Unraveling the role of dual Ti/Mg metals on the ignition and combustion behavior of HTPB-boron-based fuel
Arijit Denth , Ysh Pl ,*, Sri Nithy Mhottmnnd , Djll Trhe
a School of Aeronautical Science, Hindustan Institute of Technology and Science, Chennai 603103, India
b Department of Aerospace Engineering, B S Abdur Rahman Crescent Institute of Science and Technology, Chennai 600048, India
c Energetic Materials Laboratory (EMLab), Teaching and Research Unit of Energetic Processes, Ecole Militaire Polytechnique, BP 17, Bordj El-Bahri,16046,Algiers, Algeria
Keywords:Boron B2O3 Opposed flow burner Combustion Magnesium
ABSTRACT Metal additives play an essential role in explosive and propellant formulations.Boron(B)is widely used in propellant applications owing to its high energetic content.The addition of B to explosives and propellants increases their energy density,making them more efficient and powerful.Nevertheless,B forms oxide layers on its surface during combustion, slowing down the combustion rate and reducing rocket motor efficiency.To overcome this issue,other metal additives such as aluminum(Al),magnesium(Mg),and titanium (Ti) are revealed to be effective in boosting the combustion rate of propellants.These additives may improve the combustion rate and therefore enhance the rocket motor’s performance.The present study focused on preparing and investigating the ignition and combustion behavior of pure hydroxyl-terminated polybutadiene (HTPB)-B fuel supplemented with nano-titanium and nanomagnesium.The burn rates of HTPB-B fuel samples were evaluated on the opposed flow burner (OFB)under a gaseous oxygen oxidizer,for which the mass flux ranges from 22 kg/(m2?s)to 86 kg/(m2?s).The addition of Ti and Mg exhibited higher regression rates,which were attributed to the improved oxidation reaction of B due to the synergetic metal combustion effect.The possible combustion/oxidation reaction mechanism of B-Mg and B-Ti by heating the fuel samples at 900 °C and 1100 °C was also examined in a Nabertherm burnout furnace under an oxygen atmosphere.The post-combustion products were collected and further subjected to X-ray diffraction (XRD) and field emission scanning electron microscopy (FE-SEM) analyses to inspect the combustion behavior of B-Ti and B-Mg.It has been observed that the B oxide layer at the interface between B-Ti (B-Mg) is removed at lower temperatures, hence facilitating oxygen transfer from the surroundings to the core B.Additionally, Ti and Mg decreased the ignition delay time of B, which improved its combustion performance.
1.Introduction
The high volumetric and gravimetric heating values of B metalloids have attracted the attention of combustion researchers for their application in hybrid rocket motors and solid-fuel ramjet engines[1-3].However, despite its many advantages, B has serious problems,such as poor ignition and combustion.It is particularly sensitive to oxygen, and hence B oxide coating is easily formed on the surface of particles during ignition and combustion [4].This latter may hinder the energetic potential of B and negatively affect its reaction [4-7].The native oxide shell protects the B core from oxidizer diffusion and oxidation.It also reduces the B core oxidation rate since the oxidizer has to penetrate the shell before it can react with boron [4,7-9].Surface modification and additive/catalyst coating on B powder enhance the ignition properties and combustion efficiency[10-13].Additive/catalyst coating reduces the initial ignition temperature and increases the burning rate of B.Adding fluorine species to the combustion environment further enhances heat release and improves oxidation reactions.Boron powders naturally contain impurities that can significantly affect their ignition and combustion performance [14].To develop reliable and consistent B-based solid fuel in a variety of combustion applications,it is essential to understand their nature,origin,and influence.In B powders,impurities may affect combustion performance in a variety of ways.Firstly, impurities can affect the overall energy released during combustion, potentially altering combustion efficiency.In addition, impurities may influence the burning rate, combustion temperature,and pressure profile,thereby affecting the stability and reliability of the combustion process.As a result of these variations,practical applications such as propulsion systems or reactive materials can have substantial implications.The oxidation and combustion characteristics of B, within high-energy compositions, are influenced by several factors, including the impurities, size distribution,microstructure,chemical composition,crystalline state,and surface layer thickness of B particles [14].Various studies have successfully demonstrated the use of different additive/catalyst coatings on B for combustion applications[13,15,16].
In an alternative method by Sandalla et al.[17],the B2O3layer is slowly removed in the first stage of combustion,and then the core B particles are entirely burned with a dense flame.During this process, however, the generation of HBO2in hydrogen-containing environments reduces the energy released during the burning of B.Consequently, the conversion rate of HBO2to B2O3is likely to increase because of more residence time.An effective method has been demonstrated to diminish the oxide layer through nonthermal hydrogen plasma,in which a nanofilm containing C-F is coated over B [9,10,18].The coating containing C and F atoms promotes oxidation and increases heat release.
A study demonstrated that the use of fluorinated graphene and nano-Al(nano-aluminum)as surface-activated materials improved the ignition and combustion performance of B/KNO3particles when employed as coatings [19,20].Coating B with fluoride compounds, energetic composites, metals, and metal oxides can effectively address the challenges associated with B, including its difficult ignition and low combustion efficiency[21].These coating techniques improve the overall performance of B by enhancing its combustion efficiency,raising the flame temperature,and reducing the ignition temperature.
The oxide barrier of B can also be reduced when physically or chemically combined with different reactive metals such as Mg,Al,Ti,Cu,and other metal hydrides[10,11,22-24].The metal additives such as Mg and Ti have higher gravimetric heating values [Mg(25 kJ/g) and Ti (19.68 kJ/g)] and lower melting temperatures compared to B [10].The oxidation reactions between Mg and B produce MgB2intermetallic borides on the particle surface, which helps increase oxidation heat release by reducing B2O3oxide layer thickness through thermite reactions.Additionally, the formation of porous oxides over the surface of B during the combustion process serves as channels for the oxidizer to reach the metallic core B.Agarwal developed a method to create Mg/B solid solutions using a self-propagating high-temperature synthesis(SHS)reaction[10].A core-shell structure was formed around the B core, which includes Mg, MgO, and MgB2.The exothermic redox reaction between B2O3and Mg produced additional B compounds at the interface of B and Mg.This exothermic redox reaction increased heat release due to the synergistic interaction at the interface of B core-shell [25].The heat released from this reaction helps to enhance the reactivity of the Mg, enabling efficient combustion at higher temperatures.Ti utilizes a surface combustion mechanism,whereas Al and Mg undergo vapor-phase reactions and evaporation at a certain distance from the metal surface[26].Trunov et al.[27]performed combustion experiments on B-Ti milled mixture using a constant volume explosion technique.They reported that the higher heat release rate and combustion completeness of the B-Ti nanocomposite mixture was achieved when tested under various oxidizing environments.The addition of Ti to B particles in a solid fuel ramjet engine can enhance ignition and combustion efficiency[15].This approach takes advantage of the rapid exothermic reaction between B and Ti.This latter has a high burning temperature,which can help to heat the incoming air and fuel mixture more quickly.This leads to a more efficient combustion process,allowing for higher thrust and better performance.
This study aims to improve the ignition and combustion characteristics of HTPB-B fuel that contains Ti and Mg as combustion additives.The solid fuel samples were prepared using the vacuum casting technique.The 10 wt% of Mg, Ti, milled B-Mg, and B-Ti additives were fixed in the fuel formulations, and pure HTPB was cast as a reference sample.The burning characteristics of the prepared fuel samples were tested using an OFB set up in a gaseous oxygen environment.The possible combustion/oxidation mechanism of B-Mg and B-Ti was investigated by heating the fuel samples to 900°C and 1100°C in a Nabertherm burnout furnace under an oxygen atmosphere.The possible condensed combustion products (CCPs) were characterized using XRD and FE-SEM with energy dispersive spectroscopy (EDS).to discuss the combustion mechanism between B-Mg (B-Ti).
2.Experimental methods
2.1.Materials
Ti(with an average particle size of 30 nm and purity of 99.9%or higher) and Mg (with an average particle size of less than 120 nm and purity of 99.9%) were obtained from NanoShel in India.Boron with an average particle size of <500 nm, and purity: ≥95%, was supplied by Sigma Aldrich, USA, whereas dioctyl adipate (DOA),toluene diisocyanate (TDI), and HTPB, used in this study, were supplied by Anabond Pvt Ltd, India.
2.2.Preparation of samples
Table 1 displays the chemical composition of the different fuel formulations, where six types of fuel pellets were prepared,namely, pure-HTPB, HTPB-B (10 wt%), HTPB-Ti (10 wt%), HTPB-Mg(10 wt%), and HTPB doped with dual metals (B:Ti and B:Mg).All the samples were produced using the vacuum casting technique,as shown in Fig.1.
The dual metals were prepared by mixing B either with Ti or Mg using a ball milling process at 400 rpm for 10 h with a milling cycle of 60 min on and 10 min rest cycle.The process was conducted in a dry atmosphere, and the resulting powder was stored in a sealed container.These samples were then characterized with different analytical techniques, i.e., XRD, and FE-SEM.The X-ray diffraction pattern was obtained from the DAVINCI instrument (BRUKER Advance, USA), which was equipped with a copper target (Cu-Kα radiation 1.5418 ?).The range of scanning was set from 2θ= 10°to 80°.The particle size of milled powder was determined by measuring the intensity of the scattered light as a function of the scattering angle.A sample of approximately 200 mg was dispersed inethanol and analysed by laser diffraction using a Malvern/Nano ZS-90 (Malvern-PANalytical, Malvern, UK).To examine the surface morphology of CCPs, a scanning electron microscope (SEM) Thermoscientific Apreo-S (USA) was utilized.Additionally, to avoid overcharging, the surface of the fuel samples was coated with Au-Pd.

Table 1 Composition of HTPB-B-based solid fuels.

Fig.1.Sample preparation method.
For preparing the fuel samples as per the ratios presented in Table 1, a required amount of HTPB is taken in a glass beaker and stirred at 200 rpm for 15 min.Then,a mixture of TDI and DOA was added to HTPB and mixed thoroughly at 200 rpm for 15 min.The required amount of glycerol was subsequently added to the HTPB slurry and mixed for 20 min.After that, the respective metal additives were added to the HTPB slurry and thoroughly mixed.The slurry was stirred continuously for about 90 min to ensure a homogenous mixture.Avibrating system was used to remove voids and air gaps from the prepared mixture.The mixture was then poured into a fuel mold with a diameter of 12 mm and a length of 15 mm.The mold was placed in a vacuum chamber under 50.79 kPa for 20 min for degassing.The fuel slurry was cured for five days in an oven at 50°C, followed by 120 h of curing at ambient temperature(30°C).All samples were trimmed into 6 mm lengths and tested for regression rate and combustion analysis on an opposed flow burner.The regression rates were determined for each sample,and the obtained results were utilized to compare the performance of the fuels.
2.3.Combustion experiments
The opposed flow burner was used to evaluate the regression rate of the prepared fuels under gaseous oxygen as the oxidizer.After the components of the opposed flow burner were all aligned,the fuel sample was inserted into the pellet holder.The combustion reaction was initiated by applying a 40 V voltage to the top surface of the fuel via a nichrome wire.A rotameter was connected to the oxygen supply in order to control the mass flow rate of the oxygen through a solenoid valve, which was varied from 22 kg/(m2?s) to 86 kg/(m2?s).All the experimental processes were regulated through a Labview application to initiate the ignition of the nichrome wire and control the oxygen flow.The schematic diagram in Fig.2 accurately depicts the OFB system used in this study with all these components working together.The regression rate was estimated for all fuel samples using the length and weight difference approach.A high-speed video camera (Model: Baumer) was used to record the burning behavior of fuel samples during ignition and combustion.The video camera captured a total of 200 frames at a resolution of 800 × 620 pixels with 22 fps.The condensed combustion products ejected from the top surface should settle on the opposed flow burner’s bottom surface,but fragments were snuffed out during combustion due to a higher oxidizer mass flux.Therefore, fuel samples were burnt in a Nabertherm burnout furnace at 900°C and 1100°C under gaseous oxygen, followed by the collection of the condensed combustion products(CCPs).The CCPs were characterized by FE-SEM with EDS to identify the surface morphology and oxides of burned residues [23].
3.Results and discussion
3.1.Fuel characterizations
Figs.3(a) and 3(b) show FE-SEM images of milled B-Mg and B-Ti powders, respectively.The morphology of the milled B-Mg particles indicates that Mg and B are agglomerated and bonded together due to the milling process.The agglomerates are highly porous, and the particles are in close contact, indicating that the particles are strongly bonded.Fig.3(b) shows that the B particles surrounding Ti particles have a spherical shape, where the Ti particles are well distributed around the B particles, proving a strong interaction between them.This suggests ball milling effectively created a homogenous mixture of Ti and B particles.

Fig.2.Schematic diagram of an opposed flow burner setup.
A particle size analyzer with dynamic light scattering (with 10 MW laser input energy) was used to determine the average particle size of the milled B-Mg and B-Ti powders.Before measurements, the powdered samples were dispersed in ethanol and sonicated for 30 min to eliminate agglomeration.The distribution of milled B-Mg and B-Ti particles ranges from 4 nm to 154 nm and 2-198 nm, respectively.B-Mg particles have an average distribution (D50) of 20 nm, while B-Ti particles have an average distribution(D50)of 22 nm.In Fig.3(c),the average particle size of pure B (D50) was observed to be 27.5 nm.In the case of the B-Ti/Mg composite, the milling process has led to a decrease in the average particle size.This reduction in particle size can have several implications.Firstly, smaller particle size often corresponds to an increased surface area per unit mass.This increased surface area can be advantageous in improving the regression rates and reducing the ignition temperature, especially in fuel/propellant,where surface reactivity or surface-related properties are important.The results showed that the milled B-Mg and B-Ti particles had similar average particle sizes, indicating uniformity of the milled powders.The results also showed that the particle size distributions were relatively narrow, indicating good dispersion of the milled particles.
Fig.4 illustrates the XRD patterns of the as-received and milled powders.XRD results evident the presence of B2O3diffraction peaks at approx.2θ = 28°, as shown in Fig.4 [23].The B2O3peaks were also present in the post-milled B-Ti and B-Mg samples.The relative intensity of the peaks is significantly reduced post-milling process,suggesting that B-based compounds are more prevalent in the sample.
3.2.Average regression rates
The average regression rate of all fuel samples was evaluated using OFB under gaseous oxygen conditions.The regression rate tests were conducted on fuel samples at oxidizer mass flux levels ranging from 22 kg/(m2?s)to 86 kg/(m2?s).The average regression rate was determined by measuring the difference in length between the initial and final burned portions of the fuel sample[28,29].Fig.5 shows the average regression rates of HTPB-based fuel.It can be observed that the average regression rate of pure HTPB varied between 0.1 and 0.32 mm/s under an oxidizer mass flux range of 29.5-80 kg/(m2?s).With the addition of 10 wt%of Ti,Mg, and B, the regression rate of HTPB-based fuel varied in the range of 0.1-0.29 mm/s, 0.16-0.33 mm/s, and 0.11-0.27 mm/s,respectively.
Further,adding 10 wt%milled B-Ti and B-Mg additives into the HTPB improved the regression rate in the range of 0.2-0.36 mm/s and 0.2-0.44 mm/s, respectively.HTPB-B formulation exhibited lower regression rates compared to other tested formulations.This can be attributed to the B2O3layer formed on the surface of core B particles during the oxidation reaction, which limited the full energy releases [2,30,31].At the same time, adding Mg and Ti additives showed higher regression rates compared to HTPB-B formulations.Since the ignition temperature of Mg and Ti are lower than that of B,it helps the formulation to ignite at an earlier stage and provides more residence time in the combustion zone[27].The radiation heat released from these particles to the combustion zone helps strip more fuel from the surface and enhances regression rates.Interestingly, the regression rate of HTPB-Mg fuel is higher than that of the HTPB-Ti sample.This could be due to the fact that the thermal conductivity of Mg is higher than the Ti,which helps to transfer more heat interior of the fuel sample [22,27].At a higher oxidizer mass flux range of 75-82 kg/(m2?s), the HTPB-B-Mg and HTPB-B-Ti exhibited higher regression rates of 37.5% and 12.5%,respectively, compared to the pure HTPB sample.This could be attributed to the synergetic effects of dual metal in the HTPB matrix.There is a possibility that Mg and Ti might have played a role in promoting the oxidation reaction and enhancing the heat release rate of B particles.This could be due to the thermochemical reactivity of Mg and Ti with oxygen, which could increase the heat release rate and oxidation rate of B particles.Additionally, the presence of Mg and Ti can also lower the ignition of the HTPB-B matrix, thus facilitating the oxidation reaction.

Fig.3.(a) FE-SEM of milled B-Mg; (b) FE-SEM of milled B-Ti powder; (c) average particle size.
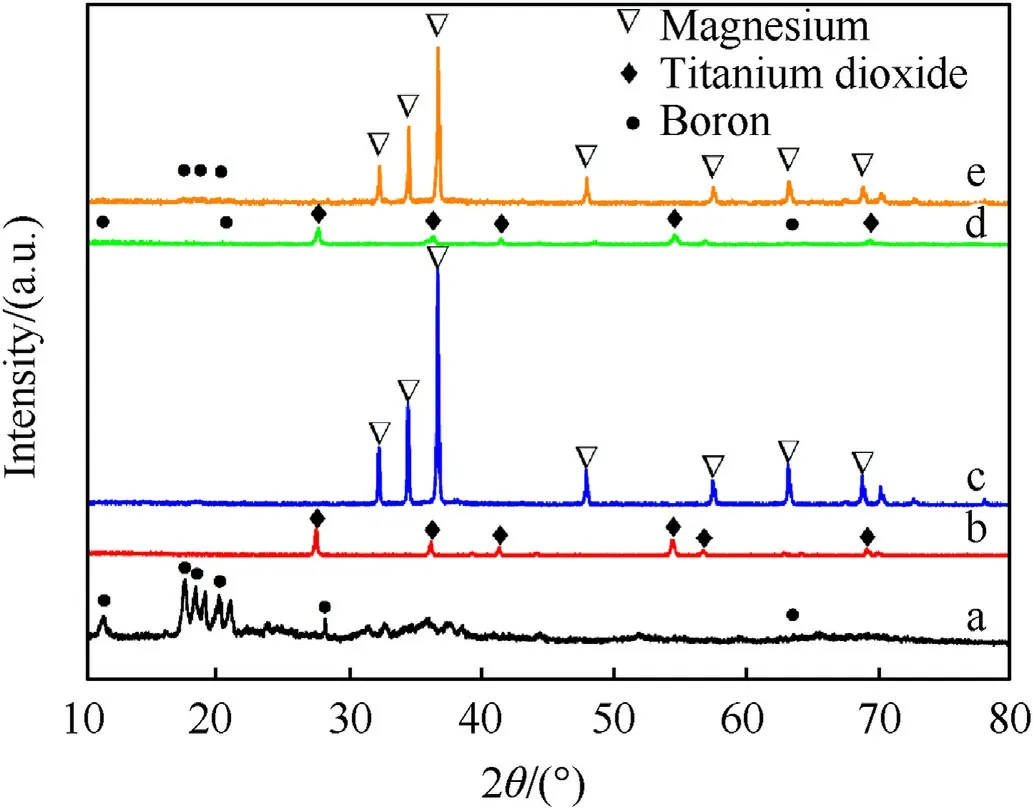
Fig.4.XRD analysis of as-received and milled powder:(a)Pure B;(b)Pure Ti;(c)Pure Mg; (d) B-Ti; (e) B-Mg.

Fig.5.Regression rate vs.oxidizer mass flux of HTPB-B-based fuels.
3.3.Ignition delay

Fig.6.Ignition and combustion frames of (a) pure HTPB; (b) HTPB-B; (c); HTPB-Mg; (d) HTPB-Ti; (e) HTPB-B-Mg; (f) HTPB-B-Ti fuel during combustion.
The ignition delay was calculated to find out how Mg and Ti affect HTPB-B matrix ignition.The interval between the firing of the ignition source and the start of combustion is known as the ignition delay time.Several factors affect the ignition delay time of solid fuels, including particle size, surface area, and chemical composition.The present study defines ignition delay time as the interval between the first electrical signal received and the time at which the flame front appears on the top surface of the fuel sample.This time interval is usually in milliseconds and measures fuel reactivity.The ignition delay time is vital for optimizing combustion processes.The ignition delay time is calculated based on two frames captured by a high-speed camera.The first frame is taken when the ignition source is initiated,and the second frame is taken when the ignition of the fuel (flame front) is observed.The difference between the two frames is the ignition delay time.
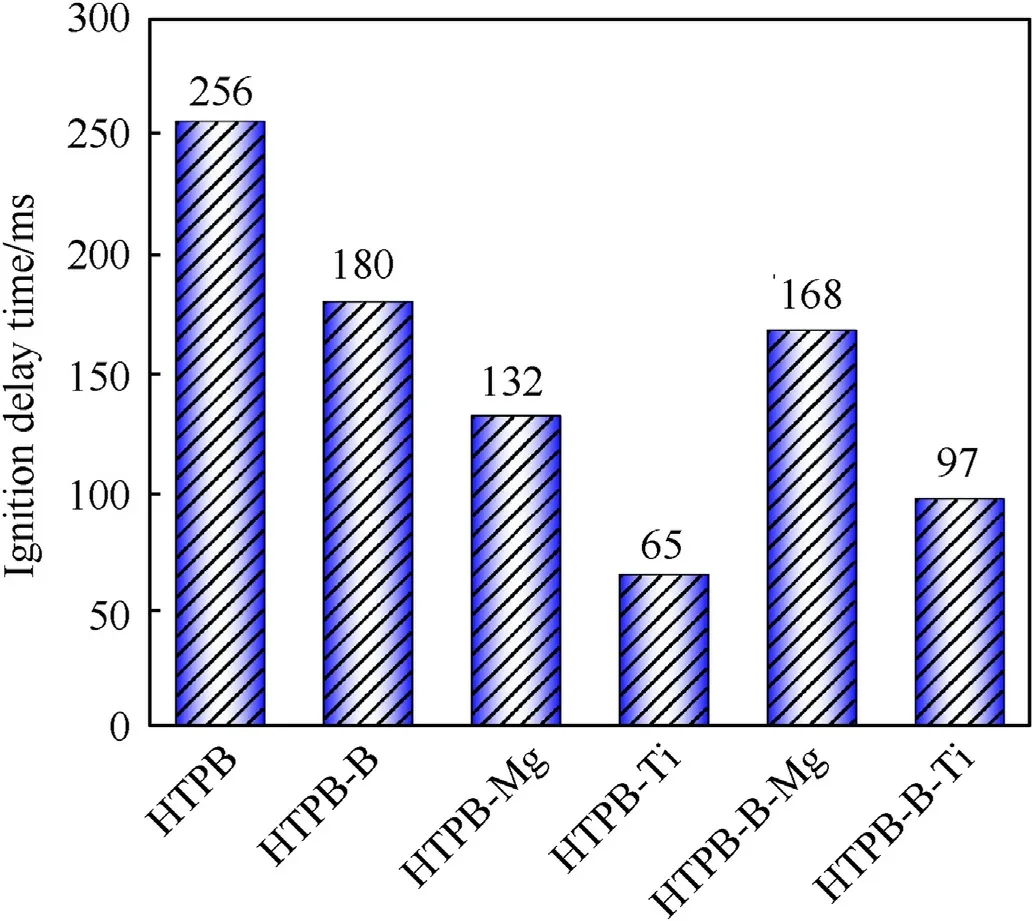
Fig.7.Ignition delay time of HTPB-based fuel samples.
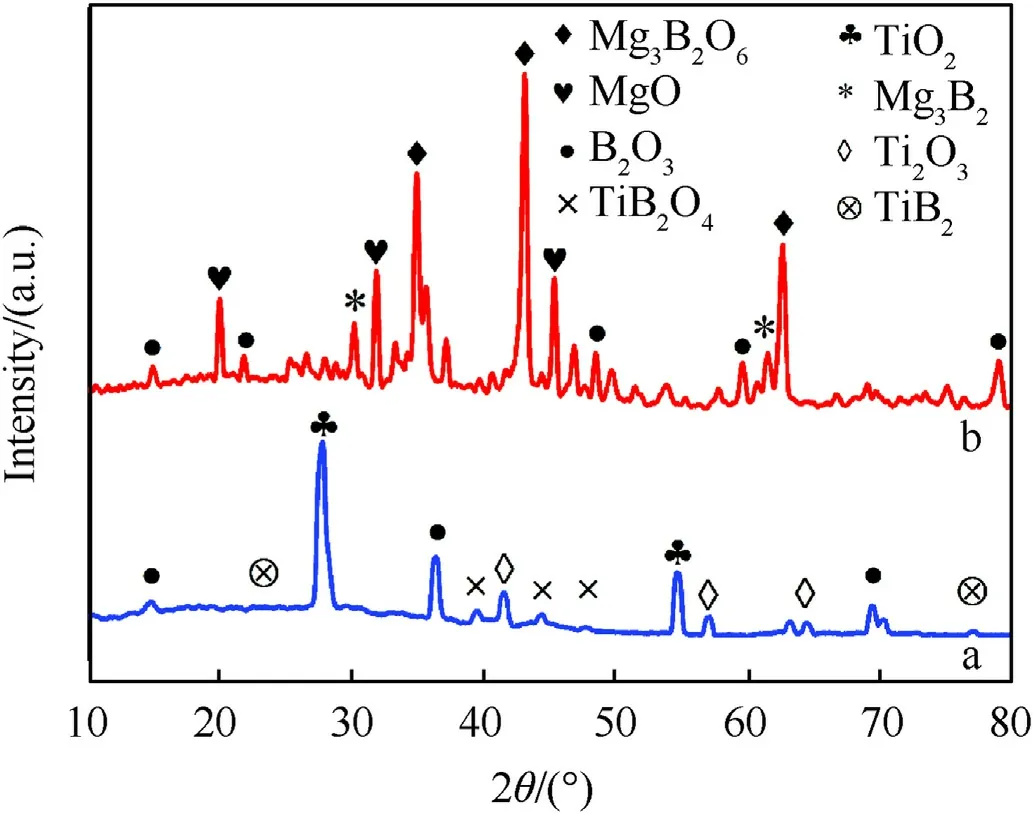
Fig.8.XRD analysis of post-combustion particles: (a) B-Mg; (b) B-Ti.
All the ignition and combustion tests were conducted on HTPBbased fuels under identical conditions.Fig.6 illustrates the ignition and combustion frames of all the fuel samples at different times,as it can be observed that the combustion process begins with ignition and moves through to the stable combustion phase and subsequent termination.During the stable combustion phase, burning agglomerates were ejected from the burning surface with a visible spark until extinction.The color of the flame for B-based samples was observed to be light green, whereas for HTPB-Mg samples, it was a more intense yellow.During the termination phase,the flame was extinguished after the burning agglomerates were completely consumed.This process was observed to take approximately 5 s.
Fig.7 shows the ignition delay time for all the tested fuel formulations.The pure HTPB has reported the longest ignition delay of 256 ms among all the tested fuel samples.Adding B,Mg,and Ti into HTPB exhibited a low ignition delay time of 180 ms,132 ms, and 65 ms, respectively.Further addition of milled B-Mg and B-Ti powders into HTPB has displayed an ignition delay of 168 ms and 97 ms, respectively.When Ti and Mg are added to the solid fuel mixture,they act as a surface catalyst/active heat transfer medium,providing active sites for the reaction to occur more rapidly and better conductive heat transfer into the fuel surface [10,27,32].In addition,the thermal conductivity of Ti is higher than the Mg and B,which could lead to lower ignition delays of the fuel samples[27,29].Ti and Mg react with the oxidizer in the fuel mixture,generating heat and forming a high-temperature environment that promotes fuel decomposition.It is crucial to consider that the ideal amount of Ti and Mg in the fuel mixture depends on the specific fuel formulation and operating conditions.If the concentration of Ti and Mg is excessive or insufficient, it can adversely affect the ignition efficiency and stability of the combustion process.Therefore, careful experimentation and testing are required to optimize the concentration of Ti and Mg for particular solid fuel applications.
3.4.Post-combustion analysis
Combustion of metalized solid fuel generates condensed combustion products that may affect the overall performance.Thus,to accurately understand the nature of the CCPs that may be formed for the above samples, it is worth investigating the combustion of the milled B-Mg and B-Ti powders first.These latter were heated at 900°C and 1100°C in a Nabertherm burnout furnace under an oxygen atmosphere to examine the possible combustion/oxidation reaction mechanisms of B.After heating,the samples were allowed to cool and examined using FE-SEM and XRD techniques.The results showed that B reacted with both Mg and Ti, forming various compounds.When milled B-Mg powder is burned in the Nabertherm burnout furnace at different temperatures, it undergoes complex oxidation reactions, forming different CCPs.The possible CCPs that are formed at 900°C temperature, along with the possible oxidation reactions between B and Mg, are B2O3, MgO,Mg3B2,and MgB2,as can be seen in Fig.8(a).The possible oxidation reactions at 900°C are presented in Eqs.(1)-(4):

Fig.9.Condensed combustion product of fuel pellets: (a) B-Mg@900 °C; (b) B-Mg@1100 °C; (c) B-Ti@900 °C; (d) B-Ti@1100 °C.

Fig.10.(a) FE-SEM of CCPs of B-Mg@1100 °C; (b) CCPs of B-Ti @1100 °C, EDS mapping of (c) B-Mg; (d) B-Mg.
At 1100°C, the possible CCPs formed are B2O3, MgO, MgB2,Mg3B2O6and MgB2.Whereas the possible oxidation reactions at 1100°C are presented in Eqs.(6)-(9):
At 900°C,the reaction is less exothermic,and so the formation of MgB2is not favored.Instead, B2O3is formed as a product.At 1100°C,the reaction is more exothermic.Therefore,the formation of Mg3B2and Mg3B2O6is favored.However, the products formed will depend on oxygen availability during combustion.The possible oxidation reactions between B and Mg involve the formation of oxides and borates [16,26].
At 900°C,Mg and B react with oxygen to form magnesium oxide(MgO) and B oxide (B2O3).These oxides can then react with each other to form Mg3B2as a white crystalline solid with a high melting point (Fig.9(a)), whereas, at 1100°C, the color of the combustion products becomes greyish.This latter could be due to CCPs of the reaction between the MgO and B2O3(Fig.9(b)).The fact that the specific color of the combustion products and oxides will depend on factors such as the purity of the reactants,the conditions under which the reaction takes place, and the presence of impurities or other factors that can affect the reaction [26].
At 900°C and 1100°C, B, and Ti can undergo several oxidation reactions,forming various oxides(Fig.8(b)).The specific oxidation reactions between B and Ti at 900°C and 1100°C depend on several factors, including the relative amounts of B and Ti present in the composition, the oxygen content during the reaction, and the duration of exposure to the high temperature.The possible oxidation reactions are presented in Eqs.(10)-(15):
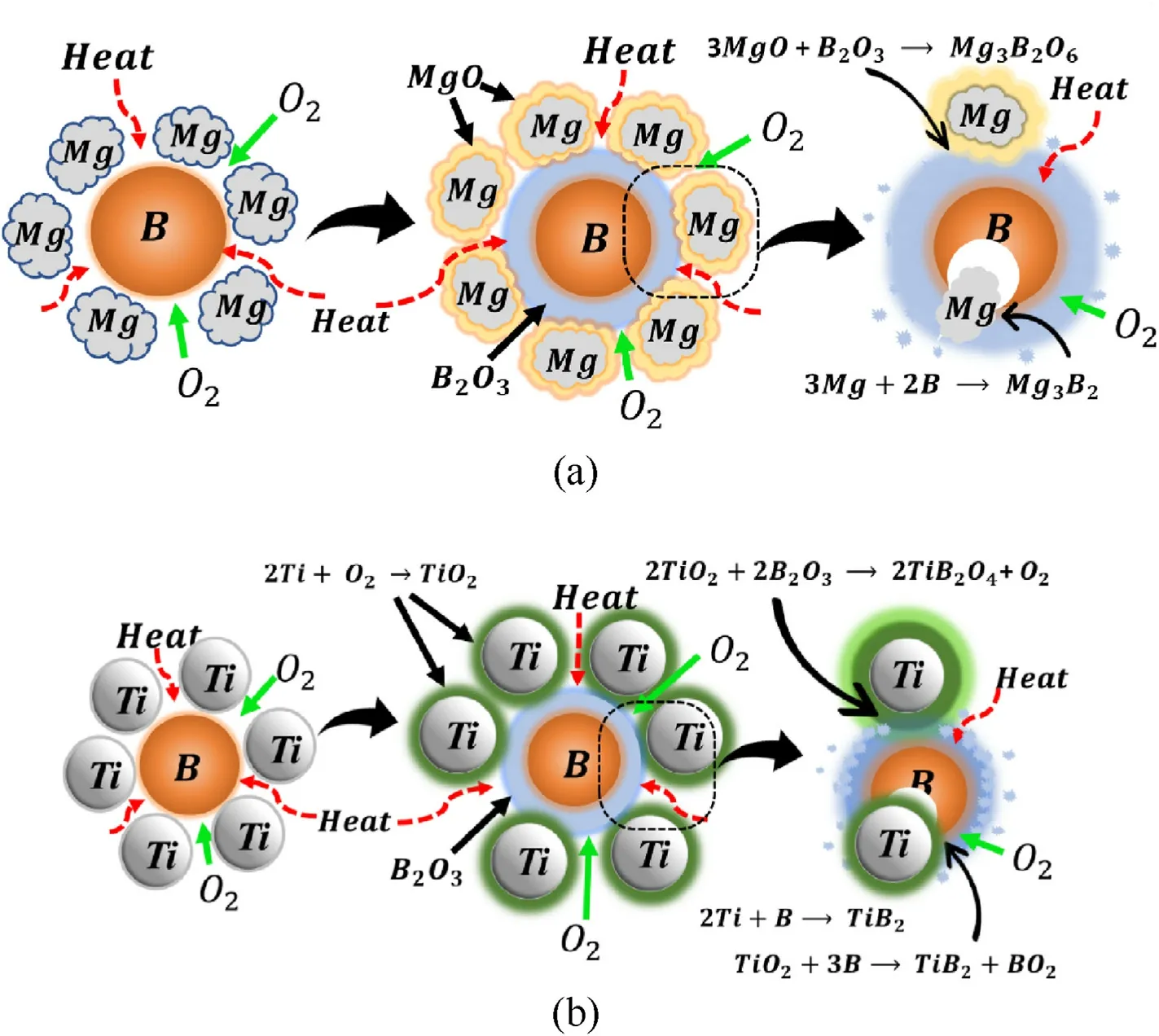
Fig.11.Schematic of combustion mechanism between (a) B-Mg and (b) B-Ti.
The reaction given in Eq.(10) occurs at temperatures above 700°C,and CCPs formed TiO2is a white crystalline solid(Fig.9(c)).Besides that, the reaction presented in Eq.(11) occurs at temperatures above 1000°C to form Ti2O3, which is a black or dark grey solid.The reactions displayed in Eqs.(12)-(15)may occur relatively slowly at low temperatures, while at higher tempertaure, these reactions may occur more rapidly.The resulting CCPs are generally dark grey solid in color (Fig.9(d)).Figs.10(a) and 10(b) shows the surface morphology of the CCPs of B-Mg/B-Ti@ 1100°C, which exhibits a range of shapes, including smooth, lumps, rough, or porous structures.As expected,the various oxides are coated on the surface of Mg and Ti,as confirmed by the EDS analysis of CCPs that revealed the existence of Ti,Mg,O,and C(Figs.10(c)and 10(d)).In contrast, the presence of Cu and Al peaks may be attributed to a small amount of impurity resulting from cross-contamination during CCP sample preparation or handling.However, the presence of quantitative or qualitative elemental B was not detected with EDS analysis.It is noteworthy that the application of EDS poses distinctive difficulties when analyzing light elements such as B,Be,and F[33-35].As a light element,B cannot be accurately estimated with the current EDS method and instrumentation.
It is important to note that these are just some of the possible oxidation reactions that could occur between B and Ti within the temperature range of 900-1100°C.The actual products of the reactions will depend on the specific conditions of the reaction, and further experimentation and analysis may be carried on the future to determine the exact products formed, which the current research will certainly promote.
An illustration of the possible combustion mechanism between B and Mg/Ti in order to provide an overall picture of the reactions involved is given in Fig.11.The combustion mechanism between B and Mg under oxygen involves a series of chemical reactions(Fig.11(a)).At the beginning of the reaction,Mg and B are heated in the presence of oxygen,leading to the formation of MgO and B2O3.The MgO and B2O3further react to form Mg3B2O6and MgB2O4[26,28,32].At higher temperatures, the oxide layers become thinner,which further increases the diffusion of heat and oxygen at the interface between core B and Mg,allowing for even more of the Mg and core B particles to react and form Mg3B2.This exothermic reaction causes the temperature to increase further and accelerates the oxidation process.This process continues until B and Mg are completely oxidized.
In the case of the combustion reaction between B and Ti, the process is initiated by the adsorption of oxygen molecules on the surface of the B-Ti mixture,starting by heating the B-Ti mixture to a sufficiently high temperature.The generated heat during the initiation step causes the reaction to propagate rapidly, leading to the oxidation of B and the reduction of Ti.The different oxidation reactions are exothermic, producing a mixture of TiO2and B2O3oxides.They involve the transfer of oxygen atoms from TiO2to B2O3, forming titanium borate (TiB2O4) [23,27,31].These reactions are also highly exothermic and produce a significant amount of heat.At high temperatures, the B2O3, the thickness of the oxide layers at the interface between B(core B)and Ti reduces,leading to enhanced diffusion of heat and oxygen.As a result, titanium diboride(TiB2)is formed,exhibiting higher reactivity between the Ti and core B particles.Combustion additives such as Mg and Ti contribute to improving oxidation reactions and combustion efficiency.This makes them suitable combustion additives for a wide range of combustion applications.
4.Conclusions
The current study investigated the ignition and combustion behavior of B doped with Ti and Mg combustion additives.The fuel samples were tested in the oxidizer mass flux range of 22-86 kg/(m2?s) using the OFB.The addition of Ti and Mg into HTPB-B exhibited higher regression rates due to improved oxidation reaction of B and dual metal combustion effect.At an increased oxidizer mass flux range between 75 kg/(m2?s)and 82 kg/(m2?s),the HTPBB-Mg and HTPB-B-Ti formulations demonstrated notable incremental regression rates of 37.5%and 12.5%,respectively,compared to the pure HTPB sample.XRD and FE-SEM analyses revealed that the B oxide layer at the interface between B-Ti(B-Mg)is removed at lower temperatures, facilitating oxygen transfer from the surroundings to the core B.When Ti and Mg are added to B, the ignition delay time is decreased, improving combustion efficiency.The untreated HTPB sample reported the highest ignition delay of 256 ms among all fuel types tested.However,adding B,Mg,and Ti to HTPB significantly reduced ignition delay times, measuring 180 ms,132 ms,and 65 ms,respectively.Further addition of milled B-Mg and B-Ti powder to HTPB resulted in even lower ignition delay times of 168 ms and 97 ms,respectively.Finally,the possible combustion mechanism/oxidation reactions between B and Mg/Ti were presented.At high temperatures,Mg and B react with oxygen to form MgO and B2O3, which further react to form Mg3B2O6and MgB2O4.Whereas, in the case of B and Ti, the oxidation reactions between these oxides produce titanium borate(TiB2O4)and B oxide(B2O3), which further react to form titanium diboride (TiB2).The addition of Mg and Ti can improve combustion efficiency, making them suitable combustion additives for various applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the Hindustan Institute of Technology and Science for their support and experimental facilities.Additionally, the authors thank the Centre for Clean Energy and Nano Convergence (CENCON), for providing the Nabertherm burnout furnace facilities.
- Defence Technology的其它文章
- Ground threat prediction-based path planning of unmanned autonomous helicopter using hybrid enhanced artificial bee colony algorithm
- Layered metastructure containing freely-designed local resonators for wave attenuation
- Predicting impact strength of perforated targets using artificial neural networks trained on FEM-generated datasets
- Construct a 3D microsphere of HMX/B/Al/PTFE to obtain the high energy and combustion reactivity
- Ignition processes and characteristics of charring conductive polymers with a cavity geometry in precombustion chamber for applications in micro/nano satellite hybrid rocket motors
- Recent research in mechanical properties of geopolymer-based ultrahigh-performance concrete: A review

