Anti-OX40 Antibody Combined with HBc VLPs Delays Tumor Growth in a Mouse Colon Cancer Model*
LIU Jia Jia, SU Qiu Dong, YI Yao, SHEN Li Ping, and BI Sheng Li
National Institute for Viral Disease Control and Prevention,Chinese Center for Disease Control and Prevention,Beijing 102206,China
Abstract Objective Combination immunotherapy strategies targeting OX40,a co-stimulatory molecule that can enhance antitumor immunity by modulating the proliferation,differentiation,and effector function of tumor-infiltrating T cells,have attracted much attention for their excellent therapeutic effects.In this study,we aimed to evaluate the antitumor efficacy of combined anti-OX40 and hepatitis B core viruslike particles (HBc VLPs) therapy using a mouse colon cancer model.Methods Humanized B-hOX40 mice were injected subcutaneously with MC38 colon tumor cells and treated with HBc VLPs+anti-hOX40 antibody.Tumor growth was monitored.Flow cytometric analysis was performed to evaluate the populations of T cell subsets in the tumors.Results The combination of anti-OX40 with HBc VLPs resulted in a significant delay in tumor growth,suggesting that a potent antitumor immunity was induced by the combination therapy.Further studies revealed that HBc VLPs+anti-OX40 treatment induced a significant increase in effector T cells (Teffs) and a significant decrease in regulatory T cells (Tregs) in the tumor microenvironment (TME),which accounted for the synergistic antitumor effect of anti-OX40 in combination with HBc VLPs.Conclusion Combination therapy of anti-hOX40 and HBc VLPs provides synergistic antitumor activity in colon cancer-bearing mice,which may represent a potential design strategy for cancer immunotherapy.
Key words: Anti-OX40 antibody;Hepatitis B core virus-like particles;Tumor;Combination therapy
INTRODUCTION
In recent years,combination immunotherapies using different immunomodulatory agents have been developed and tested in the clinic to achieve improved antitumor efficacy[1,2].Among these,OX40-targeting strategies have received much attention for their excellent therapeutic efficacy[3-6].OX40 is a member of the tumor necrosis factor receptor superfamily (TNFRSF) and is mainly expressed on activated T cells.As a co-stimulatory molecule,OX40 can enhance the antitumor immune response by promoting the proliferation,differentiation and effector function of tumor-infiltrating CD4+and CD8+T cells[6-8].Agonist antibodies against OX40 have been tested in early phase clinical trials against various types of cancer.Although administration of an OX40 agonist alone improves antitumor immunity by enhancing the function of effector T cells (Teffs)while counteracting the immunosuppressive effect of regulatory T cells (Tregs),the therapeutic efficacy is limited and insufficient to improve clinical outcomes[3,4,9].Increasing evidence suggests that OX40 agonists,together with other therapeutic modalities such as radiotherapy,chemotherapy,cytokines or immunostimulants,can effectively improve antitumor immunotherapy responses[10-14].
Hepatitis B core virus-like particles (HBc VLPs),which can serve as potent immunostimulatory agents,have been developed for the immunotherapy of cancer and have shown promising therapeutic effects[15-18].HBc VLPs are hollow,spherical particles spontaneously assembled by viral structural proteins.They are inherently biocompatible and noninfectious.In addition,HBc VLPs are highly immunogenic and are capable of eliciting humoral and cellular immune responses,which may be helpful in the eradication of cancer cells[19,20].Studies have shown that HBc VLPs,either alone or as a carrier for drug delivery,can effectively inhibit tumor growth and metastasis in tumorbearing mice through the activation of T-cellmediated antitumor immune responses[21-24].In cancer treatment,these properties of HBc VLPs make them a potential option for combining with an OX40 agonist to medicate the antitumor immune response.
In this context,we hypothesized that HBc VLPs may synergistically stimulate anti-OX40-mediated tumor responses,resulting in enhanced antitumor effects.To test the therapeutic effect of HBc VLPs and OX40 agonist combination therapy,we first produced HBc VLPs using a prokaryotic expression system.The antitumor activity of combined therapy was then evaluated using the MC38 colon cancer model.We found that HBc VLPs combined with antihOX40 induced potent antitumor immunity to delay tumor growth.Furthermore,anti-hOX40 in combination with HBc VLPs led to a favorable improvement in the ratio of Teffs to Tregs in the tumor microenvironment (TME),which provided a rationale for the improved antitumor effect induced by the combination treatment.
MATERIALS AND METHODS
Construction,Expression,and Purification of HBc VLPs
AnE.colicodon-optimized sequence of the HBc gene was designed and synthesized at Shanghai General Biotech Co.,Ltd.,and cloned into pET-43.1a(+) vector (Invitrogen) using restriction endonucleasesNdeI andXhoI.The positive plasmids were confirmed by restriction endonuclease analysis and sequencing (Figure 1A).
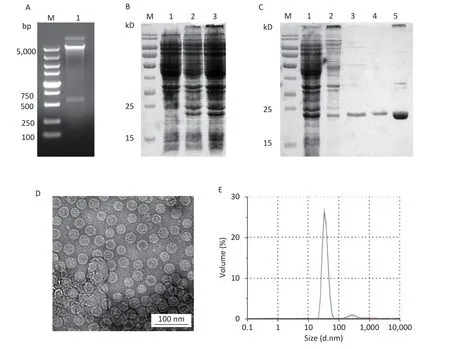
Figure 1.Expression and purification of HBc VLPs.(A) Verification of the HBc plasmid by digestion with restriction enzymes Nde I and Xho I.(B) Small-scale expression of HBc protein.Lane 1,non-induced bacteria;lanes 2-3,induced bacteria.(C) SDS-PAGE analysis of HBc protein in different stages of purification.Lane 1,total bacterial protein;lane 2,protein after ammonium sulfate precipitation;lane 3,protein eluted by SEC;lane 4,protein eluted by IEX;lane 5,HBc VLPs after density gradient centrifugation.(D) TEM image of HBc VLPs.(E) Size distribution of HBc VLPs as analyzed by DLS.HBc,Hepatitis B core virus;VLPs,virus-like particles;SDS-PAGE,sodium dodecyl sulfate polyacrylamide gel;SEC,size-exclusion chromatography;IEX,ion-exchange chromatography;DLS,dynamic light scattering.
Small-scale expression withE.coliBL21 (DE3)transformed with HBc was performed at 37 °C for 2 h.The protein expression of HBc was analyzed by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel) according to standard protocols.The expected molecular weight (MW) of HBc was 21.0 kD.Largescale preparation was performed in LB medium.Expression was induced by 1 mmol/L IPTG at 28 °C for 4 h.Cells were harvested by centrifugation (4,000×g,10 min,4 °C).Pelleted cells were resuspended in lysis buffer (500 mmol/L NaCl,10 mmol/L Tris-HCl,pH 8.0) and disrupted by sonication (300 W,25 s on,25 s off,20 times).The soluble fraction was collected by centrifugation (10,000 ×g,10 min,4 °C).For purification of HBc,saturated ammonium sulfate was added dropwise to the supernatant until the final concentration reached 20% (w/v) at 4 °C.After centrifugation (12,000 ×g,10 min,4 °C) the precipitate was dissolved in a minimal volume of 0.9% NaCl,10 mmol/L Tris-HCl,pH 8.0,and centrifuged again.The supernatant was loaded for size-exclusion chromatography (SEC) (Sepharose CL 4B,GE Healthcare,USA),a chromatographic technique that separates components in a mixture by molecular size.The flow-through fractions containing HBc were pooled and dialyzed against 100× excess of dialysis buffer (10 mmol/L Tris-HCl,pH 8.0) at 4 °C to completely remove NaCl.Then the dialyzed fractions were analyzed by ion-exchange chromatography (IEX) (DEAE Sepharose Fast Flow,GE Healthcare,USA) equilibrated with dialysis buffer.The flow-through fractions were collected while the column-bound proteins were eluted using dialysis buffer containing 100/200/400 mmol/L NaCl,respectively.After analysis by SDS-PAGE,differential ultracentrifugation with a CsCl density gradient(25%,37.5%,50%) was performed to harvest HBc VLPs.Protein concentrations were estimated by the Bradford assay.
Physicochemical Characterization of HBc VLPs
Transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses were performed to characterize the morphology and size of HBc VLPs.The samples were adsorbed on carbon-Formvar-coated copper grids and then stained with 1% phosphotungstic acid.Electron micrographs were recorded by an electron microscope (Tecain12,FEI,USA).Particle size was examined by a Zetasizer Nano ZS (Malvern,UK).
Tumor Implantation and Animal Studies
All experiments were approved by the Animal Care and Welfare Committee at the National Institute for Viral Disease Control and Prevention,Chinese Center for Disease Control and Prevention(No.20210826063).MC38 tumor cells (5 × 105) were inoculated subcutaneously into the right back of humanized B-hOX40 mice.When tumor volume reached 0.5-0.7 cm in the largest diameter,B-hOX40 mice were randomized to the experimental groups(n=8 mice per group) and injected intravenously(i.v.) using a standard protocol on days 1,7,and 14.HBc VLPs and anti-hOX40 were administered at a dose of 2.5 mg/kg and 0.15 mg/kg,respectively.All mice were sacrificed on day 17 to harvest tumors for flow cytometry analysis.Tumor size was monitored with a digital caliper every three days after inoculation and calculated as volume=length ×width2/ 2.Mice were sacrificed when the tumor volume reached 3,000 mm3as per guidelines.Mental status,nutrition and tumor growth were monitored daily during the experiment.
Anti-hOX40 antibody and B-hOX40 mice were provided by Baccetus Pharmaceutical Technology Co.,Ltd.Anti-hOX40 is a recombinant humanized OX40 IgG1 monoclonal antibody that binds OX40 with high affinity and good specificity.B-hOX40 mice is a powerful preclinical model forin vivoevaluation of anti-hOX40.Preliminary results indicate that antihOX40 is safe and effective in the treatment of advanced solid tumors.At present,anti-hOX40 Phase I clinical trial (NCT05169697) has also been approved and is underway.
Flow Cytometric Analysis of Tumor-infiltrating Lymphocytes
Tumors were excised from mice and cut into small pieces using sterile scissors.The fragments were then incubated with digestion mix and dissociated using a tissue homogenizer (Gentle MACS,Miltenyi Biotec,GER).After centrifugation(300 ×g,10 min,4 °C),a single cell suspension was obtained for flow cytometric analysis.Fluorochromelabeled antibodies were purchased from Biolegend or eBioscience,including anti-mCD45-APC/Cy7,antimCD3e-PerCP/Cy5.5,anti-mCD4-FITC,anti-mCD8a-BV605,anti-mCD16/32,anti-m/rFoxp3-PE/Cy7,antihCD134(OX40)-PE,and mIgG1-PE.Cell staining was in accordance with the manufacturer's protocol.Results were analyzed on an Attune NxT flow cytometer (Thermo Fisher,USA).
Statistical Analysis
Results are presented as Mean ± SD.Statistical significance between two groups were determined by One way ANOVA,andP≤ 0.05 were considered to be statistically significant and indicated in the figures(*P≤ 0.05,**P≤ 0.01,***P≤ 0.001).
RESULTS
Preparation and Characterization of HBc VLPs
In small-scale expression,the expected HBc protein of 21.0 kD was observed,suggesting that HBc protein could be efficiently expressed inE.coli(Figure 1B).Large-scale expression products were treated with sonic disruption for SDS-PAGE analysis.HBc protein was present in the supernatant in a soluble form.After salting out with saturated ammonium sulfate,HBc VLPs was purified by SEC and IEX (Figure 1C).Ammonium sulfate precipitation resulted in co-precipitation of the HBc protein with other host cell proteins.Fractions eluted by SEC removed most host cell proteins,but some contaminating proteins were still present.To reduce the content of contaminating proteins as much as possible,IEX was performed for further purification.After identification by SDS-PAGE,density gradient ultracentrifugation was performed to obtain purified HBc VLPs.TEM and DLS results (Figure 1D and E)showed that HBc VLPs were spherical monodisperse particles with a diameter of 32.1 ± 1.5 nm,which were consistent with the previous reports.The purified HBc VLPs were filtered using a 0.44 μm filter and stored at 4 °C at a concentration of 1 mg/mL until further analysis.
Efficacy of HBc VLPs Combined with Anti-hOX40 against Colon Cancer
The MC38 colon cancer mouse model was established by subcutaneous implantation of MC38 tumor cells into B-hOX40 mice.HBc VLPs and antihOX40 were then injected intravenously (i.v.)(Figure 2A).Tumor volume measurements were performed every three days after tumor inoculation.Body weight and daily activities of mice in each group were monitored during the treatment.Mice in each group maintained normal diet and activity throughout the observation period,and body weight changes were not significantly different for each treatment (Figure 2B).Tumor growth curves showed that treatment with HBc VLPs (P< 0.05),anti-hOX40(P< 0.05),and HBc VLPs+anti-hOX40 (P< 0.01)resulted in delayed tumor growth compared to the control group (Figure 2C and D).Of note,the tumor size in the combination treatment group was much smaller than that in the HBc VLPs group (P< 0.05;Figure 2C and D) and in the anti-hOX40 group (P<0.05;Figure 2C and D).These results indicate that HBc VLPs synergized with anti-hOX40 to induce potent antitumor tumor growth inhibition.
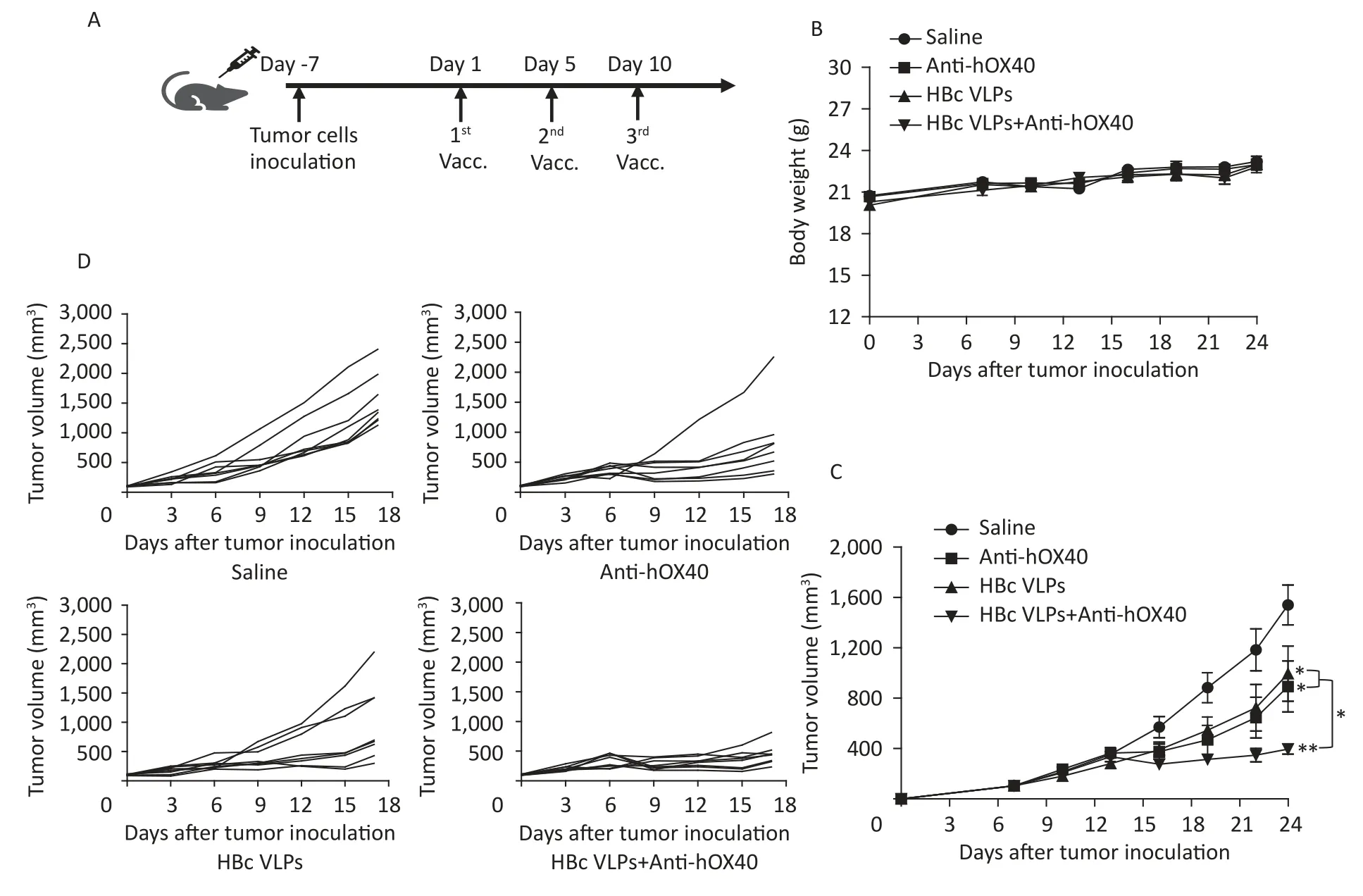
Figure 2.Injection of HBc VLPs in combination with anti-hOX40 induced a delay in tumor growth.(A)Treatment schema.MC38 tumor cells (5 × 105) were inoculated subcutaneously into the right back of BhOX40 mice.When tumors reached 0.5-0.7 cm in the largest diameter (usually on days 6 to 7 after inoculation),HBc VLPs (2.5 mg/kg) and anti-hOX40 antibody (0.15 mg/kg) were injected (i.v.) on day 1,7,14.Tumors sizes were measured every three days with a caliper.(B) Body weight changes during treatment (n=8 mice per group).(C) Tumor growth curve (n=8 mice per group).(D) Tumor volume in individual mice (n=8 mice per group).HBc,Hepatitis B core virus;VLPs,virus-like particles.Data were determined as means ± SD.*P < 0.05,**P < 0.01,***P < 0.001.
HBc VLPs Combined with Anti-hOX40 Increase T Cells Infiltration in the TME
Effective antitumor immunity requires the presence of both CD4+and CD8+T cells[10].MC38 tumor-bearing mice in all groups were sacrificed on day 3 after the last treatment for analysis of tumorinfiltrating CD4+and CD8+T cells by flow cytometry to determine how HBc VLPs combined with antihOX40 treatment affected the TME.As shown in Figure 3,treatment with HBc VLPs alone and antihOX40 alone had no significant effect on the proportions of CD4+and CD8+T cells in the TME compared with the control group.However,treatment with HBc VLPs+anti-hOX40 resulted in a significant increase in tumor-infiltrating CD4+(P<0.001;Figure 3A) and CD8+(P< 0.05;Figure 3B) T cells,which may be responsible for the synergistic antitumor effect induced by the combination therapy.These results suggest that HBc VLPs or anti-OX40 alone are not sufficient to induce a robust antitumor immune response,whereas HBc VLPs combined with anti-OX40 can enhance the immune response by increasing the populations of tumorinfiltrating CD4+and CD8+T cells.

Figure 3.HBc VLPs combined with anti-hOX40 increase CD4+ and CD8+ T cells infiltration in the TME.BhOX40 mice (n=8 mice per group) were treated as Figure 1,mice were sacrificed at day 3 after the last treatment and tumors were harvested for flow cytometry analysis.(A) Percentage of CD4+ T cells in CD45+T cells.(B) Percentage of CD8+ T cells in CD45+ T cells.Data were determined as means ± SD.*P < 0.05,**P< 0.01,*** P < 0.001.
HBc VLPs Combined with Anti-hOX40 Increase the Ratio of Teffs to Tregs in the TME
Tregs (Foxp3-CD4+),an immunosuppressive subset of CD4+T cells,play a critical role in selftolerance and immune homeostasis[25,26].It has been reported that OX40 signaling induced by anti-OX40 antibody can inhibit the function of Tregs and promote the activation of Teffs (Foxp3+CD4+),thus improving the efficacy of antitumor immunotherapy.To further understand the mechanism of immune enhancement induced by HBc VLPs and anti-hOX40 combination therapy,the proportions of Teffs and Tregs in the tumor were evaluated.The results of flow cytometry showed that HBc VLPs therapy alone and anti-hOX40 therapy alone induced a slight decrease in the number of tumor-infiltrating Tregs.However,the combination therapy of HBc VLPs and anti-hOX40 resulted in a significant decrease of Tregs compared to the monotherapies (P< 0.01;Figure 4A).Furthermore,the ratio of effector CD4+and CD8+T cells to Tregs,a critical determinant of immunotherapy success,was significantly upregulated in HBc VLPs+anti-hOX40 treated mice compared to monotherapy groups (P< 0.001 andP<0.05;Figure 4B and C).These results demonstrate that HBc VLPs combined with anti-hOX40 can shift the intratumoral immune landscape toward a highly effective antitumor state by modulating the ratio of Teffs to Tregs in the TME.This further reflects the positive antitumor effect achieved by combination therapy.

Figure 4.HBc VLPs combined with anti-hOX40 increase the ratio of Teffs to Tregs in the TME.B-hOX40 mice (n=8 mice per group) were treated as Figure 1,mice were sacrificed 3 days after the last treatment and tumors were harvested for flow cytometry analysis.(A) Percentage of Tregs (Foxp3- CD4+) in CD4+ T cells.(B) Ratio of effector CD4+ T cells to Tregs.(C) Ratio of effector CD8+ T cells to Tregs.Data were determined as means ± SD.*P < 0.05,**P < 0.01,***P < 0.001.
DISCUSSION
Strategies targeting the OX40 system have shown great potential for the treatment of cancer patients,especially in combination therapy.Various combinations of immunostimulatory agents with OX40 agonist have been developed to improve the efficacy of antitumor immunotherapy in preclinical models of cancer[27-29].A potential combination therapy of HBc VLPs and anti-hOX40 was developed in this study.The therapeutic efficacy and the antitumor immunity induced by this combination were evaluated in a murine colon cancer model.Our data showed that HBc VLPs combined with antihOX40 elicited the strongest antitumor effect,manifested by significant tumor growth delay in HBc VLPs+anti-hOX40 treated mice.Since the goal of antitumor immunity is to elicit strong,durable tumor-specific T cell responses capable,we compared the recruitment of T cells into tumors between the treatment groups by flow cytometry.Consistent with the observed antitumor effect after the combined treatment with HBc VLPs and antihOX40,this combination led to a significant increase in the number of tumor-infiltrating CD4+and CD8+T cells compared to the monotherapies.A critical role in antitumor immunity is known to be played by CD4+and CD8+T cells.CD8+T cells are usually considered to be the primary immune cells due to their ability to lyse tumor cells,while CD4+T cells have received more attention for their contribution to antitumor immunity[30,31].Previous data have shown that activated CD4+T cells can not only stimulate CD8+T cells to exert a cytolytic function against tumor cells,but can also directly kill tumor cells[32-34].The increase in tumor-infiltrating CD4+and CD8+T cells suggests that HBc VLPs combined with anti-hOX40 could enhance antitumor immunity.In addition,OX40 agonist has been reported to increase the function of effector CD4+and CD8+T cells and to counteract the function of Tregs in the TME,but the mechanism of the effect of anti-OX40 treatment on the function of these T cells is not consistent.Some studies have suggested that anti-OX40 enhances the antitumor response by directly activating Teffs and supporting their survival[7,35,36].However,it has also been suggested that anti-OX40 indirectly promotes Teffs function by impairing the suppressive function of Tregs through Tregs depletion[37-40].In our study,we found that combined treatment with HBc VLPs and anti-hOX40 reduced the number of tumor-infiltrating Tregs and resulted in increased ratios of both effector CD4+and CD8+T cells to Tregs in the TME compared to anti-hOX40 therapy alone.This is consistent with our findings of increased intratumoral T cells and enhanced antitumor efficacy in HBc VLPs+anti-hOX40-treated mice,as reduced Tregs infiltration has been correlated with enhanced antitumor immunity.
Taken together,the enrichment of T cells and depletion of Tregs in the tumor area induced by HBc VLPs+anti-OX40 treatment suggest that HBc VLPs synergized with anti-OX40 shift the intratumoral immune landscape toward a highly effective antitumor state.However,it is unclear how HBc VLPs provide co-stimulatory signals for anti-OX40 mediated antitumor immune responses.One possible explanation is that HBc VLPs increase the population of effector tumor-infiltrating T cells,making anti-OX40 responses more effective.As an immunostimulatory agent,HBc VLPs contain a large number of T cell and B cell epitopes that are capable of stimulating both humoral and cellular immune responses[23,41].Several studies showed that HBc VLPs could induce antigen-specific antitumor immunity,inhibiting tumor growth and metastasis in a subcutaneous tumor model[17,21-24].The immunostimulatory ability of HBc VLPs may enhance the antitumor effects of anti-OX40.However,our study is still at the exploratory stage.To validate this hypothesis,more detailed studies are needed to determine the exact process that occurs after treatment with HBc VLPs and anti-OX40.
Combination immunotherapy is a promising but also a highly complex topic.The critical question in combination therapy is which treatment strategies induce the best systemic antitumor immune responses,as indicated by numerous preclinical and clinical studies[42,43].In particular,the sequencing and timing of combined therapy is an important consideration.Several studies have shown that the simultaneous treatment with anti-OX40 and PD-1 inhibitor does not lead to a therapeutic benefit,whereas the sequential administration of these drugs promotes the antitumor activity[44,45].In addition,the route of administration and the dosage also complicate the efficacy of combination therapy[46].Although our current combination regimen of HBc VLPs+anti-OX40 positively affects T-cell-mediated tumor immune response,the exact mechanism by which this occurs is unclear,and much work remains to be done to explore the antitumor effect induced by HBc VLPs and anti-hOX40.
To conclude,we provide evidence that HBc VLPs plus OX40 agonist positively modulates the intratumoral immune state by increasing effector CD4+and CD8+T cells infiltration and decreasing Tregs infiltration in the TME,leading to improved therapeutic efficacy in colon cancer mice model.This finding suggests that the combination of HBc VLPs and OX40 agonist may be a potential combined strategy to reduce intratumoral immunosuppression and ultimately enhance antitumor immunity.
CONFLICT OF INTEREST
The authors declare that they have no competing interests in this paper.
ACKNOWLEDGEMENTS
The authors would like to thank Dr.YY and his colleagues in Baccetus Pharmaceutical Technology Co.,Ltd (Beijing) for providing technical support in the mouse experiments.
AUTHOR CONTRIBUTIONS
BI Sheng Li and LIU Jia Jia designed this work;SU Qiu Dong,YI Yao and LIU Jia Jia performed the expression and purification of HBc VLPs;BI Sheng Li,SHEN Li Ping and LIU Jia Jia conducted the experiments in mouse;LIU Jia Jia wrote the manuscript.All authors read and approved the final manuscript.
Received: April 10,2023;
Accepted: October 11,2023
 Biomedical and Environmental Sciences2024年2期
Biomedical and Environmental Sciences2024年2期
- Biomedical and Environmental Sciences的其它文章
- Metabolomic Analysis in Saliva and Different Brain Regions of Older Mice with Postoperative Delirium Behaviors*
- Association between Gene Polymorphisms and SNP-SNP lnteractions of the Matrix Metalloproteinase 2 Signaling Pathway and the Risk of Vascular Senescence*
- lnferring Mycobacterium Tuberculosis Drug Resistance and Transmission using Whole-genome Sequencing in a High TB-burden Setting in China*
- Effectiveness of Histopathological Examination of Ultrasoundguided Puncture Biopsy Samples for Diagnosis of Extrapulmonary Tuberculosis*
- Comparative Study on the Immunogenicity and Efficacy of Different Post-exposure Intramuscular Rabies Vaccination Regimens in China
- Relationship of Retinal Nerve Fiber Layer Thickness and Retinal Vessel Calibers with Cognitive lmpairment in the Asymptomatic Polyvascular Abnormalities Population*
