Multi-omics analysis reveals the molecular regulatory network underlying the prevention of Lactiplantibacillus plantarum against LPS-induced salpingitis in laying hens
Dan Song ,Aike Li ,Bingxu Chen ,Jia Feng ,Tao Duan ,Junlin Cheng ,Lixian Chen,Weiwei Wang*and Yuna Min*
Abstract Background Salpingitis is one of the common diseases in laying hen production,which greatly decreases the economic outcome of laying hen farming.Lactiplantibacillus plantarum was effective in preventing local or systemic inflammation,however rare studies were reported on its prevention against salpingitis.This study aimed to investigate the preventive molecular regulatory network of microencapsulated Lactiplantibacillus plantarum (MLP) against salpingitis through multi-omics analysis,including microbiome,transcriptome and metabolome analyses.Results The results revealed that supplementation of MLP in diet significantly alleviated the inflammation and atrophy of uterus caused by lipopolysaccharide (LPS) in hens (P<0.05).The concentrations of plasma IL-2 and IL-10 in hens of MLP-LPS group were higher than those in hens of LPS-stimulation group (CN-LPS group) (P<0.05).The expression levels of TLR2,MYD88,NF-κB,COX2,and TNF-α were significantly decreased in the hens fed diet supplemented with MLP and suffered with LPS stimulation (MLP-LPS group) compared with those in the hens of CN-LPS group (P<0.05).Differentially expressed genes (DEGs) induced by MLP were involved in inflammation,reproduction,and calcium ion transport.At the genus level,the MLP supplementation significantly increased the abundance of Phascolarctobacterium,whereas decreased the abundance of Candidatus_Saccharimonas in LPS challenged hens(P<0.05).The metabolites altered by dietary supplementation with MLP were mainly involved in galactose,uronic acid,histidine,pyruvate and primary bile acid metabolism.Dietary supplementation with MLP inversely regulates LPSinduced differential metabolites such as LysoPA (24:0/0:0) (P<0.05).Conclusions In summary,dietary supplementation with microencapsulated Lactiplantibacillus plantarum prevented salpingitis by modulating the abundances of Candidatus_Saccharimonas,Phascolarctobacterium,Ruminococcus_torques_group and Eubacterium_hallii_group while downregulating the levels of plasma metabolites,p-tolyl sulfate,o-cresol and N-acetylhistamine and upregulating S-lactoylglutathione,simultaneously increasing the expressions of CPNE4,CNTN3 and ACAN genes in the uterus,and ultimately inhibiting oviducal inflammation.
Keywords Laying hen,Metabolome,Microbiome,Microencapsulated Lactiplantibacillus plantarum,Salpingitis,Transcriptome
Background
According to the findings of the Food and Agriculture Organization (FAO) in 2021,poultry accounts for 38.6%of the overall global meat consumption.In terms of egg production,the global output reached 86.3878 million tons in the same year,with China alone contributing 34.4% to worldwide production [1].It is projected that in the forthcoming decade,poultry consumption will surpass half of the global meat consumption growth [2].Demand for poultry products have been shifting gradually from quantity to quality [3].The use of antibiotics is restricted in laying hens because of their possible residues in eggs.Meanwhile,the lack of antibiotics in laying hens increases the risk of bacterial inflammation such as salpingitis,which brings down the laying performance and egg quality [4].Salpingitis is a common and frequent disease in laying hens and is characterized by distension and inflammation of the oviduct and caseous exudates[5],which also is an infection of the oviduct by bacteria from the intestinal lumen and respiratory tract,resulting in 10% to 25% of the elimination rate in chickens [6].And the formation and coloring of eggshells were directly affected by inflammation in the uterus of the oviduct.This disease led to decreased animal welfare,considerable economic losses,and the risk of horizontal and vertical transmission of pathogenicE.coli[7].Consequently,preventing salpingitis through appropriate nutritional regulation is an urgent need in poultry production.Probiotics is the commonly used feed additive for nutritional regulation against inflammation,in whichLactiplantibacillus plantarumis widely used and taken as the antibiotic alternative [8].
Dietary supplementation withLactiplantibacillus plantarumincreased laying rate,improved intestinal microbiota composition [9],enhanced intestinal barrier and immune function,inhibited apoptosis [8,10],and reduced ammonia emissions [11].Lactiplantibacillus plantarumregulated the intensity of inflammatory response by enhancing mucosal barrier function and phagocytosis ability of phagocytes,stimulating immune cells to produce anti-inflammatory cytokines and antibodies,and inhibiting the secretion of pro-inflammatory cytokines [12-14].Research had shown thatLactiplantibacillus plantarumcould effectively preventE.coliinfection,regulated intestinal immune function,and effectively treated intestinal infections caused byE.coli[15].Cao et al.[16] demonstrated thatLactiplantibacillus plantarum1.2567 suppressed necrotizing enteritis by regulating intestinal mucosal immune response,improving villus structure and antioxidant defense system in broilers,and achieved anti-inflammatory effects by inhibiting neutrophil recruitment and reducing the release of inflammatory mediators.
Host-microbe interactions are closely related to health and disease.The key mechanism underlying these interactions is that metabolites derived from the microbiota not only provide the host with nutrients,but also involve in the regulation of immune development.However,it remains unclear whetherLactiplantibacillus plantarumprevents salpingitis and whether it is correlated with the altered blood metabolites derived from gut microbiome.Salpingitis of laying hens is mainly induced byE.coli,in which lipopolysaccharide (LPS) is its principal pathogenic component [17].Lipopolysaccharide,a prominent bioactive constituent of the cell wall of Gram-negative bacteria,is known to play a pivotal role in triggering immune responses,acute inflammation,and tissue damage,and is commonly used to induce local or systemic inflammation models [18].We invented a salpingitis model as a patent using LPS fromE.coliO55:B5 in hens [19].This study aims to elucidate the multi-omics molecular regulatory network for prevention of microencapsulatedLactiplantibacillus plantarum(MLP) against LPS-induced salpingitis through microbiome,metabolome and transcriptome analyses.
Materials and methods
Animal ethics
All experimental procedures were approved by the Animal Care and Use Committee of Northwest A&F University,Yangling,China.
Experimental materials
The MLP (1.0 × 1010CFU/g) was prepared by our patented emulsion technology (ZL202011208280.7).This technique used multilayer coating to enhance the stress resistance ofLactiplantibacillus plantarum[20].Lipopolysaccharide obtained fromE.coliO55:B5 was purchased from Sigma Aldrich (Shanghai,China).
Experimental design and sample collection
A total of 270 34-week-old healthy Hy-Line Brown laying hens were randomly divided into 3 groups with 6 replicates in each group and 15 birds in each replicate for 24 weeks.The hens in the control group (CN) and LPS-stimulation group (CN-LPS) were fed a basal diet,while the hens in the MLP with LPS-stimulation group(MLP-LPS) was given a diet containing 0.1% MLP.After a 24-week feeding period,a total of 3 birds were selected from each replicate (n=18).Empty capsules and capsules containing LPS at 2 mg/kg body weight were placed into the oviducts of the hens in the control (CN) and experimental groups (CN-LPS and MLP-LPS),respectively.
For the collection of blood from wing veins,one laying hen was randomly selected from each replicate after the 12 h LPS challenge.Blood samples were centrifuged for 10 min at 4 °C at 3,000 r/min and then stored at -20 °C.The laying hens were exsanguinated by inhalation anesthesia using carbon dioxide (CO2) after blood sample collection,and the oviduct was dissected immediately.In addition,a piece of uterus measuring approximately 1 cm3was fixed in formalin buffer and another piece was frozen immediately in liquid nitrogen at -80 °C for future RNAseq analysis.In the concurrent phase,fresh cecal contents were also collected for further microbiota analysis [21].
Diets and management
The basal diet was prepared based on NRC (1994) [22].The composition and nutrient levels of the basal diet were shown in Table S1,Additional file 1.It was provided with artificial light for 16 h/d and routine immunizations for the hens in a three-story stepped cage,3 hens per cage.It was kept at a temperature of 21-24 °C.During the entire experimental period,diets and water were available ad libitum.
Uterine histological assessment
After more than 24 h fixing of uterine tissue in 4% formalin buffer,the tissue samples were embedded in paraffin,sectioned,and stained with hematoxylin eosin(HE).After staining with HE,the sections of tissue samples were examined under bright field light microscope(BX43,Olympus,Tokyo,Japan) at a magnification of 200 for histopathological changes [23,24].In this study,the scoring criteria for pathology were modified from those originally published by Liu et al.[25].Pathological symptoms were classified into two categories: glandular atrophy and interstitial inflammation.Each symptom was scored separately,and the sum of the scores for each symptom was the total pathological score of the tissue.The pathological scoring criteria for glandular atrophy were as follows: 0 was normal and 1-4 points represented the percentage of < 25%,25%-50%,50%-75% and> 75% for the atrophied mucosa of the entire lamina propria,respectively.The criteria for scoring interstitial inflammation pathologically were as follows: 0 indicated normal,1 indicated mild cell infiltration,2 indicated mild cell infiltration with inflammatory lesions and capillary congestion,and 3 indicated moderate cell infiltration with inflammatory lesions and capillary congestion.
Measurement of serum cytokine concentrations
The concentrations of cytokines (IL-2,interleukin-2,Cat#: MK5249A;IL-6,interleukin-6,Cat#: MK5250A;IL-10,interleukin-10,Cat#: MK5023A; INF-γ,interferon-γ,Cat#: MK2660A) were determined using chicken-specific ELISA kits (Beijing Zhongke Kaiser Technology Co.,Ltd.,China).
Real-time PCR
The protocol for real-time PCR analysis was conducted according to the previous study [26].Total RNA extraction from uterus was performed using the RNA Easy Fast Tissue/Cell kit DP451 (Tiangen Biochemical Technology Co.,Ltd.,Beijing,China),OD260/280and OD260/230ratios were used to determine the quality of the RNA [21].Thereafter,cDNA was synthesized by PrimeScript?RT reagent kit (TaKaRa,Dalian,China).Real-time PCR was performed according to the TB Green?Premix Ex Taq?kit (TaKaRa,Dalian,China).Results of RT-qPCR were calculated through 2-ΔΔCtmethod to quantify relative gene expression [27].The sequences of primers used for real-time PCR were shown in Table S2,Additional file 1.
Transcriptome data analysis
Transcriptome analysis included RNA extraction,library preparation,and Illumina Nova seq 6000 sequencing,read mapping and differential expression analysis and functional enrichment.Using FASTQ,the raw sequencing read data were transformed into quality checks[21].The filtered reads were compared to the reference genome (Gallus_gallus,GCF_016700215.1) using HISAT2 software.To identify differentially expressed genes (DEGs),DESeq2 was applied with the parameters |log2FC| ≥ 1 andPvalue < 0.05 [28].The R language ggplots2 was employed to map the volcanoes.After finishing DEG analysis,Gene Ontology (GO) functional enrichment as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using Goatools (https://github.com/tangh aibao/Goato ols) and KOBAS (http://kobas.cbi.pku.edu.cn/home.do).In addition,theP-value was calculated by hypergeometric distribution method with significant enrichment criterionP-value < 0.05 [29].Finally,cluster analysis was performed on the selected differentially expressed genes.
Gut microbiome analysis
16S rRNA high-throughput sequencing methods included DNA extraction,PCR amplification,fluorescence quantification,Illumina library construction,and sequencing [30].For sequencing,a MiSeq PE300 platform from Illumina was used (Shanghai Majorbio Bio-Pharm Technology Co.,Ltd.,China).Data analysis was carried out on the Megisense cloud computing platform(https://cloud.major bio.com).The alpha diversity indices,including Shannon,Simpson,Chao1 and ACE,were calculated with Mothur 1.30.1 using OTU information.The linear discriminant analysis (LDA) effect size (LEfSe)was calculated to identify the significantly abundant taxa(phylum to genera) of bacteria among different groups(LDA score > 2,P<0.05) [31].
Metabolomic profiling of plasma
Analysis of non-targeted metabolomics by ultra-performance liquid chromatography-tandem time of flight mass spectrometry (UPLC/Q-TOF-MS/MS;Shanghai Majorbio Bio-pharm Technology Co.,Ltd.,China).A 100-μL liquid sample was extracted using a 1:1 solution of methanol-acetonitrile.LC-MS analysis was performed using UHPLC-Q Exactive HF-X system(Thermo Fisher Scientific),according to Li et al.[30].Data were pre-processed and analyzed on Megisense’s cloud computing platform (http://cloud.major bio.com/).PLS-DA (partial least squares-discriminant analysis) was performed by R package ropls (Version 1.6.2).Based on Student’st-test (VIP-Pred-OPLS-DA > 1,P<0.05) and Kruskal-Wallis H test (multiple group comparisons),differential metabolites were screened.The final screened differential metabolites were used to build a metabolic set on which VIP value analysis and KEGG enrichment were performed.
Statistical analysis
The data were analyzed using SPSS 20.0 (SPSS Inc.,Chicago,IL,USA).Duncan’s multiple comparison test was used to compare the differences between experimental groups to determine the effects of dietary treatment on the measured variables.The probability value ofP<0.05 was considered to be statistically significant.The data were expressed as means and pooled SEM,and graphs were generated by GraphPad Prism 8.0 software.The correlation analysis was conducted using Pearson’s correlation coefficient.
Results
Pathological evaluation of uterus and inflammatory cytokines in plasma and expression of immune-related genes of uterus
HE staining was used to observe pathological lesions of the uterus.In the CN-LPS group,multifocal inflammatory lesions were present in the interstitium of the lamina propria and the mucosal folds of the uterus showed partial atrophy.The atrophic area accounted for 25%—50% of the total lamina propria area.According to pathological score and histological analysis,the inflammation and atrophy were relieved after the addition of MLP,and the atrophic area accounted for less than 25% of the total lamina propria area (Fig.1A and B).Compared with the control group (CN),the plasma concentration of IL-6 was significantly increased and the plasma concentrations of IL-2 and IL-10 were decreased after LPS stimulation (P<0.05).The plasma concentrations of IL-2 and IL-10 in MLP-LPS group were higher than those in CN-LPS group (P<0.05).Dietary supplementation with MLP and LPS stimulation did not significantly affect the concentration of IFN-γ(P>0.05) (Fig.1C).As depicted in Fig.1D,the expression levels ofTLR2,INOS,COX2,MYD88,NF-κBandTNFαwere higher than those in CN-LPS group (P<0.05).The expression levels ofTLR2,MYD88,NF-κB,COX2,andTNF-αwere significantly decreased in the MLP-LPS group,compared with CN-LPS group (P<0.05).
Transcriptome analysis
Based on a screening for differentially expressed genes usingP<0.05,FC ≥ 2 and FC ≤ 0.5,there were 233 upregulated and 233 down-regulated DEGs in the CN-LPS groups compared to the CN group (Fig.2A).In comparison to the CN-LPS group,202 up-regulated and 71 down-regulated DEGs were detected in the MLP-LPS group (Fig.2A).Further screening of the DEGs by comparing the three groups resulted in the identification of 24 genes that were subjected to KEGG enrichment analysis (Fig.2B).It revealed that the most enriched pathways among the DEGs included PI3K-Akt signaling pathway,chemical carcinogenesis-receptor activation,inflammatory mediator regulation of TRP channels,and MAPK signaling pathway.All the pathways were related with inflammation.In addition,the expression levels ofSPP1,HK2,CYP2C18,NGF,GNG4,CHGA,MOGAT1andVTNwere upregulated in the CN-LPS group compared to the CN group,while the expression levels ofESR1,KLHL1,CA4,CPNE4,CNTN3andACANwere downregulated.In contrast to the CN-LPS group,the aforementioned genes were inversely regulated in the MLP-LPS group.(Fig.2C).

Fig.2 Analysis of uterine transcriptome data of laying hens stimulated with LPS by dietary MLP.A Volcano plot of differentially expressed genes(DEGs) in the uterus of laying hens.B KEGG signaling pathways enriched by DEGs.The size of the dots indicates the number of DEGs,and the color of the dots represents the P-value.C The heatmap of DEGs on CN,CN-LPS,and MLP-LPS groups,where the red indicates up-regulated genes,and the blue indicates down-regulated genes.CN,Basal diet;CN-LPS,Basal diet+LPS;MLP-LPS,Basal diet+MLP+LPS
To validate the results of transcriptome analysis,the relative gene expressions of candidate genes (CA4,SPP1,HK2andSOCS1) were assessed by quantitative real-time PCR (qRT-PCR).In comparison to the RNAseq results,qRT-PCR results showed a similar expression pattern,indicating reliability of the transcriptome results (Fig.S1).
Microbiological analysis
A total of 666,504 V3 16S rRNA amplicon sequence reads were analyzed.The maximum number of sequences per sample was 64,120 and the minimum number of sequences was 31,723.Alpha diversity analysis in Table S3 showed that the richness (ACE and Chao) and diversity(Shannon and Simpson) of cecal microflora were not significantly affected by dietary supplementation of MLP and LPS challenge (P>0.05),but those in the CN-LPS group had a higher abundance than the other groups.The Venn diagram showed that all three groups shared 754 common OTUs (Fig.3A).Subsequently,the composition of the cecal microbiota was analyzed at the phylum and genus levels.Bacteroidota and Firmicutes were the dominant phyla in each group.The abundance of Bacteroidota was higher and that of Actinobacteriota was lower in the CN-LPS group than those in the other treatment groups,while the abundance of Firmicutes in the CN-MLP group was higher than that in the CN-LPS and CN groups (Fig.3B).At the genus level,the abundances ofBacteroidesandRikenellaceae_RC9_gut_groupwere higher,while the abundances ofRuminococcus_torques_groupandPhascolarctobacteriumwere lower in the CN-LPS group than those in the CN and MLP-LPS groups.The abundances ofLactobacillus,FaecalibacteriumandMegamonasin MLP group were higher than those in the other treatment groups (Fig.3C and D).According to the LEfSe analysis,the microbiota of the control group was mainly enriched inCHKCI002,Eubacterium,Campylobacter,norank-f-Muribaculaceae,andPhascolarctobacterium;the microbiota enriched in the CN-LPS group wereElusimicrobiumandCandidatus-Saccharimonas,and those enriched in the MLP-LPS group wereGCA-900066575andRhodococcus(Fig.3E).Comparing the three groups,it was found that the CN-LPS group significantly increased the abundance ofCandidatus-Saccharimonaswhile decreasing the abundance ofPhascolarctobacterium(P<0.05).In contrast,compared to the CN-LPS group,the MLP-LPS group significantly increased the abundance of beneficial bacteriaPhascolarctobacterium,while decreasing the abundance ofCandidatus_Saccharimonas(P<0.05) (Fig.3F).
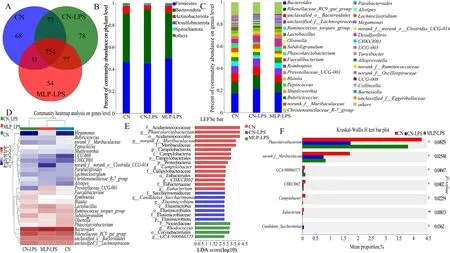
Fig.3 Effects of MLP on the cecal microbiota of laying hens challenged with LPS derived from Escherichia coli.A Venn diagram of operational taxonomic units (OTUs).B and C The cecal bacterial community compositions in control,LPS,and MLP groups at phylum and genus levels.The different colors of the bars represent different species,and the length of the bars represents the proportion of the species.D The heatmap showing the composition of the genus-level microbiota combined with the results from the cluster analysis.E Histogram of the LDA score reveals the most diferentially abundant taxa in CN,CN-LPS,and MLP-LPS groups. F Differential microorganisms were tested using the Kruskal-Wallis H test in control,LPS,and MLP groups.CN,Basal diet;CN-LPS,Basal diet+LPS;MLP-LPS,Basal diet+MLP+LPS
Metabolome analysis
PLS-DA analysis revealed that metabolites in the CN,CN-LPS,and MLP-LPS groups were clearly classified into three categories (Fig.4A).Based on the volcano plots,the CN-LPS group had 60 up-regulated and 20 down-regulated metabolites compared to the CN group.Similarly,compared with the CN-LPS group,there were 62 upregulated and 75 downregulated metabolites in the MLP-LPS group (Fig.4B).VIP analysis of the differential metabolites revealed thatp-cresol glucuronide had the largest difference between CN vs.CN-LPS and CN-LPS vs.MLP-LPS (Fig.4C).After the threegroup comparison,22 differentially expressed metabolites were further screened.Among them,organic acids and derivatives accounted for 42.11% of the differential metabolites in CN,CN-LPS,and MLP-LPS,organic heterocyclic compounds accounted for 21.05%,lipids and lipid-like molecules at 15.79%,organic oxygen compounds at 15.79%,and aromatic compounds at 5.26%(Fig.4D).KEGG enrichment analysis showed that the main enriched pathways included primary bile acid biosynthesis,galactose metabolism,ascorbate and aldarate metabolism,pyruvate metabolism and histidine metabolism (Fig.4E).As shown in Fig.5,compared with the CN group,the metabolites 2-pyrrolidinone,glycyl-lysine,hydroxylatedN-acetyl desmethyl frovatriptan,L-gulonic gamma-lactone,LysoPA (24:0/0:0),N-acetylhistamine,o-cresol,p-cresol glucuronide,andp-tolyl sulfate were significantly upregulated (P<0.05) in the CN-LPS group,while 6’-malonylcosmosiin and 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3,4,5-trihydroxyoxan-2-yl)-4Hchromen-4-one were significantly downregulated(P<0.05).The supplementation of MLP in the diet significantly alleviated the changes of metabolites induced by CN-LPS group (P<0.05),with no significant difference compared to the control group (P>0.05).

Fig.4 Effects of MLP on the plasma metabolites of laying hens challenged with LPS derived from Escherichia coli.A Partial least squares-discriminant analysis (PLS-DA) model of plasma metabolites.The x-and y-axes denote the first and second principal components,respectively.B Volcano plot of differentially plasma metabolites.C VIP scores analysis for differential plasma metabolites.Top 20 metabolites among VIP scores > 1 were selected and ranked based on VIP scores.The length of the bar indicates the value of the contribution of this metabolite to the difference between the two groups.The color of the bar indicates the significance of the difference between the two groups of samples.* means P<0.05,** means P<0.01 and *** means P<0.001.D Classification of differential metabolites in the HMDB database.E KEGG signaling pathways enriched by different metabolites (P<0.05,VIP-Pred-OPLS-DA > 1).The size of the dots indicates the number of differentially abundant metabolites and the color of the dots represents the P-value.CN,Basal diet;CN-LPS,Basal diet+LPS;MLP-LPS,Basal diet+MLP+LPS
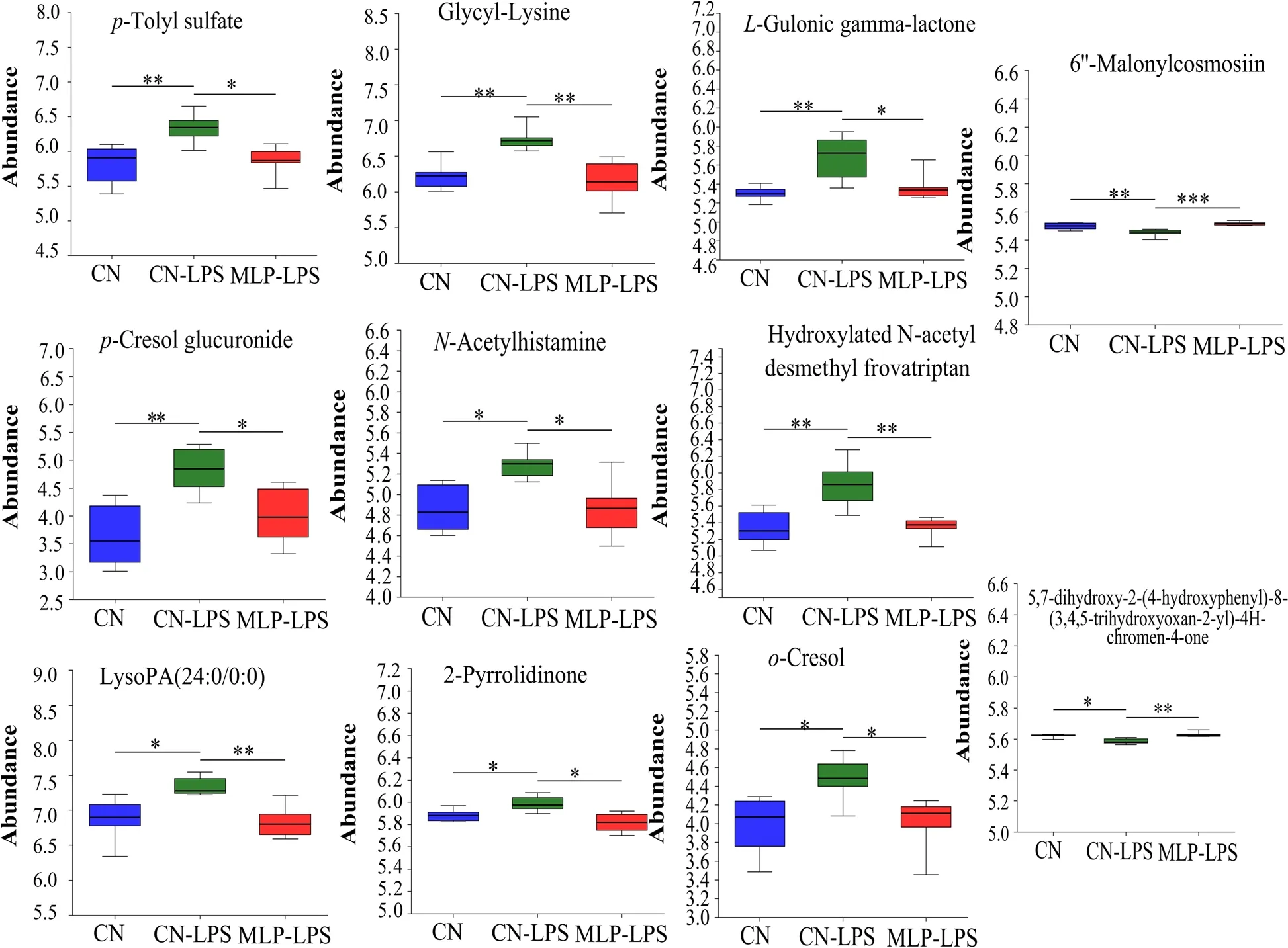
Fig.5 Significant changes in plasma differential metabolites were observed in the CN,CN-LPS and MLP-LPS groups.Significance was calculated by the Kruskal-Wallis H test.* means P<0.05,** means P<0.01 and *** means P<0.001.CN,Basal diet;CN-LPS,Basal diet+LPS;MLP-LPS,Basal diet+MLP+LPS
Correlation analysis of microbiome,metabolome and transcriptome
To further analyze the preventive mechanism of MLP supplementation in preventing LPS-induced salpingitis,a correlation analysis of the microbiome,metabolome and transcriptome was performed.S-lactoylglutathione was significantly negatively correlated withCandidatus_Saccharimonas,Alistipes,CYP2C18,VTN,andMOGAT1,while it was significantly positively correlated withMuribaculaceae,CNTN3,CPNE4,andESR1.p-Tolyl sulfate was significantly negatively correlated withCampylobacter.CPNE4,andACAN,o-cresol was significantly negatively correlated withEubacterium_hallii_group,CPNE4,CA4,andACAN,and bothp-tolyl sulfate ando-cresol were significantly positively correlated withCandidatus_Saccharimonas,Rikenellaceae_RC9_gut_group,Elusimicrobium,andVTN.N-acetylhistamine was significantly negatively correlated withPhascolarctobacterium,Ruminococcus_torques_group,Eubacterium_hallii_group,CNTN3,andACAN(Fig.6A and B).

Fig.6 Combined multi-omics analysis.Correlation analysis between significantly differential bacteria in the cecum and differential metabolites in the plasma (A),significantly differential metabolites in the plasma and expressed genes (DEGs) in the uterus (B),and effects of MLP on microorganisms,metabolites,and genes after lipopolysaccharide stimulation (C).Red indicates a positive correlation;the blue indicates a negative correlation.***,**,and * represent levels of significance (P<0.001,P<0.01,and P<0.05,respectively).DHTH,5,7-dihydroxy-2-(4-hydroxyp henyl)-8-(3,4,5-trihydroxyoxan-2-yl)-4H-chromen-4-one
Discussion
Salpingitis,a common and frequent disease of laying hens,was caused by infections with biological pathogens,particularlyEscherichia coli[32].The purpose of this study was to investigate the molecular regulatory network ofLactiplantibacillus plantarumin preventing salpingitis induced by LPS derived from pathogenicEscherichia coli.The serious inflammation and atrophy were observed in the uterus of the oviduct after LPS stimulation.In parallel,it was observed that the levels of pro-inflammatory cytokine IL-6 were significantly elevated while the levels of anti-inflammatory cytokine IL-10 in plasma were markedly reduced.LPS-induced inflammation mainly activated the expression of genes related to MAPK/NF-κB pathway [33].This study found that the expression levels ofTLR2,INOS,COX2,MYD88,NF-κBandTNF-αwere significantly increased in CNLPS group.Recently,research had indicated that the activation of the NF-κB pathway resulted in a significant inflammatory response [34].Dietary supplementation with MLP significantly alleviated LPS-induced inflammation and atrophy and changes in cytokine concentrations,which was consistent with Gong et al.[35] who found thatLactiplantibacillus plantarumimproved inflammation induced byClostridium perfringens.Compared with CN-LPS group,the expression levels ofTLR2,COX2,MYD88,NF-κBandTNF-αwere significantly decreased in MLP-LPS group.Zhao et al.[36] reported thatL.plantaruminduced M1 polarization and suppressedSalmonellae-induced inflammation by triggering TLR2/NF-κB.The inhibition by MLP against NF-κB activation may lead to lower levels of inflammatory cytokines and higher levels of anti-inflammatory cytokines,ultimately mitigating the inflammation and atrophy of oviduct caused by LPS.
To gain further insight into the mechanisms of salpingitis remission,RNA-Seq was used to assess transcriptomic changes in salpingitis alleviation in the current study.According to KEGG enrichment analysis,PI3KAkt signaling pathway was the pathway with the most enriched genes,includingSPP1,VTN,CNG4,NGF,etc.PI3K and Akt were previously shown to be involved in biological signal transduction,such as cell apoptosis [37].Akt was the direct downstream target of PI3K.NF-κB was activated by Akt,which entered the nucleus and activated target genes to release inflammatory mediators and induce inflammatory responses [38].CN-LPS group induced salpingitis by activating the PI3K-Akt signaling pathway in this study.This finding was consistent with that of Ni et al.[39] who found that sodium glucan sulfate induced ulcerative colitis through PI3K/AKT/NF-κB signaling pathway.VTN,an adhesive multifunctional glycoprotein,stimulated tissue repair and regeneration and promoted cell adhesion,migration and degradation of the matrix [40].The NF-κB signaling pathway was a positive regulator ofVTNexpression [40],hense,these results suggested that activation of the NF-κB pathway led to higher expression ofVTN.SPP1induces a proinflammatory microenvironment by recruiting leukocytes to sites of inflammation,regulating the production of cytokines and cell activation,and inhibiting lymphocyte apoptosis [41].CYP2C18is a metabolic enzymeencoding gene that is up-regulated when the body is stimulated by inflammation or toxic substances [42].The present study indicated that that CN-LPS group induced salpingitis by up-regulating the expression ofVTN,SPP1andCYP2C18.MOGAT1,a gene involved in lipid metabolism,is upregulated leading to fatty liver [43].HK2has been linked to malignant growth in many types of cancer,where it plays a key role in aerobic glycolysis[44].CA4plays a crucial role in maintaining acid-base balance in the internal environment and assists MCT4 in transportingL-lactic acid outside the cell,accelerating acidification in the external environment [45].However,downregulation ofCA4is mainly associated with tumors and cancers,such as colon cancer [46].With down-regulation ofCNTN3,EGFRmay be abnormally activated and initiate ErbB signaling to promote tumor growth and invasion [47].ACANis a major component of the extracellular matrix.Excessive production of proinflammatory factors led to increased production of matrix metalloproteinases (MMPs),other cytokines and prostaglandins,which would lead to decreased synthesis ofACANand ultimately degradation of the extracellular matrix [48].In this study,HK2andMOGAT1were upregulated andCA4,CNTN3,andACANwere downregulated in the CN-LPS group,suggesting that LPSinduced salpingitis caused abnormal lipid and sugar metabolism and altered the acid-base balance of the internal environment,degrading the extracellular matrix.Dietary supplementation with MLP inversely modulated the above genes,suggesting that MLP was effective in suppressing salpingitis induced by LPS.The oviduct is the main reproductive organ of laying hens,and the induction of salpingitis directly affects the reproductive performance of laying hens.ESR1is a ligand-dependent nuclear receptor,which is closely related to the reproductive performance of laying hens.It had been shown that chickens that were deprived of estrogen and nuclear receptors had reproductive defects,such as fewer follicles,slower ovarian follicle growth,and a reduced ability to ovulate [49].In this study,ESR1expression was highly modulated in the MLP-LPS group compared to the CNLPS group,suggesting that MLP mitigates the damage of salpingitis on the reproductive performance of laying hens through high expression ofESR1.The uterus serves as the primary site for eggshell formation;thus the regulation of calcium was crucial in the uterus.The calcium-related genes involved in this study areKLHL1,CPNE4,CHGA.KLHL1interacted with channel proteins to regulate the function of calcium ion channels,and its deletion resulted in different changes in calcium current and neuronal excitability [50].CPNE4,a gene that encodes a calcium-dependent phospholipid binding protein,is suppressed in wound infections [51].CHGAis a signaling factor secreted by chromaffin granules of neurosecretory cells and involved in Ca2+and catecholamine metabolism [52].CHGAindirectly promotes the development of intestinal inflammation by modulating epithelial repair through selective activation of macrophages [53].The results of our study indicated that CN-LPS group affected calcium ion regulation by up-regulatingCHGAand inhibiting the expression ofKLHL1andCPNE4.This suggested that salpingitis might lead to abnormal eggshell formation by affecting the expression of genes involved in calcium ion regulation.The effect of salpingitis on eggshells in the MLP-LPS group might be mitigated by down-regulatingCHGAand up-regulatingKLHL1andCNPE4.In summary,Lactiplantibacillus plantarumprevented salpingitis by inhibiting the expression of inflammatory genes,promoting the expression of anti-tumor genes,and increasing the expression of calcium-regulated genes.The effect of MLP on the expression of related genes was likely due to promoting the growth of beneficial bacteria and inhibiting the overgrowth of harmful bacteria [3,54].
The gut microbiota played a key role in intestinal ecology [55].This study indicated that dietary supplementation with MLP increased the abundance of Firmicutes,which were involved in the digestion of various nutrients and improved host health by producing SCFAs,inhibiting inflammation [56,57].Due to the increase of Firmicutes,the immune response was weakened and the intestinal barrier function was enhanced [58].By analyzing the composition of gut microbiota at the genus level,the hens in CN-LPS had higher levels ofBacteroidesandRikenellaceae_RC9_gut_group,whileRuminococcus_torques_groupandPhascolarctobacteriumwere lower than those in the CN and MLP-LPS groups.The abundances ofLactobacillus,FaecalibacteriumandMegamonasin MLP-LPS group were higher than those from other groups.Additionally,LEfSe analysis revealed that the most abundant discriminant taxon wasCandidatus-Saccharimonasin the CN-LPS group andRhodococcusin the MLP-LPS group.The benefits ofLactobacillusincluded maintaining intestinal microbiota,improving immunity,regulating cholesterol metabolism,and promoting nutrient absorption [15,59].The bacteriumFaecalibacteriumcould produce large amounts of butyrate and anti-inflammatory factors,such as shikimic and IL-10,as well as inhibit the activation of NF-κB and the production of pro-inflammatory cytokines [60,61],which also explained why MLP supplementation enhanced the plasma IL-10 level in the LPS-induced hens in this study.Megamonasinduced regulatory T-cell differentiation by producing propionate to enhance host health [62].Furthermore,Rhodococcushad a wide range of metabolic abilities,including the degradation of toxic chemicals and the synthesis of valuable compounds [63].The abundance ofBacteroideswas positively correlated with the expression of pro-inflammatory cytokines,such as IL-1β and TNF-α,which were increased when the gut was pathologically damaged and promoted inflammation,which might disrupt the intestinal barrier [59,64].Rikenellaceae RC9 gut groupwas positively correlated with harmful bacteria,and its abundance increase might further induce dysbiosis [65].TheCandidatus Saccharimonasinhibited TNF production within macrophages and was associated with inflammatory mucosal diseases[66].Ruminococcus_torques_groupwas closely associated with obesity and metabolic syndrome,and that it was beneficial for metabolism by increasing deoxycholic acid production and improving obesity through the bile-acidadipose TGR5 axis [61].Phascolarctobacteriumreduced inflammation and protected the intestinal mucosal barrier by producing propionate [67].In conclusion,Bacteroides,Rikenellaceae_RC9_gut_groupandCandidatus Saccharimonashad been found to promote the proliferation of pathogenic bacteria by disrupting the intestinal barrier,which may lead to an increase in toxic metabolites production [68,69].Conversely,Ruminococcus_torques_groupandPhascolarctobacteriumhad been shown to improve metabolism of fatty acids and bile acids and enhanced the integrity of the intestinal barrier [61,67].This explained why the upregulation of beneficial bacteria,such asPhascolarctobacterium,relieved the inflammatory response and intestinal barrier damage caused by LPS challenge.
Recently,it was shown thatLactobacillus plantumGIM17 improved colonic inflammation by increasing the relative abundance of beneficial bacteria (Actinomycetes) and raising the levels of beneficial serum metabolites [70].In addition,altered blood metabolites,such as SCFAs,derived from changed gut microbiota could ameliorate apoptosis of ovarian granulosa cells [71].To investigate the connection between the gut microbiome and distal uterine inflammation,we performed an analysis of plasma metabolite features using untargeted metabolite profiles and demonstrated significantly different plasma metabolite profiles in the CN,CN-LPS,and MLP-LPS groups.In the present study,LysoPA (24:0/0:0),p-tolyl sulfate,2-phenylethanol glucuronide,p-cresol glucuronide,N-acetylhistamine,dulcitol and taurocholic acid were significantly higher in CN-LPS group,whileS-lactoylglutathione and glycocholic acid were significantly lower expressed.LysoPA (24:0/0:0) was a lysophosphatidic acid.Rancoule et al.[72] stated that lysophosphatidic acid (LPA) was highly expressed in pathological inflammation,which was consistent with the results in this study.The high expression of LysoPA (24:0/0:0) was due to increased pro-inflammatory factors (TNF) induced by LPS,which produced a large amount of lysophospholipase D autotaxin (ATX),and ATX produced LPA [72].Dietary supplementation with MLP reduced the level of LysoPA (24:0/0:0),presumably because of reducing the level of pro-inflammatory cytokines.p-Tolyl sulfate andp-cresol glucuronide were generated by phenylalanine and tyrosine decomposition with anaerobic bacteria,which promoted the expressions of inflammatory genes,such as interleukin-6 (IL-6) [73,74].This explained why elevated levels ofp-tolyl sulfate andp-cresol glucuronide were associated with increased inflammation in the CNLPS group.In pyruvate metabolism,S-lactoylglutathione could be hydrolyzed into glutathione by hydroxyacylglutathione hydrolase [75].In this study,the CN-LPS group might reduce the amount of glutathione by loweringS-lactoylglutathione,which led to increased inflammation.In CN-LPS group,high expression ofN-acetylhistamine,a metabolite of histidine,led to abnormal histidine metabolism,which in turn impinged on histidine’s antiinflammatory effects [76].2-Phenylethanol glucuronide and dulcitol are toxic products produced by the abnormal metabolism of uronic acid and galactose,and a significant increase in the concentration of the former may result in a lack of glucose,and a high expression of the latter can trigger inflammation [77,78].Li et al.[79]showed thatL.plantarumsignificantly reduced the relative content of 2-phenylethanol glucuronide,suggesting thatL.plantarumNCU116 has a potential effect on improving metabolism of uronic acid,which was consistent with the present study.In addition,MLP altered the metabolism of primary bile acids,indicating a decrease in taurocholic acid and an increase in glycocholic acid.A previous study had pointed out that high levels of taurocholic acid caused epithelial paracellular permeability and barrier dysfunction of the ileum [80].Taken together,MLP group alleviated salpingitis by altering the composition of beneficial and harmful bacteria,downregulating galactose,uronic acid,and histidine,upregulating pyruvate,and improving primary bile acid metabolism,and then inversely regulating the expressions of LPS-induced genes involved in inflammation,reproduction,and calcium ion transport (Fig.7).

Fig.7 Schematic diagram of regulatory network of MLP for prevention of salpingitis.Arrows beside microbes,genes,and metabolite names indicate increases (upward) or decreases (downward) in microbial abundance,gene expression,and metabolite content.Blue arrows indicate CN-LPS vs.CN and green arrows indicate MLP-LPS vs.CN-LPS.The group after ‘vs.’ represents the control group
The effect ofLactiplantibacillus plantarumon salpingitis was further elucidated by analyzing the correlation of differential metabolites,microorganisms and genes.Based on the results of the correlation analysis,it was observed that the levels ofPhascolarctobacterium,Eubacterium_hallii_group,andRuminococcus_torques_groupwere elevated,while the levels ofCandidatus_SaccharimonasandRikenellaceae_RC9_gut_groupwere reduced by MLP supplementation.These changes in the microbial composition contributed to the improvement of the intestinal barrier and the reduction of inflammation [65-67,81].The intestinal barrier was improved to reduce toxic substances in the plasma,which explained the reduced levels ofN-acetylhistamine,p-tolyl sulfate,ando-cresol in the plasma [68,69].Subsequently,changes in these metabolites resulted in elevated levels of the genesACAN,CNTN3andCPNEand decreased levels ofVTN,CYP2C18andMOGAT1.Changes in these genes alleviated inflammation and cytoplasmic degradation and improved metabolism [40,42,43,47,48] (Fig.6C).
Conclusions
In summary,the molecular regulatory network of microencapsulatedLactiplantibacillus plantarumfor prevention of salpingitis is to reduce the abundance of harmful bacteriaCandidatus_Saccharimonasand increase the abundance of beneficial bacteriaPhascolarctobacterium,Ruminococcus_torques_groupandEubacterium_hallii_group,which could increase the content of beneficial metabolitesS-lactoylglutathione and reduce the content of toxic metabolitesp-toyl sulfate,o-cresol,andN-acetylhistamine.Ultimately,it promoted the expression of anti-tumor genes,increased the expression of calciumregulated genes,and reduced the degradation of extracellular matrix.This study provides valuable information for studying the prevention of salpingitis byLactiplantibacillus plantarum.
Abbreviations
ACAN Aggrecan
ASIC4 Acid sensing ion channel subunit family member 4
BIRC5 Baculoviral IAP repeat containing 5
CA4 Carbonic anhydrase 4
CHAC1 ChaC glutathione specific gamma-glutamylcyclotransferase 1
CHGA Chromogranin A
CISH Cytokine inducible SH2 containing protein
CNTF Ciliary neurotrophic factor
CNTN3 Contactin 3
COX2 Cytochrome c oxidase subunit II
CPNE4 Copine 4
CYP2C18 Cytochrome P450 family 2 subfamily C member 18
ELISA Enzyme-linked immunosorbent assay
ESR1 Estrogen receptor 1
FGF7 Fibroblast growth factor 7
GNG4 G protein subunit gamma 4
HK2 Hexokinase 2
IL-10 Interleukin-10
IL18BP Interleukin 18 binding protein
IL-1β Interleukin-1β
IL-2 Interleukin-2
IL-6 Interleukin-6
INF-γ Interferon-γ
INOS Nitric oxide synthase 2
KLHL1 Kelch like family member 1
MOGAT1 Monoacylglycerol O-acyltransferase 1
MYD88 Myeloid differentiation primary response 88
NF-κB Nuclear factor kappa B
NGF Nerve growth factor
RPS6KA2 Ribosomal protein S6 kinase A2
SOCS1 Suppressor of cytokine signaling 1
SPP1 Secreted phosphoprotein 1
SRM Spermidine synthase
TLR2 Toll like receptor 2
TLR4 Toll like receptor 4
TNF-α Tumor necrosis factor alpha
VTN Vitronectin
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1186/s40104-023-00937-x.
Additional file 1: Table S1.Ingredients and nutrient composition of the basal diet.Table S2.Sequences of real-time PCR primers.Table S3.Effect of dietary MLP on cecal microflora diversity in laying hens challenged with LPS.Fig.S1.Validation of relative expression levels of transcriptome candidate genes.
Acknowledgements
We are grateful to all the members who worked on sample collection and data statistics.We would like to thank the reviewers and editors for their valuable comments and suggestions and for their careful revision of our manuscript.
Authors’ contributions
DS conducted experimental design,animal experiments,sample analysis and wrote the manuscript.BXC participated in sample analysis.TD and JLC participated in animal feeding and sampling.LXC provided MLP.WWW,AKL,JF and YNM reviewed and edited the manuscript and contributed to the development of conceptual ideas.
Funding
This study was funded by the National Natural Sciences Foundation of China(No.32002192),Research Fund for National Non-profit Research Institution(grant number JY2016) and China Agriculture Research System of MOF and MARA (CARS-40-S20).
Availability of data and materials
Datasets used or analyzed in this study are available by reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
All experimental procedures were approved by the Animal Care and Use Committee of Northwest A&F University,Yangling,China.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Author details
1College of Animal Science and Technology,Northwest A&F University,Shaanxi,People’s Republic of China.2Key Laboratory of Grain and Oil Biotechnology of National Food and Strategic Reserves Administration,Academy of National Food and Strategic Reserves Administration,Beijing,People’s Republic of China.
Received:25 May 2023 Accepted:4 September 2023

 Journal of Animal Science and Biotechnology2024年1期
Journal of Animal Science and Biotechnology2024年1期
- Journal of Animal Science and Biotechnology的其它文章
- Integrins and their potential roles in mammalian pregnancy
- The biological functions and metabolic pathways of valine in swine
- Hepatic steatosis is associated with dysregulated cholesterol metabolism and altered protein acetylation dynamics in chickens
- Improving the nutritional values of yellow mealworm Tenebrio molitor (Coleoptera:Tenebrionidae) larvae as an animal feed ingredient: a review
- Associations of genome-wide structural variations with phenotypic differences in cross-bred Eurasian pigs
- Insights into genetic diversity and phenotypic variations in domestic geese through comprehensive population and pan-genome analysis
