An effective catalyst carrier SiO2:Enhancing catalytic and combustion properties of CuFe2O4 on energetic components
Li Ding , Chong Wn , Suhng Chen ,**, Zho Qin , Kngzhen Xu ,*
a School of Chemical Engineering/Xi'an Key Laboratory of Special Energy Materials, Northwest University, Xi'an, 710069, China
b Xi’an Modern Chemistry Research Institute, Xi'an, 710065, China
Keywords:Copper ferrite Silicon dioxide Combustion catalyst Thermal decomposition Laser ignition
ABSTRACT To enhance the catalytic activity of copper ferrite(CuFe2O4) nanoparticle and promote its application as combustion catalyst,a low-cost silicon dioxide(SiO2)carrier was employed to construct a novel CuFe2O4/SiO2 binary composites via solvothermal method.The phase structure,morphology and catalytic activity of CuFe2O4/SiO2 composites were studied firstly, and thermal decomposition, combustion and safety performance of ammonium perchlorate (AP) and 1,3,5-trinitroperhydro-1,3,5-triazine (RDX) with it affecting were then systematically analyzed.The results show that CuFe2O4/SiO2 composite can remarkably either advance the decomposition peak temperature of AP and RDX, or reduce the apparent activation energy at their main decomposition zone.Moreover, the flame propagation rate of RDX was promoted by about 2.73 times with SiO2 content of 3 wt%, and safety property of energetic component was also improved greatly, in which depressing the electrostatic discharge sensitivity of pure RDX by about 1.89 times.In addition,the effective range of SiO2 carrier content in the binary catalyst is found to be 3 to 5 wt%.Therefore,SiO2 opens a new insight on the design of combustion catalyst carrier and will promote the application of CuFe2O4 catalyst in solid propellant.
1.Introduction
Solid propellant is widely used in aerospace and military fields,one of the most effective methods to improve the combustion performance of solid propellant is to advance the thermal decomposition process of energetic components (such as ammonium perchlorate (AP) and 1,3,5-trinitroperhydro-1,3,5-triazine (RDX))by adding a few combustion catalysts (3%-5%) [1-4].Nowadays,several combustion catalysts, such as metals [5-8], metal oxides[9-13],bimetals[14,15],and GO-based composition[3,16,17],have been proved to present compelling advantage in reducing the pressure exponent and forming platform combustion effect.
The copper ferrite(CuFe2O4)nanoparticle,as a typical magnetic semiconductor with narrow band gap,has been extensively applied as gas-sensing materials [18], supercapacitor electrodes [19], and efficient catalysts [20-23] owing to its magnetic properties,excellent electronic conductivity and high thermal stability.Therefore,its application in solid propellent has been concerned as well.Zhang et al.prepared hollow CuFe2O4nanosphere by hydrothermal method and confirmed its higher catalytic activity on RDX and FOX-7 than that of CuO and Fe2O3[24]; Liu et al.further introduced graphene oxide (GO) to synthesize CuFe2O4/GO composite by self-assembly method and demonstrated that it could reduce the apparent activation energy of RDX by 98.71 kJ/mol[25].However,CuFe2O4particles are inevitably to aggregate into clusters because of its nanometers size effect, which is detrimental for combustion of solid propellant[26,27].The most common strategy for inhibiting aggregation and improving the dispersion of CuFe2O4nanoparticles is to introduce appropriate nanoparticles carriers.Besides, pursuing the carrier that could display certain catalytic synergy properties with CuFe2O4, improve the combustion performance and anti-electrostatic discharge sensitivity of energetic components are more preferably.
Many researchers have devoted themselves in seeking for layer carbon materials with two-dimension(such as GO)as right catalyst carrier, whereas the high-price, complex processing technology and waste acid contamination limit its engineering application[2,3].But for silicon-based composites,holding abundant resources,low prices and facile preparation process,have been widely studied in solid propellent[28-30].Kline et al.investigated the influences of thermally conducting (graphite) and insulating (SiO2) particles on film propagation rate of solid propellent,and demonstrated that the latter has a higher burning rate with low mass percentage additives [31]; Wang et al.demonstrated that Al/AP/SiO2(7 wt%)composite particles was more reactive and that is approximately 7 times higher than Al/AP particles [32]; Chen et al.successfully prepared AP/RDX/SiO2nanocomposite via the sol-gel method and confirmed that SiO2could accelerate the decomposition of AP and reduce the sensitivity of energetic materials [33].Therefore, using SiO2as combustion catalyst carrier will produce many positive effects in thermal decomposition,combustion and safety properties.
For CuFe2O4/SiO2composite combining silica and copper ferrite through in situ growth or sol-gel method,has been widely applied in fields of peroxidase and visual biosensing [34], magnetic materials[35],chemical looping gasification[36]and coupled with Li2O as anode material [37], yet there isn’t any report of CuFe2O4/SiO2applied in solid propellent so far.On the basis of above analysis,we speculated that CuFe2O4/SiO2composite would be an excellent candidate material as combustion catalysts for AP and RDX.
In this work, CuFe2O4/SiO2binary nanocomposite was successfully prepared via the solvothermal method.This material was further used as combustion catalyst and its effect on thermal decomposition of AP and RDX was studied.Moreover, the combustion and safety properties of RDX over CuFe2O4/SiO2nanocomposite were investigated.All results indicated that CuFe2O4/SiO2nanocomposite could produce excellent catalytic effect on energetic components of solid propellent.
2.Experiment
2.1.Reagents and materials
All chemical reagents are of analytical grade and were used without further purification.FeCl3?6H2O and CuCl2?2H2O were purchased from Sinopharm Chemical Reagent Co., Ltd.Anhydrous CH3COONa and polyvinylpyrrolidone (PVP) were sourced from Damao Chemical Reagent Co., Ltd.Ethylene glycol was purchased from Kelong Chemical Reagent Co., Ltd.SiO2(micrometer grade)was obtained from Macklin Biochemical technology Co., Ltd.The energetic materials (AP and RDX) were provided by Xi’an Modern Chemistry Research Institute.
2.2.Synthesis of CuFe2O4/SiO2 composite
CuFe2O4/SiO2composite was synthesized through a versatile solvothermal method [3],and the preparation process is shown in Fig.1.Firstly,10 mmol FeCl3?6H2O and 5 mmol CuCl2?2H2O were separately dissolved in ethylene glycol (35 mL) accompanied by magnetic stirring.Secondly,different proportions SiO2(1 wt%,3 wt%, 5 wt%, 7 wt%) were dissolved in FeCl3?6H2O solutions, respectively.Meanwhile,1 g PVP was dissolved in CuCl2?2H2O solution as a dispersant [38].Those solutions were dispersed by ultrasonication under ultrasonic powder of 600 W for 1 h.Thirdly, the Fe3+solution was mixed with the Cu2+solution under continuous magnetic stirring until the complete mixture was achieved.Then,2.46 g CH3COONa, used as a sedimentation agent to promote the formation of the final product, was added into the mixture accompanying with vigorous stirring.Subsequently, the obtained homogenous reaction solution was sealed in a 100 mL Teflon-lined stainless-steel autoclave and maintained at 180°C for 12 h.The final product was rinsed by ethanol and deionized water for three times, respectively, the suspension was centrifuged and dried at 60°C for 12 h.In contrast, pure CuFe2O4nanoparticles were prepared under the same condition without adding SiO2carrier.
2.3.Structure and morphology characterization
The phase structures of as-obtained samples were investigated by X-ray diffraction (Rigaku, Smart LAB SE); The surface morphology, size and element component were recorded by Scanning electron microscopy (Zeiss SIGMA) coupled with energy dispersive spectrometry (Oxford 51-XMX); Functional groups on the surface of samples were investigated by Fourier transform infrared (Shimadzu, IRAffinity-1S); Element composition and chemical valence state were determined via X-ray photoelectron spectra (ThermoFisher, NEXSA); Zeta potential analysis (Anton Paar, Litesizer?500) of SiO2carrier was determined by Dynamic Light Scattering method; The specific surface areas and pore size distributions were measured by Nitrogen adsorption/desorption(Micromeritics, ASAP 2460) through the Brunauer-Emmett-Teller method.
2.4.Catalytic and combustion properties studies
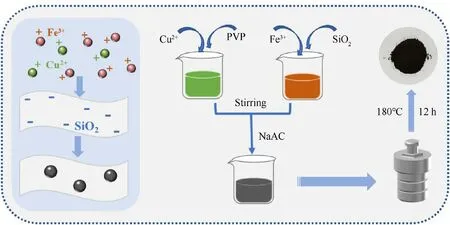
Fig.1.Schematic diagram for synthesis of CuFe2O4/SiO2 composite.
The catalytic decomposition behavior of energetic materials was studied by Differential scanning calorimetry(Netzsch,DSC-200F3),the N2flow rate is 100 mL/min, the heating rate is 10°C/min and temperature ranges are 30-460°C and 30-310°C for AP and RDX,respectively; The ignition characteristics were recorded by a CO2laser ignition system (SLC 110) at air atmosphere.The stacking density of sample in crucible is around 0.76 g/cm3;The electrostatic discharge sensitivity (EDS) was analyzed by a JGY-50(III) electrostatic test apparatus [39-41].The test energy was determined by the energy calculation equation: E50= 1/2CV2, in which C is the capacitance of the capacitor, V is the charge voltage in volts [42].The charge capacitance was set as 10,000 pF,and the electrode gap length was set as 0.12 mm.In the above three types of characterizations,the catalyst was physically mixed with energetic materials at a mass ratio of 1:4.
3.Results and discussion
3.1.Structure and component characterization
SEM was used to observe the loading of CuFe2O4on the surface of SiO2from apparent morphology, as shown in Fig.2(a)-2(d).Fig.2(a) shows the SiO2substrate with a micrometer sheet structure,while the CuFe2O4nanoparticles in Fig.2(b)are spherical with an average particle size of 160-170 nm.Through further observation of CuFe2O4/SiO2-3% composite in Fig.2(d), CuFe2O4nanoparticles are uniformly distributed on the surface of SiO2substrate.In comparison with CuFe2O4/SiO2-1% composite in Fig.2(c), the agglomeration phenomenon of CuFe2O4nanoparticles is effectively suppressed.Besides,the elemental mapping images in Fig.2(d-1)-2(d-4) reveal that the elements of Fe, Cu, Si, and O are distributed on the surface of samples,which further demonstrates the uniform loading of CuFe2O4particles on the surface of SiO2substrate.
XRD patterns and FT-IR spectra are often applied to study the composition and chemical bonding information of materials.The crystal structures of CuFe2O4, SiO2and CuFe2O4/SiO2composite were verified via XRD,as shown in Fig.2(e).Feature peaks at 18.50°,30.17°,35.64°,43.04°,57.05°and 62.77°can be connected well with(111), (220), (311), (400), (511) and (440) panels of CuFe2O4,matching well with the standard ICDD card NO.025-0283[26,27,43,44].This proves that high purity CuFe2O4was successful prepared.Meanwhile, feature peaks at 20.88°, 26.58°, 50.08°,60.02°and 67.86°are correspond to (100), (101), (112), (211) and(212) panels of SiO2, which are well compatible with the standard ICDD card NO.001-0649 [43].It is essential that the intensity of feature peak at 26.58°presents a gradually increasing tendency with larger SiO2content in CuFe2O4/SiO2composite.These typical feature peaks of CuFe2O4and SiO2co-exist in CuFe2O4/SiO2binary composite, indicating that CuFe2O4nanoparticles are successfully coupled with SiO2substrate.Furthermore, the FT-IR spectrums of as-obtained samples are shown in Fig.2(f), the bands around 1662 cm-1and 3400 cm-1belong to stretching vibrations of absorbed water molecules and hydroxyl group, respectively.The strong absorption bands at 468 cm-1,798 cm-1and 1060 cm-1are assigned to oscillatory vibration, symmetric stretching vibration and asymmetric stretching vibration of Si-O-Si bond,respectively[20,37,45,46].The Si-O-Si bond at 451 cm-1exists in the tetrahedral position is overlapped with metal oxides vibration[37].The absorb peak at 543 cm-1is attributed to metal-oxygen stretching[26,27,37].XRD and FT-IR results demonstrate the successful synthesis of CuFe2O4/SiO2composite.

Fig.2.SEM images of (a) SiO2; (b) CuFe2O4; (c) CuFe2O4/SiO2-1%; (d) CuFe2O4/SiO2-3%; (d:1-4) element mapping of CuFe2O4/SiO2-3%; (e) XRD pattern and (f) FTIR spectra of CuFe2O4, SiO2 and CuFe2O4/SiO2 (3 wt%, 5 wt%, 7 wt%) composites.
In the survey spectrum of CuFe2O4/SiO2-3% composite in Fig.3(a),the Fe,Cu, Si and O elements can be seen clearly,there is no extra element in comparison with the survey spectrum of CuFe2O4(Fig.3(d)).The broad and asymmetric peak of O1s(Fig.3(b)) spectrum implies that there can be more than one chemical state for O element,the two peaks centered at 530.16 eV and 532.91 eV are assigned to lattice oxygen in CuFe2O4and nonmetal oxides (Si-O), respectively [27].Whereas, the peak centered at 531.55 eV belongs to the surface hydroxyl group(-OH)[43].As shown in Fig.3(c), the peaks centered at 103.38 eV and 100.30 eV indicate that Si element is presented in the form of SiO2[30].The Cu2p spectrum (Fig.3(e)) exhibits four peaks located at 933.92 eV for Cu2p3/2, 953.40 eV for Cu2p1/2, 941.58 eV for the satellite feature of Cu2p3/2and 962.56 eV for the satellite feature of Cu2p1/2[43].Meanwhile,the Fe2p spectrum(Fig.3(f))can be fitted into three contributions(FeO,Fe2O3and Fe3O4),the peaks at 711.08 and 724.13 eV are assigned to the binding energies of 2p3/2and 2p1/2 of Fe3+, 714.73 and 726.93 eV are attributed to the binding energies of 2p3/2and 2p1/2of Fe2+.In addition, the peak locating at 718.63 eV indicates that Fe3+and Fe2+coexist in the CuFe2O4/SiO2-3% composite [27,35].XPS results further confirm the successful preparation of CuFe2O4/SiO2composite.
To illustrate the combination state between CuFe2O4nanoparticles and SiO2substrate, Zeta potential measurement of SiO2particles was carried out at room temperature (25°C) and laser wavelength of 660 nm.SiO2particles were firstly suspended in ethylene glycol solution and then sonicated for 2 h to form a homogeneous dispersion.The measurement was calculated for three times and results are shown in Fig.4(a) and 4(b).The large zeta potential value (-54.0 mV) of SiO2particles indicates its good dispersion stability in ethylene glycol solution, and suggests that particles repel each other and do not undergo flocculation[47].The negatively charged surface of SiO2supplies active sites to absorb cations of Fe3+and Cu2+through electrostatic attraction.Results demonstrate that SiO2can be an effective carrier for CuFe2O4nanoparticles.
The specific surface areas of as-obtained catalysts were investigated by a nitrogen adsorption/desorption instrument and calculated by the multipoint Brunauer-Emmett-Teller (BET) method, nitrogen adsorption-desorption isotherms curves and pore size distributions are shown in Fig.4(c) and 4(d), respectively.Pure CuFe2O4has a relatively small specific surface area(~42.6125 m2/g),but the specific surface areas of CuFe2O4/SiO2composites progressively increases to 49.0999 m2/g, 70.7566 m2/g, 56.7653 m2/g and 53.6471 m2/g with SiO2content increasing from 1 to 7 wt%.Moreover, the maximum value(70.7566 m2/g)achieved at 3 wt%content of addition suggests that 3 wt% content of SiO2carrier possess enough capacity for dispersing CuFe2O4nanoparticles.The results further suggest that the SiO2carrier can inhibit the aggregation of CuFe2O4nanoparticles,which is consistent with SEM image in Fig.2(d).Additionally, the adsorption-desorption isotherms curve in Fig.4(c) present typically type IV hysteresis loops when P/P0range is 0.4-1.0 [34,44].The maximum pore size can reach 148 nm(Fig.4(d)),indicating that the introduction of SiO2carrier increases the pore content,pore size and specific surface area of catalysts,thus greatly improving the catalytic activity.Large specific surface area of CuFe2O4/SiO2could offer more adsorption and reaction sites, which consequently result in better catalytic activity on energy components[43].
3.2.Catalytic decomposition properties
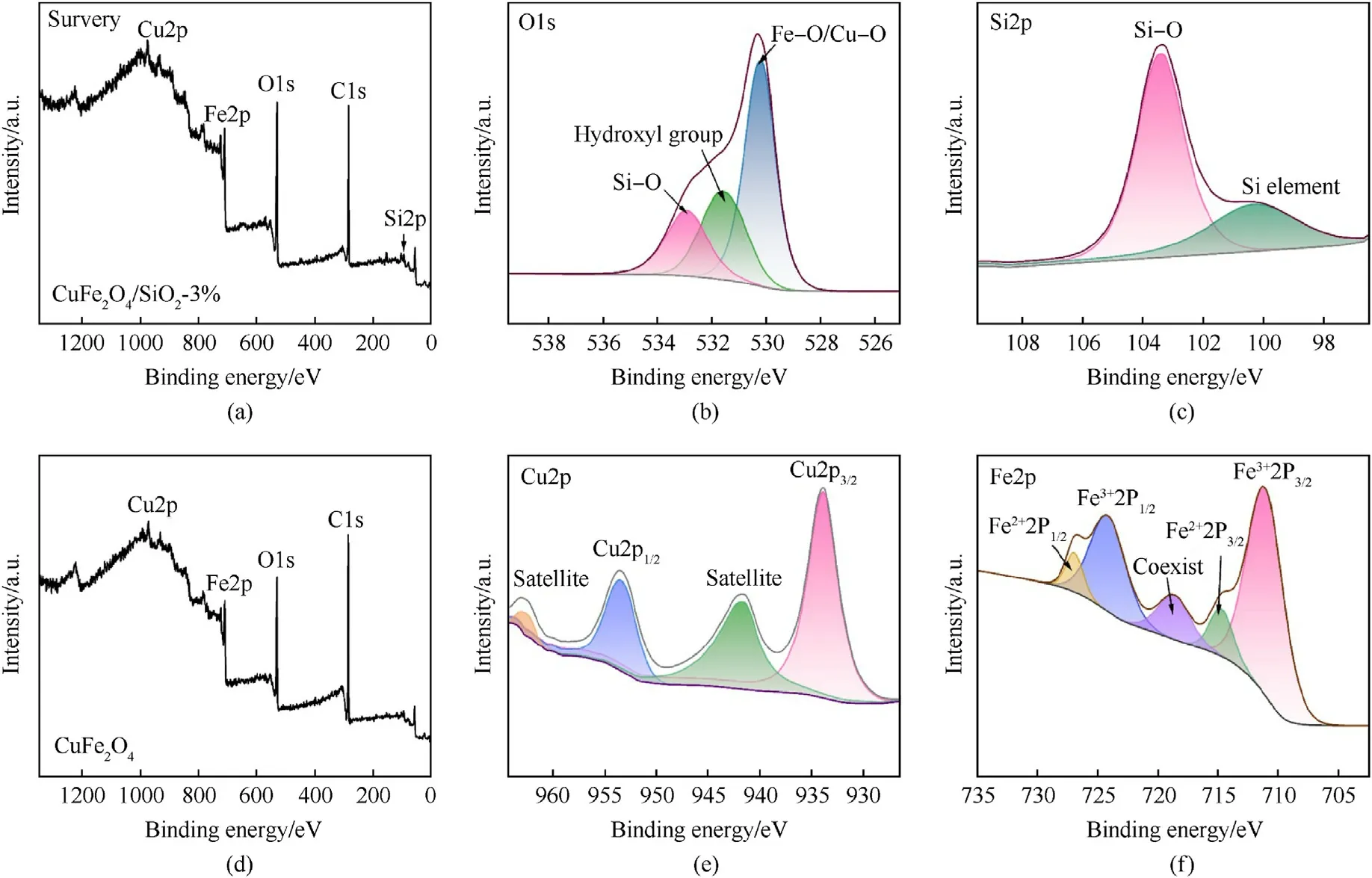
Fig.3.(a)-(c), (e), (f) XPS spectrum of CuFe2O4/SiO2-3% composites and (d) CuFe2O4.

Fig.4.(a), (b) Zeta potential of SiO2 particles in ethylene glycol solution; (c) N2 adsorption/desorption isotherms and (d) pore size distribution of samples.
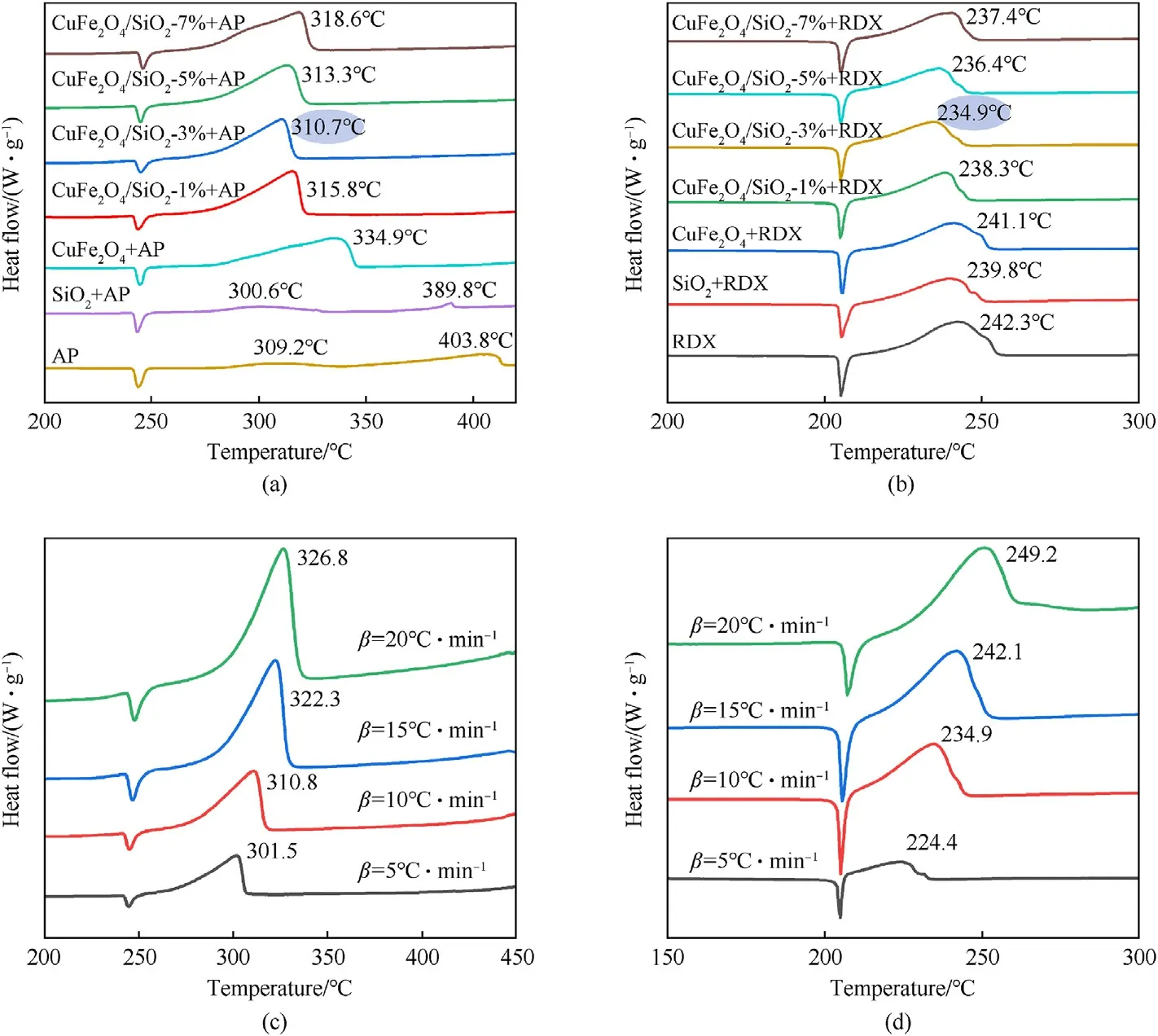
Fig.5.DSC curves of CuFe2O4/SiO2 composites on (a) AP and (b) RDX; DSC curves of CuFe2O4/SiO2 (3 wt%) on (c) AP and (d) RDX at different heating rates.
The catalytic abilities of CuFe2O4/SiO2composites on AP and RDX were investigated and shown in Fig.5(a)and 5(b).DSC curves in Fig.5(a) show a distinct endothermic peak of AP at 245°C assigning to transformation from orthorhombic phase to cubic phase [26].The pure SiO2advances the high temperature decomposition (HTD) and the low temperature decomposition(LTD) of AP from 403.8°C and 309.2°C to 389.8°C and 300.6°C,respectively, presenting a weaker catalytic decomposition on AP.Comparatively, the two exothermic peaks merge into one broad peak under the action of CuFe2O4nanoparticles, and the peak temperature is advanced to 334.9°C.It can be seen clearly in Fig.5(a) that there exists a remarkably synergistic catalytic effect after CuFe2O4and SiO2combining, the exothermic peak temperatures of AP decrease to 315.8°C,310.7°C,313.3°C and 318.6°C with SiO2content increasing from 1 to 7 wt%.In comparison with pure CuFe2O4(334.9°C)and SiO2(389.8°C),the 3 wt%content of SiO2in CuFe2O4/SiO2composites can achieve the optimum catalytic effect and the exothermic peak temperatures of AP is advanced by 24.2 and 79.1°C, respectively.
Taking good catalytic effect of CuFe2O4/SiO2composites on AP thermal decomposing into consideration, its catalytic activity on RDX was further investigated, as shown in Fig.5(b).The peak temperature of RDX exothermic decomposing is advanced to 241.1°C and 239.8°C under the catalytic effects of CuFe2O4and SiO2, respectively, and the exothermic peak temperature of RDX decreases to 238.3°C, 234.9°C, 236.4°C and 237.4°C, with SiO2content increasing from 1 to 7 wt%.Interestingly, the variation tendency of catalytic effect of CuFe2O4/SiO2composites on RDX is consistent well with that of AP,in which the peak temperatures of them all decrease firstly and then increase accompanied with the increasing of SiO2content in CuFe2O4/SiO2composites.
The synergistic effect of CuFe2O4and SiO2can be explained as:(1)The uniform dispersion of CuFe2O4nanoparticles on the surface of SiO2by electrostatic interaction and the effective inhibition of aggregation of nanoparticles proved by SEM analysis in subsection 3.1, both RDX (234.9°C) and AP (310.7°C) realized the lowest thermal decomposition peak temperature under the catalytic effect of CuFe2O4/SiO2(3 wt%)composites;(2)The specific surface area of CuFe2O4/SiO2composite achieves the maximum at the carrier content of 3 wt%, as described in subsection 3.1, which generates more active sites and higher reaction activity, resulting in better catalytic ability; (3) CuFe2O4nanoparticles show more obvious catalytic effect than pure SiO2carrier for thermal decomposition of AP and RDX,hence higher content of SiO2(5 wt%and 7 wt%)would weaken the synergistic catalytic effect.
In addition, the apparent activation energy (Eα) is an essential indicator reflecting the difficulty of decomposition process of energetic materials, which is of great significance for the study of catalytic decomposition performance [48,49].In order to contrast the catalytic effect of CuFe2O4/SiO2on AP and RDX,the Eα values of AP + CuFe2O4/SiO2(3 wt%)and RDX +CuFe2O4/SiO2(3 wt%) were calculated from DSC data recorded at different heating rates (β =5.0, 10.0, 15.0 and 20.0°C/min) using Kissinger method [50] and Flynn-Wall-Ozawa method[51].As shown in Fig.5(c)and 5,(d),the peak shape remains unchanged while the decomposition peaks advance to high temperature successively with heating rate increasing.With the addition of CuFe2O4/SiO2catalyst, the Eα values of AP and RDX decrease from 161.0 kJ/mol and 239.6 kJ/mol to 139.3 kJ/mol and 113.6 kJ/mol for Kissinger method,141.7 kJ/mol and 116.1 kJ/mol for Flynn-Wall-Ozawa method, respectively.Comparing with previous research [26], CuFe2O4/SiO2(3 wt%) decreases the Eα value of AP and RDX by 21.7 kJ/mol and 126 kJ/mol,respectively.It can be seen in Table 1 that fewer catalyst carrier(3 wt%) but higher catalytic activity is achieved, which illustrates the prominent catalyst effect of CuFe2O4/SiO2composites on AP and RDX, and further confirms that SiO2can be used as an effective carrier for CuFe2O4nanoparticles.
Multiple catalysts have been used to improve the thermal decomposition properties of AP and RDX,results all confirmed the catalytic effects on advancing the high exothermal peak temperature, reducing the apparent activation energy, and even transforming the slow two-stage decomposition process into a rapid one-step process.For comparison, the catalytic capacities of several catalysts or the combination of them are listed in Table 2,which further verifies the excellent synergistic catalytic effect of the CuFe2O4/SiO2composites on both AP and RDX.

Table 1 The apparent activation energy (Eα) values of samples.

Table 2 Comparison of catalysts effects on AP and RDX in previous works.
3.3.Laser ignition performance
Both ignition delay time and flame propagation velocity are important parameters to investigate the ignition and combustion performance of energetic materials [56], in which the former is defined as the time interval between the triggering of laser and the appearance of igniting flame[39,40],and the latter is related to the amount and rate of gas production.
As shown in Fig.6(a), the ignition delay time of all samples decreases sharply with the increase of laser power density.CuFe2O4nanoparticles can shorten ignition delay time of RDX in lower power density of 109.3 W/cm2and improve the ignition properties of RDX, revealing that CuFe2O4nanoparticles tend to be more sensitive to laser radiation.However, the ignition delay time of RDX increases after introducing SiO2and higher SiO2content is corresponding to longer ignition delay time.Moreover,the ignition delay time values of all composites tend to be close to each other after the power density exceeding 155.3 W/cm2.In Fig.6(b), the flame propagation velocity of RDX + CuFe2O4is investigated, which exhibits a lower flame propagation velocity than that of pure RDX but the longest combustion time (272 ms).Besides, the flame propagation velocity of CuFe2O4/SiO2(3 wt%) is 2.73 times faster than pure RDX.Considering the thermally insulating property of SiO2carrier, the abnormal phenomenon may be explained as (1) The poor thermal conductivity of SiO2makes it a barrier for thermal conduction, therefore the temperature at the vicinity of SiO2particles increases rapidly and results in multiple ignition points for combustion, thereby promoting the combustion progress [57,58];(2) Once ignited, particulate product is ejected along with highpressure gas to increase the size of the flame, facilitating laser feedback and heat transfer [31,32]; (3) It is worthy to note that higher content(5 wt%)of SiO2may decrease the energy density and reactivity because of heat-sink effect [32].
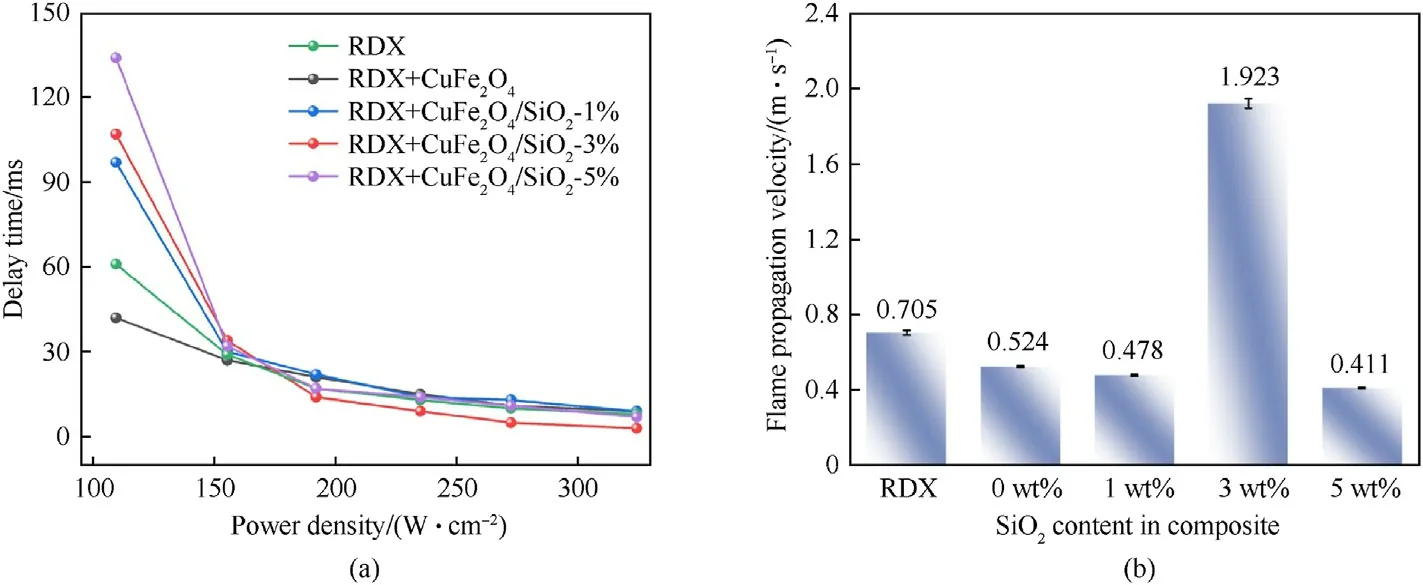
Fig.6.Ignition delay time and Flame propagation rate of composites at different SiO2 content.

Fig.7.Combustion process of(a)pure RDX;(b)RDX+CuFe2O4;(c)RDX+CuFe2O4/SiO2(1 wt%);(d)RDX+CuFe2O4/SiO2(3 wt%)and(e)RDX+CuFe2O4/SiO2(5 wt%)composites.
To illustrate the combustion performance of CuFe2O4/SiO2composites, the burning snapshotting of RDX, RDX +CuFe2O4and RDX + CuFe2O4/SiO2(1 wt%, 3 wt%, 5 wt%) composites were recorded under the same condition for comparison.As shown in Fig.7(a),the combustion flame of pure RDX is dark red and weakly propagated, while the flame of RDX + CuFe2O4is more luminous and lasts for 272 ms in Fig.7(b), proving that CuFe2O4could promote burning process of RDX.This might be attributed to the good thermal diffusivity of CuFe2O4nanoparticle which facilitates heat transfer and accelerates combustion progress.Yet the combustion time decreases to 213 ms (Fig.7(c)) after the introduction of SiO2carrier (1 wt%).As shown in the 5 ms picture (Fig.7(d)) of RDX + CuFe2O4/SiO2(3 wt%), the mixture immediately forms an accelerated flame propagation fronter at the highest velocity and the combustion process completes in the shortest time (103 ms).The reaction intensity in Fig.7(e) becomes weaker and flame propagation velocity declines sharply, which might be due to the high content of SiO2in composite.All results reveal that the influence of reaction front area is greater than that of thermal diffusion at low mass percentage (3 wt%) additives in solid propellent [32].Therefore, it is necessary to control the appropriate content of SiO2carrier to achieve the expected effect in practical application.
3.4.Electrostatic discharge sensitivity
The production,transportation and storage process of energetic material has high requirements due to its high energy density,therefore the sensitivity regulation is particularly important [59].The electrostatic discharge sensitivities (EDS) of samples were investigated by apparatus JGY-50(III) introduced in subsection 2.4.As shown in Fig.8, the introduction of CuFe2O4nanoparticles produces a lower EDS value of RDX, which may easily produce security problems.However, E50values of RDX + CuFe2O4/SiO2composites increase to 7.32 ± 0.05 mJ as the SiO2content rises to 7 wt%, indicating that the EDS value decreases by 189% and 230%when compared it with pure RDX (3.87 ± 0.05 mJ) and RDX + CuFe2O4(3.20 ± 0.05 mJ), respectively.Additionally, the value of electrostatic discharge sensitivity of RDX+SiO2(100 wt%)decreases by 253% compared with that of pure RDX, which might be attributed to the micron-size structure of SiO2which facilitates the dissipation of energy when electrostatic forces act on the composites [33,41], and the charge accumulation caused by aggregation of CuFe2O4nanoparticles is eliminated to a great extent after introducing the SiO2.Therefore, in carrier content range of 3-5 wt%, the CuFe2O4/SiO2catalyst not only possess excellent catalytic and combustion properties, but also present stable safety characteristics.

Fig.8.EDS results of samples, 0 wt%: RDX +CuFe2O4,100 wt%: RDX + SiO2.
4.Conclusions
In this work, CuFe2O4/SiO2composite was successfully synthesized through solvothermal method, proving by phase structure,morphology and chemical bond.The effect of SiO2content on the catalytic property of CuFe2O4/SiO2composite was explored, and it is found that CuFe2O4/SiO2(3 wt%) reduced the exothermic decomposition temperature of AP and RDX by 93.1°C and 7.4°C,respectively.In addition, the promotion effect of CuFe2O4/SiO2composite on the combustion and safety performance of RDX were investigated and the results indicate that SiO2also plays a significant role in accelerating the flame propagate and enhancing the anti-electrostatic ability of RDX, in which the flame propagate velocity increases from 0.705 ± 0.005 to 1.923 ± 0.025 m/s after introduction of SiO2carrier (3 wt%) and the E50value rises from 3.87±0.05 to 7.32±0.05 mJ after introduction of SiO2carrier(7 wt%).Therefore, SiO2is confirmed to be an excellent carrier that can synergistic with CuFe2O4to form combustion catalysts and be used in solid propellants.Furthermore,the range of SiO2carrier content in which the catalyst exhibits remarkably catalytic performance,certain combustion promotion effect,and controllable electrostatic discharge sensitivity is found to be 3 wt% to 5 wt%.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This investigation received financial assistance from the National Nature Science Foundation of China (Grant Nos.21673178,22105160), the Natural Science Foundation of Shaanxi Province(Grant No.2023-JC-ZD-07), the Foundation of Key Laboratory of Defense Science and technology (Grant No.6142603032213) and the Key Science and Technology Innovation Team of Shaanxi Province (Grant No.2022TD-33).
- Defence Technology的其它文章
- Ground threat prediction-based path planning of unmanned autonomous helicopter using hybrid enhanced artificial bee colony algorithm
- Layered metastructure containing freely-designed local resonators for wave attenuation
- Predicting impact strength of perforated targets using artificial neural networks trained on FEM-generated datasets
- Construct a 3D microsphere of HMX/B/Al/PTFE to obtain the high energy and combustion reactivity
- Ignition processes and characteristics of charring conductive polymers with a cavity geometry in precombustion chamber for applications in micro/nano satellite hybrid rocket motors
- Recent research in mechanical properties of geopolymer-based ultrahigh-performance concrete: A review

