples and concentrations of dissolved alloying elements in the impure NaCl–KCl–MgCl2 salt before and after corrosion
Figure 3 presents the weight changes of 316H SS samples and the concentrations of metallic elements in the impure NaCl–KCl–MgCl2salt before and after corrosion.316H SS samples underwent weight loss in the impure salt.The average weight changes per area were 4.6 ± 0.5,8.7 ± 1.0, and 7.1 ± 0.3 mg/cm2for 100, 500, and 1000 h,respectively.Fe, Cr, Ni, and Mo concentrations in the impure NaCl–KCl–MgCl2salt after corrosion remarkably increased because of the dissolution of alloying elements from the alloy matrix into molten salts.After 100 h, the concentrations of elemental Fe and Cr dissolved in the salt increased from 45 to 5582 mg/kg and from 2 to 1265 mg/kg, respectively.After corrosion for 1000 h, Fe and Cr increased progressively to 27,961 and 2141 mg/kg, respectively.Meanwhile, the conventional noble elements Ni and Mo were also corroded, and the concentrations of Ni and Mo dissolved in the salt increased from 68 to 974 mg/kg and from 24 to 728 mg/kg when the time was increased from 100 to 1000 h,respectively.The significant decrease in the weight of 316H SS samples and the increased concentration of metallic elements in the molten salt indicate that 316H SS suffered severe corrosion damage in the impure NaCl–KCl–MgCl2salt at 700 °C.
Figure 4 shows the surface morphology of 316H SS samples corroded in the impure NaCl–KCl–MgCl2salt at 700 °C for 100–1000 h.The 316H SS exhibited significant intergranular corrosion, with the alloy surface relatively rough and grain boundaries increasingly looser.EDS results showed that the contents of Cr, Fe, Ni, and Mo in the alloy surface were notably reduced compared to the original composition of 316H SS, indicating the outward diffusion of these elements from the alloy matrix to molten salts during the corrosion process.Some oval particles were deposited in the grain boundaries and marked by red lines.Combining with the EDS mappings in Fig.5, it can be assumed that these particles were relatively enriched in elements Cr, Mg,and O and poor in Fe, Ni, and Mo.Figure 6 is the XRD pattern of 316H SS samples before and after corrosion in the impure NaCl–KCl–MgCl2salt.The characteristic peaks of(111), (200), and (220) of austenite structure in the 316H SS were retained largely after corrosion.The new peaks in the XRD patterns of 316H SS after corrosion corresponded to the phase of MgCr2O4.It was deduced that the deposited precipitates enriched in Cr, Mg, and O elements in the grain boundaries should be MgCr2O4, consistent with the previous report [10, 33].

Fig.4 (Color online) Surface SEM images and the content (in weights,.wt%) of major elements in the surface of 316H SS after corrosion in the impure NaCl–KCl–MgCl2 salt at 700 °C for a 100 h, b 500 h, and c 1000 h
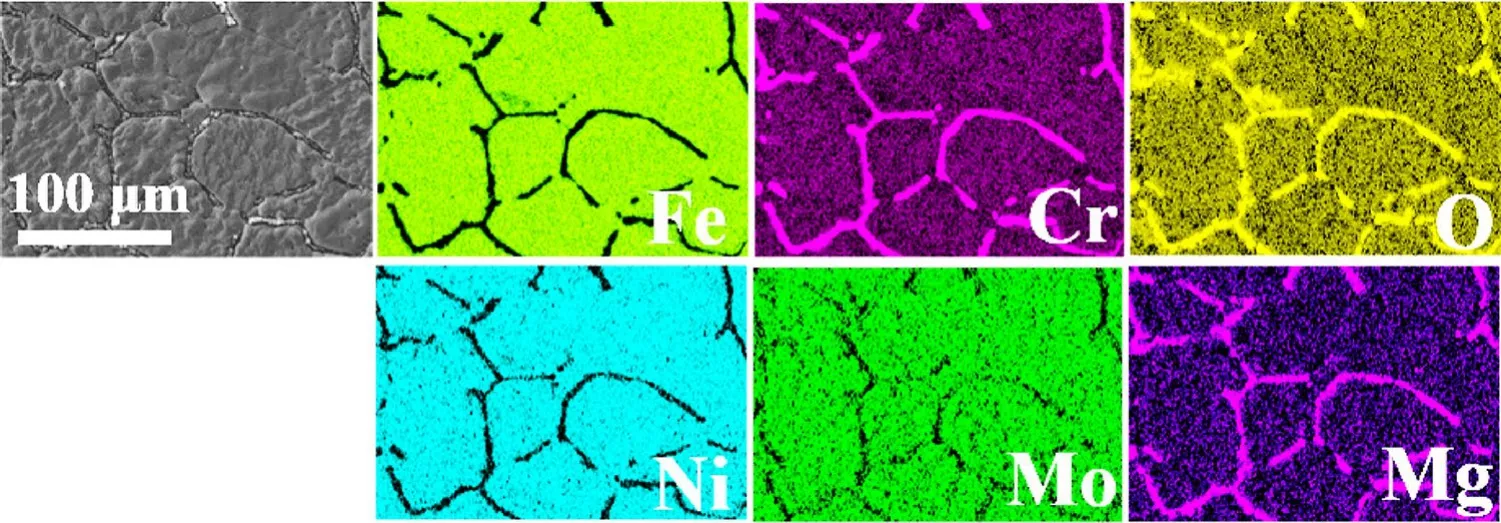
Fig.5 (Color online) SEM images and EDS mappings of elements in the 316H SS surface exposed in the impure NaCl–KCl–MgCl2 salt for 1000 h

Fig.6 XRD patterns of 316H SS samples a before corrosion and after corrosion in the impure NaCl–KCl–MgCl2 salt at 700 °C for b 100 h, c 500 h, and d 1000 h
The cross-sectional SEM images in Fig.7 reveal that all 316H SS samples underwent severe intergranular corrosion in the impure NaCl–KCl–MgCl2salt.Numerous corrosion voids were distributed along the grain boundaries.The corrosion depth of 316H SS samples was approximately 90,120, and 130 μm for 100, 500, and 1000 h, respectively.The EDS mappings (Fig.8) demonstrate that the depletion of Cr,Fe, Ni, and Mo of 316H SS samples was mainly along the grain boundaries.The Cr-depletion layer in the cross section was ~ 100 μm after corrosion for 100 h, slightly higher than the depth of intergranular corrosion voids (90 μm).Considering corrosion voids resulted from clustering vacancies, formed by the continuous depletion of alloying elements.Thus, the thickness of the Cr-depletion layer was thicker than that of corrosion voids.
Meanwhile, some precipitates enriched in Cr, Mg, and O were observed in the near-surface region, mainly attributed to the formation of MgCr2O4, confirmed by XRD and EDS results.Within the region of corrosion voids, some particles enriched in elements Mg and O were distributed along grain boundaries, which resulted from the deposition of MgO during the corrosion process.The above results indicate that 316H SS had poor corrosion resistance and exhibited intergranular corrosion in the impure NaCl–KCl–MgCl2salt; the degree of intergranular corrosion was increased with increasing time.It is shown that the impure NaCl–KCl–MgCl2salt was highly corrosive and caused extreme corrosion damage to 316H SS.Therefore, the NaCl–KCl–MgCl2salt without purification is not suitable for engineering applications.
3.2 Corrosion behavior of 316H SS in the purified NaCl–KCl–MgCl2 salt
The results in Sect.3.1 indicate that the strong corrosiveness of the impure NaCl–KCl–MgCl2salt hinders its engineering application.Therefore, Mg metal was applied to purify the molten chloride salt.Then, the corrosion behavior of 316H SS in the purified NaCl–KCl–MgCl2salt was investigated to evaluate the effectiveness of the purification treatment.

Fig.7 (Color online) The crosssectional SEM images of 316H SS samples after corrosion in the impure NaCl–KCl–MgCl2 salts at 700 °C for a 100 h, b 500 h, and c 1000 h
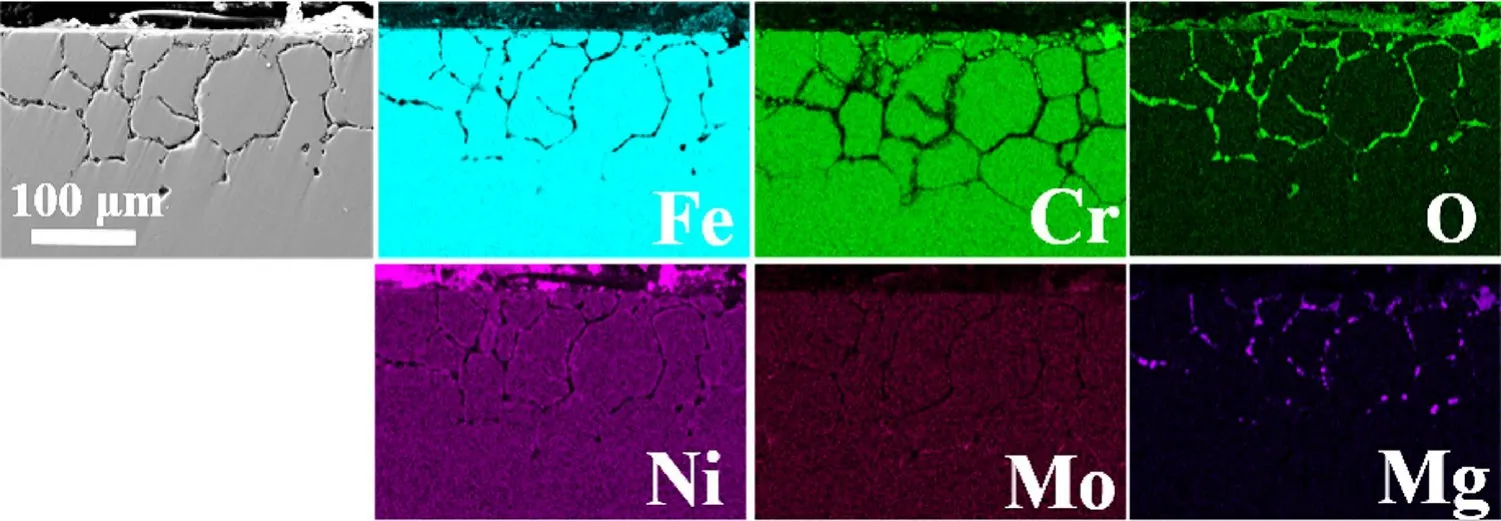
Fig.8 (Color online) Crosssectional SEM images and EDS mappings of alloying elements in the 316H SS sample after corrosion in the impure NaCl–KCl–MgCl2 salt at 700 °C for 1000 h
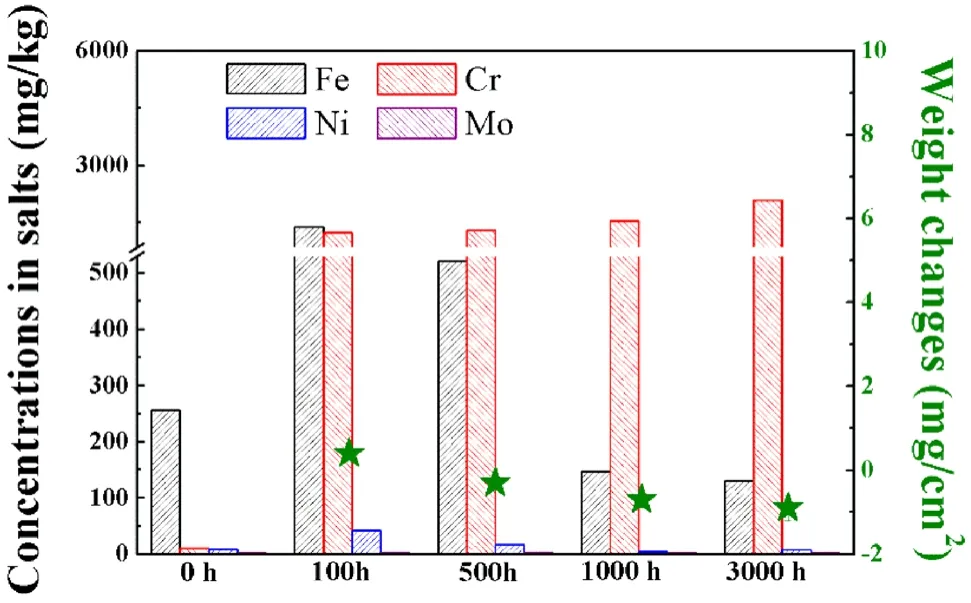
Fig.9 Weight changes per area of 316H SS samples and concentrations of dissolved alloying elements in the purified NaCl–KCl–MgCl2 salt before and after corrosion
The weight changes of 316H SS samples and concentrations of metallic elements after corrosion in the purified NaCl–KCl–MgCl2salt are shown in Fig.9, where the minus sign (-) indicates an increase in weight after corrosion.The average weight changes of 316H SS samples were 0.4 ± 0.1, - 0.3 ± 0.2, - 0.7 ± 0.2, and - 0.9 ± 0.3 mg/cm2for 100,500, 1000, and 3000 h corrosion, respectively.By comparison, the weight variation of 316H SS samples in the purified NaCl–KCl–MgCl2salt was much smaller than that obtained in the impure salt.For 100 h immersion, the weight changes in the purified salt (0.4 ± 0.1 mg/cm2) was approximately one–tenth of that in the impure NaCl–KCl–MgCl2salt (4.6 ± 0.5 mg/cm2), indicating the Mg treatment could effectively mitigate the corrosion degree of 316H SS.Moreover, the weight gain of 316H SS samples was observed after corrosion from 500 to 3000 h, which was different from the severe weight loss in the impure NaCl–KCl–MgCl2salt.Given that the dissolution of alloying elements results in weight loss, and the deposition of corrosion products may lead to an increase in weight, it is deduced that the corrosion mechanism of 316H SS in the purified salt has been changed when compared to impure NaCl–KCl–MgCl2salt.
Fe, Cr, Ni, and Mo concentrations in the purified NaCl–KCl–MgCl2salt before corrosion were 256, 10, 9,and 2 mg/kg, respectively.The high concentration of Fe was derived mainly from the stainless steel crucible, which was used to prepare the purified salt during the heating process with a Mg rod.After corrosion for 100 h, Fe, Cr, and Ni increased to 1378, 1242, and 42 mg/kg.Then, Cr was increased continually to 2090 mg/kg for 3000 h corrosion,but Fe and Ni decreased slightly with time, and the concentration of Mo in the salt remained almost unchanged.Speculatively, the corrosion of 316H SS in the purified salt was mainly dominated by the dissolution of the active Cr element, and Mg treatment distinctly alleviated the corrosion rate of 316H SS in molten NaCl–KCl–MgCl2salt.

Fig.10 Surface SEM images of 316H SS and the contents of major elements (in weights, wt%) after corrosion in the purified NaCl–KCl–MgCl2 salt at 700 °C for a 100 h, b 500 h, c 1000 h, and d 3000 h
Figure 10 shows the surface morphology of 316H SS corroded in the purified NaCl–KCl–MgCl2salt.The relatively smooth surface of the corroded samples indicated that 316H SS underwent a slight corrosion attack at 700 °C for 100–3000 h immersion.The Cr content on the alloy surface decreased, and the Fe content increased compared to the composition of the 316H SS matrix.This phenomenon was attributed to the depletion of Cr from the alloy and the accumulation of Fe on the alloy surface during the corrosion process.The cross-sectional SEM images (Fig.11)also demonstrate that 316H SS suffered minor corrosion with a maximum corrosion depth of approximately 3 μm in the purified NaCl–KCl–MgCl2salt.Figure 12 presents EDS mappings of 316H SS after corrosion for 1000 h and 3000 h.The obvious depletion of Cr and the evident enrichment of Fe were observed through the distribution of alloying elements, which was consistent with the results in Fig.10.Therefore, the reaction between Fe ions in molten salt (256 mg/Kg) and the Cr atoms in the alloy surface can be simplified asxCr +yFeClx=yFe +xCrCly(x,y= 2/3),resulting in the accumulation of Fe atoms and the depletion of Cr atoms in the corroded surface.A layer of corrosion products with a depth of 2 μm (Fig.11d) was observed on the alloy surface for 3000 h corrosion, which was confirmed to be MgO (Fig.12b) and has been reported by Ding[27] and Gregoire [11].Due to the low solubility in molten chloride salt, MgO would precipitate from the salt to form a continuous oxide layer.The relatively dense MgO layer could protect the alloy bulk against further corrosion from the molten salt.
To assess the extent of the corrosion attack, the linescan mode in EDS measured the maximum Cr-depleted thickness, and the thickness of the Cr depletion layer with the corrosion duration is summarized in Fig.13.The results showed that a rapid increase of the Cr-depleted layer in the early stage, and then a slow increase to a steady state in the stable corrosion period.In Fig.13, the maximum Cr-depleted layer was about 5 μm for 3000 h, andextrapolation indicated that the corrosion rate of 316H SS was less than 10 μm/year in the purified NaCl–KCl–MgCl2salt.The above experimental results show that the purification of the molten chloride salt through the Mg metal could significantly reduce its corrosiveness, and 316H SS exhibited excellent corrosion resistance in the purified NaCl–KCl–MgCl2salt.

Fig.11 Cross-sectional SEM images of 316H SS samples corroded in the purified NaCl–KCl–MgCl2 salt at 700 °C for a 100 h, b 500 h, c 1000 h, and d 3000 h

Fig.12 (Color online) Crosssectional SEM–EDS mappings of 316H SS samples after corrosion in the purified NaCl–KCl–MgCl2 salt at 700 °C for a 1000 h and b 3000 h

Fig.13 The thickness of the Cr depletion layer of 316H SS after corrosion for different exposing durations in the purified NaCl–KCl–MgCl2 salt at 700 °C
4 Discussion
The above experimental results reveal that 316H SS suffered severe intergranular corrosion in the impure NaCl–KCl–MgCl2salt.The corrosiveness of the salt was significantly inhibited when purified by Mg metal.Interestingly, the concentration of metallic elements in the purified NaCl–KCl–MgCl2salt was considerably higher than in the impure salt (Table 1).In contrast, the corrosiveness of the impure salt was much stronger than the purified salt.It was deduced that the corrosiveness of metallic impurities in molten salts is relatively weak, and the corrosion effects of metallic impurities are negligible.The role of Mg metal during the purification treatment was elaborated to clarify the corrosion behavior of 316H SS in molten chloride salt.
The analytical grade NaCl, KCl, and MgCl2salts were employed to prepare the ternary eutectic salt.For the impure NaCl–KCl–MgCl2salt, these analytical salts were mixed in the air atmosphere and dried at 100 °C for 24 h.The hygroscopic MgCl2absorbed moisture from the air to form hydrated compounds MgCl2·6H2O, which XRD is verified in Fig.2.The corrosion tests were conducted at 700 °C, MgCl2·6H2O occurred the hydrolysis reaction,and decomposed to H2O, HCl, and MgO, above 304 and 415 °C during the heating process through Eqs.2 and 3[11, 32].As strong oxidizing impurities, H2O and HCl both drive the corrosion of structural alloys through possible reactions shown in Table 2 [34].Gibbs free energies show that reactions among H2O, MgCl2, HCl, and major alloying elements can drive the dissolution of Fe/Cr atoms to form corresponding metal ions, resulting in the evident weight loss of 316H SS.The depletion of alloying elements was preferentially outwardly diffused along grain boundaries, resulting in the severe intergranular corrosion of 316H SS.Reactions among CrClx(x= 2, 3), H2O, and Ni/Mo can drive the corrosion of Ni/Mo to form NiCl2/MoCl3, leading to the increased concentration of Ni and Mo in salts (Fig.3), although both Ni and Mo are noble elements.During the pyrohydrolysis process, the generated O2-would react with Cr atoms to produce Cr2O3owing to the strong affinity between Cr3+and O2-ions.The formed MgO in Table 2 was mainly deposited on the alloy surface and then diffused into the corrosion voids due to its small solubility in the molten chlorides [9, 10].Besides, MgO could further react with Cr2O3to form MgCr2O4, confirmed by XRD and EDS results, mainly distributed along grain boundaries.
When the impure NaCl–KCl–MgCl2salt was purified by Mg metal at 600 oC for 24 h, some Mg atoms were dissolvedinto the molten chloride salt with a concentration of about 250 ± 50 mg/Kg (~ 0.025 wt%).Possible reactions in Table 3 show that Mg metal can react with oxidizing impurities,such as H2O, HCl, and metallic ions, to reduce their concentrations in molten chloride salts.These hazardous impurities have been pre-reacted with the dissolved Mg to form MgO and H2.Theoretically, the reactivity of active Mg metal with impurities was greater than that of alloying element with impurities because the electromotive force (EMF) of Mg2+/Mg (- 2.646 V vs.Pt) was more negative than that of Cr3+/Cr (- 1.181 V vs.Pt) and Fe3+/Fe (- 0.852 V vs.Pt) in NaCl–KCl–MgCl2eutectic salt [35].When corrosion experiments were conducted at 700 oC, oxidizing impurities could preferentially react with the dissolved Mg metal rather than alloying elements (Cr/Fe) in the 316H SS; Mg metal acted as the corrosion inhibitor to lower theconcentration of impurities and reduce the corrosiveness of molten NaCl–KCl–MgCl2salt.Besides, the salt chemistry of NaCl–KCl–MgCl2was changed through Mg treatment to reduce its corrosiveness [22].Because there was no filtering of MgO from the molten salt, the generated MgO would deposit and accumulate on the alloy surface, forming a layer of MgO to inhibit further corrosion of 316 H SS in Fig.12b.Therefore, Mg metal can effectively purify the molten NaCl–KCl–MgCl2salt, and the dissolved Mg metal(~ 0.025 wt%) used as the corrosion inhibitor to mitigate the corrosion of structural alloys.The corrosion rate of 316H SS in the purified NaCl–KCl–MgCl2salt was below 10 μm/year, which could meet the requirement of commercial applications (< 30 μm/year).Through the purification treatment of Mg metal, NaCl–KCl–MgCl2salt has the potential for engineering applications to MCFR reactors and CSP plants.

Table 2 Possible reactions and the corresponding standardGibbs free energies at 700 °C during the corrosion process of 316H SS in the impure NaCl–KCl–MgCl2 salt

Table 3 Possible reactions between Mg and impurities during the purification process in NaCl–KCl–MgCl2 salt at 600 °C and the corresponding Gibbs free energies
5 Conclusion
The corrosion behavior of 316H SS in the impure and purified NaCl–KCl–MgCl2salt at 700 °C was investigated to elaborate on the role of purification in mitigating the corrosiveness of NaCl–KCl–MgCl2salt.Results show that 316H SS suffered obvious weight loss and severe intergranular corrosion in the impure NaCl–KCl–MgCl2salt; the major metallic elements Fe, Cr, Ni, and Mo were depleted and diffused into molten salt.The corrosion depth of 316H SS was up to 130 μm for 1000 h.The strong corrosiveness of NaCl–KCl–MgCl2salt is attributed mainly to the absorbed H2O in the form of MgCl2·6H2O.This hydrated compound MgCl2·6H2O could decompose into hazardous impurities(H2O and HCl) at elevated temperatures and lead to the corrosion of 316H SS.Fortunately, the corrosiveness of the NaCl–KCl–MgCl2salt can be alleviated distinctly by the purification treatment with Mg metal.The Mg metal would act as the corrosion inhibitor to react with oxidizing impurities (H2O, HCl, metallic impurities) to reduce the corrosiveness of chloride salts.Experimental results confirmed that 316H SS exhibited excellent corrosion resistance in the purified NaCl–KCl–MgCl2salt, and the corrosion was attributed mainly to the dissolution of active Cr from the alloy matrix into molten salt.There was no obvious corrosion attack in the 316H SS.The corrosion rate of 316H SS in the purified NaCl–KCl–MgCl2salt was less than 10 μm/year, which is promising for its commercial application to the MCFR and CSP systems.
Author contributions All authors contributed to the study conception and design.Material preparation, data collection, and analysis were performed by Hua Ai, Xin-Mei Yang, and Yan-Jun Chen.The first draft of the manuscript was written by Hua Ai, and all authors commented on previous versions of the manuscript.All authors read and approved the final manuscript.
Data availability The data that support the findings of this study are openly available in Science Data Bank at https:// www.doi.org/ 10.57760/ scien cedb.j00186.00331 and https:// cstr.cn/ 31253.11.scien cedb.j00186.00331.
Declarations
Conflict of interest The authors declare that they have no competing interests.
 Nuclear Science and Techniques2023年12期
Nuclear Science and Techniques2023年12期
- Nuclear Science and Techniques的其它文章
- A machine learning approach to TCAD model calibration for MOSFET
- BL02U1: the relocated macromolecular crystallography beamline at the Shanghai Synchrotron Radiation Facility
- Coin-structured tunable beam shaping assembly design for accelerator-based boron neutron capture therapy for tumors at different depths and sizes
- Simulation study of BESIII with stitched CMOS pixel detector using ACTS
- Advances in nuclear detection and readout techniques
- Enhancing betavoltaic nuclear battery performance with 3D P+PNN+multi-groove structure via carrier evolution
