Clinical features and possible pathogenesis of multiple evanescent white dot syndrome with different retinal diseases and events: a narrative review
Chun-Li Chen, Yi-Zhe Cheng,3, Zhi-Han Zhang, Ge Wang, Xiao-Yan Peng
1Department of Ophthalmology, Beijing Tongren Hospital,Capital Medical University, Beijing 100730, China
2Beijing Ophthalmology and Visual Science Key Laboratory,Beijing 100730, China
3State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510600, Guangdong Province, China
Abstract
● KEYWORDS: multiple evanescent white dot syndrome;punctate inner choroidopathy; multifocal choroiditis;secondary multiple evanescent white dot syndrome
INTRODUCTION
Multiple evanescent white dot syndrome (MEWDS) is an evanescent and multifocal chorioretinopathy first reported by Jampolet al[1]in 1984.This disorder typically occurs in young healthy individuals, particularly in women,with its incidence estimated to be 0.22 per 100 000 population annually[2-3].Patients with MEWDS usually present with multiple symptoms, such as moderate-to-severe vision loss,photopsia, and enlargement of physiologic scotoma.Clinically,MEWDS (primary MEWDS) is typically characterized by small white dots at the deep retina of the posterior pole and peripheral retina, papillary edema, foveal granularity, and retinal vascular sheathing[2].Sometimes vitreous inflammation also could be observed (Figure 1)[4].In addition, more than half of patients have myopia.Approximately 50% of patients have a history of respiratory tract infection, viral infection,vaccination, or show prodromes of systematic infection before the occurrence of MEWDS (about 4wk before onset)[1,5-8].
The etiology of MEWDS remains elusive.It is suspected that MEWDS is associated with viral infection.The diagnosis primarily relies on medical history, clinical features, andancillary multimodal imaging (MMI)[9].Generally, MEWDS exhibits a self-limiting course with a duration of 6 to 8wk,mostly without permanent complications.Therefore, local/retinal steroids and/or systemic steroid therapy have not been regularly recommended, except for some complications that require symptomatic treatment and related supportive therapy[2].With the rapid advancement of diagnostic techniques and profound awareness of the disease, more and more patients with MEWDS secondary to or cooccurring with other retinal diseases or ocular events have been reported in the era of MMI.Essilfieet al[10]recently proposed a term, secondary MEWDS, that may be triggered by a macular disease or iatrogenic injury, distinguishing from the primary MEWDS described by Jampol[1].Secondary MEWDS refers to classical MEWDS features triggered by choroidal antigen exposure after varied causes and diseases possibly disrupting the retinal pigment epithelium-Bruch membrane-choriocapillariscomplex (RPE-BrM-CC)[11-14].They believed that secondary MEWDS seems to be an epiphenomenon that may be seen in diseases disruptive to RPE-BrM-CC.On our review of current literature, secondary MEWDS could be seen in other else white dot syndromes[15-19], such as punctate inner choroidopathy(PIC), multifocal choroiditis (MFC), acute zonal occult outer retinopathy (AZOOR), andetc., retinal diseases like best vitelliform macular dystrophy (BVMD), pseudoxanthoma elasticum (PXE), and retinal insults induced by ocular events(history of scleral buckle for retinal detachment, idiopathic retinochoroiditis, traumatic subretinal hemorrhage, choroidal rupture, penetrating trauma of the fellow eye or the choroid insults)[20-25].What’s more, MEWDS secondary to MFC/PIC is prone to form focal choroidal excavation (FCE)[26-28]and choroidal neovascularization (CNV)[23,29-34].In secondary MEWDS, the manifestations affiliated with primary MEWDS are still evanescent, and treatment and prognosis depend on the severity of concomitant diseases.Herein, the accurate diagnosis of secondary MEWDS can guide the treatment and evaluation of prognosis to avoid severer complications.In this article, we summarized the features, clinical manifestations,and possible pathogenesis of secondary MEWDS in the aforementioned entities.

Table 1 Difference between spots and dots
Overview of Primary Multiple Evanescent White Dot SyndromeIt is necessary to have an understanding of primary MEWDS before describing secondary MEWDS.The primary MEWDS commonly occurs in young and mid-aged females.The male-to-female ratio is 1:5.The patients often present with acute or subacute vision loss, enlarged blind spots, photopsia,and floaters.We reviewed previous studies and reports about MEWDS and summarized the imaging features in different examinations as follows: 1) Color fundus photograph (CFP)shows that multifocal, round, and grayish-white spots, 100-200 μm in diameter, were located in proximity to areas of the posterior pole, macula, and optic disc.The lesions could extend from the posterior pole to the mid-peripheral retina at occurrence (Figure 2) and fade from the periphery to the posterior pole in the phase of resolution (Figure 3).Foveal granularity is another characteristic manifestation of CFP and is associated with the unique anatomical structure of the fovea[2,35-37].2) Fluorescein angiography (FFA) helps the diagnosis of MEWDS.Early FFA shows varying sizes of tiplike hyperfluorescence arranged in a wreath-like shape in 80%of patients.On late FFA, no significant fluorescence leaks from the capillaries around the disc.The hyperfluorescence of the optic disc is noted in a few patients[2,35,38].Finn and Khurana[39]first defined the lesions in MEWDS as dots and spots on FFA and indocyanine green angiography (ICGA).They defined dots as small lesions of approximately 100 μm and spots as larger lesions of more than 200 μm.Dots are correlated to the wreath-like hyperfluorescence on early FFA, and spots showed ill-defined and uneven hyperfluorescence on FFA (Table 1).3) ICGA is also a significant auxiliary examination to provide better visualization of choroidal vessels.Early -ICGA shows no abnormalities of choroidal background fluorescence and medium-to-large vessels.But in the middle and late stages(after roughly 10min), multiple and scattered hypofluorescent spots in varying sizes and with poorly-defined borders were seen in the macula and mid-peripheral choroid as well as around the optic disc, surrounded by a light gray halo in the boundary.Subsequently, the hypofluorescence was fused in sheet-like patterns on late-ICGA[2].4) Fundus autofluorescence(FAF) imaging is a rapid and noninvasive technique to evaluate retinal pigment epithelium (RPE) function.On FAF,mottled and scattered hyperautofluorescent lesions are in the vicinity of the optic disc and in the posterior pole with scattered hyperautofluorescent dots at the edges of lesions.5)Optical coherence tomography (OCT) is an important and noninvasive tool to observe the structure of the retinain vivo.The OCT shows disruption of the ellipsoid zone (EZ) in the fovea and dome-like accumulation of subretinal hyperreflective material (SHRM) overlying RPE.The SHRM has been considered debris of the insulted outer segments in previous studies.En-faceOCT noted the dots in the outer nuclear layer and spots in the EZ/interdigitation zone (IZ), and also show‘dots on spots’ in proximity to the optic disc (Figure 4)[2].6)OCT angiography (OCTA) is an advanced imaging technique to produce high-resolution images of blood flow of all the vascular layers in a rapid and non-invasive fashion[40-41].In the acute phase of MEWDS, OCTA shows completely normal choriocapillary morphology.The hypofluorescent areas on ICGA also show no dilation or occlusion of choriocapillary,inferring that the lesions in the acute phase of MEWDS are limited to the outer segment of the photoreceptor[42].7) The visual field examination exhibits an enlarged physiological scotoma in MEWDS.The aforementioned imaging features help us make an acute diagnosis clinically.

Figure 1 Anterior segment photographs of a 25-year-old female who complained of decreased vision with fixed scotoma for one week and presented with vitreous cells in the right eye (A and B)She was diagnosed with MEWDS in the right eye.MEWDS: Multiple evanescent white dot syndrome.
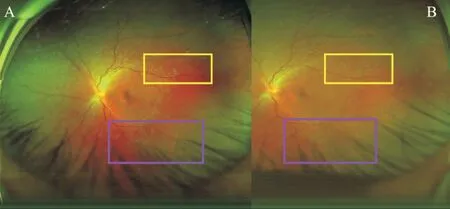
Figure 2 Ultrawide fundus images of a 33-year-old female who was diagnosed with MEWDS in the left eye with a complaint of decreased vision for 1wk A: Plenty of white dots and spots; B:Increased numbers of dots and spots from the posterior pole to the peripheral over time (yellow and purple box).MEWDS: Multiple evanescent white dot syndrome.
As for the prognosis, due to its self-limiting course, the vision will return to the pre-onset level in 95% of the patients with primary MEWDS, with a resolution of structural change on MMI[2].However, enlargement of the scotoma, photopsia,and chromatic aberration may persist in a minority of patients[23,29-34,43].

Figure 3 Ultrawide fundus images of a 33-year-old female who is diagnosed with MEWDS in the lefteye with a complaint of decreased vision for one week A: White spots and dots in the peripheral after one week; B: White spots start to fade from the peripheral after 2wk.MEWDS: Multiple evanescent white dot syndrome.

Figure 4 En-face OCTA of a 28-year-old female who complained of acute vision loss for one week and was diagnosed with MEWDS in the lefteye En-face OCTA shows the typical dots and spots.A: Dots are located in the outer nuclear layer (red oval); B: Spots represent EZ disruption (yellow ovals).OCTA: Angio optical coherence tomography; EZ: Ellipsoid zone; MEWDS: Multiple evanescent white dot syndrome.
Multiple Evanescent White Dot Syndrome Secondary to Retinal Diseases

Figure 5 Color fundus photographs and FFA of a 35-year-old male presented with bilateral floating shadows and complained of vision decline.He was diagnosed with PIC and MEWDS in the lefteye A-D: The fundus imaging at the first visit.A: Varying sizes of whitish-yellow lesions with pigmentation in the posterior pole and mottled yellow lesions in the mid-peripheral (blue arrows); B: Diffused hyperautofluorescence in the posterior pole with scattered hypoautofluorescence on FAF; C: Wreath-like hyperfluorescence on early FFA (blue arrows) and hypofluorescent PIC lesions with hyperfluorescent margin (yellow triangle); D: Fluorescence staining of PIC lesions in the posterior pole on late FFA.E-H: The follow-up at the time of 3wk later.E: The disappearance of the mid-peripheral lesions; F: Hypoautofluorescent PIC lesions in the posterior pole on FAF, and some of them are surrounded by the hyperautofluorescence at the border, while the diffused hyperautofluorescence in the posterior pole faded; G: Hypofluorescent lesions with a hyperfluorescent border in the posterior pole; H: Fluorescence staining of PIC lesions in the posterior pole on late-FFA.PIC: Punctate inner chorioretinopathy; MEWDS: Multiple evanescent white dot syndrome; FAF: Fundus autofluorescence; FFA: Fundus fluorescence angiography.
Multiple evanescent white dot syndrome secondary to multifocal choroiditis/punctate inner choroidopathyIn addition to the already recognized manifestations of primary MEWDS, secondary MEWDS is referred to classical MEWDS features triggered by choroidal antigen exposure after varied causes and diseases possibly disrupting the RPE-BrM-CC.Cases of MEWDS secondary to MFC/PIC have been reported in the recent literature.MFC mostly affects bilaterally and occurs in young myopic women presented with photopsia,blurred vision and visual field defects[44-45].When inflammation infiltrates deep choroid, the affected eyes may be presented with inflammatory cells of the vitreous cavity and anterior chamber, macular serous detachment, optic disc edema or congestion, electroretinogram (ERG) changes, and so forth.Idiopathic MFC is a generic term encompassing all three diseases, including PIC (Figure 5), MFC with panuveitis and progressive subretinal fibrosis.Despite sharing demographic features and symptoms, MFC differs from MEWDS in several aspects[1].White spots in MEWDS are usually unilateral and present clinically in the deep retina or RPE layer, rather than the choroid.Vitreous inflammation could occur, but it is milder and more evanescent than MFC.Besides, leakage and macular detachment usually do not occur in MEWDS.Compared to MFC, white spots and visual field defects usually resolve spontaneously within 4 to 6wk with no significant residual lesions.Visual acuity usually remains good, although macular granularity tends to persist.
Regarding the similar demographic features and symptoms of MEWDS and MFC, a common host susceptibility likely exists between these two distinct entities.The overlap seen in these 2 entities can be explained in several ways.Kuznetcovaet al[46]hypothesized a collective mechanism of episodes of choriocapillaris before MEWDS and MFC, and then this recurrent asymptomatic choriocapillaris may eventually evolve into MFC or other entities of WDS.Regarding MEWDS and MFC, previous literature has reported several cases of patients with MEWDS who subsequently exhibited typical manifestations of MFC with/without CNV during continuous observation[15,47].However, the opposite sequence is rarely seen(MFC evolving to MEWDS), with only two cases identified in the literature.In these two cases, MEWDS occurred in patients with pre-existing peripheral retinal scars that may be induced by prior MFC[15].Whether the manifestation of inflammatory diseases is initially of MFC pattern or MEWDS pattern, most likely depends on the identity of the stimulating antigen or infectious agent and the host susceptibility.Some patients with MEWDS may be presented with overlapping MFC in the same or fellow eye[18].Whether the two entities in the same patient exhibit a common susceptibility or common pathological mechanisms, there is considerable evidence of overlap among many idiopathic inflammatory choroidal entities, not just MFC and MEWDS[18].MFC and MEWDS may be affiliated with the same disease spectrum and are more closely related to each other than other entities of WDS.MFC and MEWDS may be mediated by the same immune process that triggers one or the other, or both[48].An MEWDS-like reaction may be triggered by the previous or simultaneous damage to the outer retina.Therefore, more studies about the genetic predisposition and molecular basis of MEWDS-like reactions will help to further understand this disease.
MEWDS secondary to MFC/PIC exhibits not only features of primary MEWDS but also includes additional conditions as below: 1) Isolated juxtafoveal yellowish-white inflammatory PIC lesion is visible on CFP.2) Bruch’s membrane (BrM)disruption and SHRM are visible on OCT.Choroidal thickening can be observed on enhanced depth imaging OCT(EDI-OCT) in the acute phase, suggesting the presence of inflammation in the outer retina and choriocapillaris.3) The neovascularization in the optic disc or macula could be seen at the initial presentation on FFA, ICGA, and OCTA.A detailed description of MEWDS secondary PIC/MFC could be found in our team’s previous work[13].The common complications of MFC/PIC have been referred to as FCE and CNV[26-34,43].Therefore, some previous cases showed cooccurrence of MEWDS and FCE/CNV.We reviewed these cases and summarized them as follows.
Secondary MEWDS with focal choroidal excavationFCE was first identified on time-domain OCT and then named by Margoliset al[49].FCE mostly exhibits a stable state during a 1-year or 3-year follow-up period[50].Although the pathogenesis remains hypothetical, it is suspected that FCE is in association with congenital or acquired abnormalities of choroidal capillary, including the choroidal malformation and developmental defects , inflammatory diseases[27-28,51]and choroidal vascular disease[52].There are three reported cases of secondary MEWDS with yellowish-white lesions developing into FCE[26-28].Their common features are the increased choroidal thickness at the lesion’s site initially, and the separation of the RPE from BrM with SHRM with/without mild BrM disruption subsequently.A case of FCE succeeding occurrence of MEWDS, reported by Jabbarpoor Bonyadiet al[27], was given a small dose of short-term steroid therapy and then BrM returned to structural integrity at the last followup, whereas the excavation was gradually deepened and morphologically stabilized.It is suggested that the severity of inflammation-induced damage and the plasticity of the RPEBrM-CC may be related to the different types of acquired FCE.The mechanisms underlying MEWDS with FCE seem to consist of the following pathogenic events.First, inflammation occurring in the outer retina/inner choroid causes impairment of RPE-BrM-CC leading to adhesion of retinal and choroidal tissues through the RPE-BrM-CC ruptures with the increase of choroidal thickness.Then, contraction of the fibrotic lesions in combination with intraocular pressure leads to retinal herniation into the choroid.In addition, it has also been suggested that focal choroidal scar is the starting point for the pathogenesis of FCE and the imbalance of intraocular pressure and choroidal pressure may play a role in the formation of FCE[52].
Secondary MEWDS with choroidal neovascularizationCNV is caused by perturbation of RPE, presented with vision loss due to intraretinal exudate, subretinal fluid, hemorrhage,or fibrosis.CNV is an uncommon complication of primary MEWDS and usually occurs in secondary cases, such as the recurrent forms or MEWDS with marked inflammatory sy mptoms[15,29-34,43,47,53-58].In the evolution of recurrent forms,infiltration and persistence of inflammation may lead to the formation of chorioretinal scar, then predisposing to CNV.On the review of the literature, we found that CNV occurred ranging from 4wk before to 13y after the occurrence of MEWDS, especially concurred with CNV in 6 cases[15,29-34,43,47,53-58].The CNV is mainly located in the juxtafovea, with two cases located around the optic disc[29,33].It is suggested that acute changes of CNV are related to the occurrence of MEWDS.Of note, there are six atypical cases of MEWDS in three publications presented with juxtafoveal yellowish-white lesions[29,32-33].Our team reported a case series of MFC/PIC cases with MEWDS-like features[13].In our study,all the cases showed the MEWDS features of multifocal deep retinal grayish-white spots, with the addition of juxtafoveal yellowish-white lesions (determined as CNV by OCTA),increased choroidal thickness, and SHRM.
The reason why MEWDS concurred with CNV is uncertain.It is suspected that the ischemia at the RPE-BrM-CC may trigger neovascularization.The evanescent choroidal hypoperfusion corresponding to hypofluorescence on late-ICGA in typical MEWDS[2], is not sufficient to cause permanent structural damage to the RPE and outer retina.Nonetheless, in secondary MEWDS cases, inflammation in the choroid leads to the formation of pachyvessels in the Haller layer and ruptures of BrM, and then choriocapillaris and scatter layer suffer a sufficient compression exerted by pachyvessels to produce a local ischemic microenvironment in conjunction with BrM ruptures predisposing to CNV.
Treatment is needed in cases of inflammatory CNV followed by secondary MEWDS.It is suspected that timely and effective steroid treatment is beneficial to the repair of elastic and collagen fibers of BrM, reducing the pulling of fibrous tissue against the formation of CNV[13,59].The inflammatory CNV preceding MEWDS suggests an asymptomatic phase of choriocapillaris.CNV may trigger the occurrence of MEWDS,probably due to the pro-inflammatory environment generated by the retinal tissue surrounding CNV.The pro-inflammatory environment acts as a trigger effect in susceptible eyes.These triggers can induce CNV and inflammatory changes in choriocapillaris or evanescent ischemia of choriocapillaris.Secondary MEWDS occurs more likely in patients with chorioretinitis with BrM ruptures[24-25].The yellowish-white lesion in the juxtafovea may be the precursor of inflammatory CNV, and that timely and effective glucocorticoid treatment may reduce the progression of inflammatory lesions transforming into FCE or CNV and protect visual function as much as possible.MEWDS with Acute Zonal Occult Outer RetinopathyAZOOR is an outer retinal dysfunction extending from the optic disc, mainly characterized by acute vision loss or enlargement of a blind spot with photopsia, unilateral presentation, and often combined with myopia[33].In the early stage of AZOOR, the ocular examination in 90% of patients, is almost completely normal, whereas atrophy of RPE and choroidal formed in the later stage.Different from MEWDS, AZOOR has a progressive course and a relatively aggressive prognosis.MMI is a helpful tool in diagnosing and differentiating MEWDS and AZOOR.FAF shows the characterized pattern of zonal fused hyperautofluorescence surrounding the optic disc.FFA shows no abnormalities in AZOOR patients, and ERG contributes to assessing if there is rod or cone cell dysfunction by evaluating the function of the outer retina.OCT shows the disappearance of EZ,corresponding to areas of visual field defect[24].
The presence of MEWDS and AZOOR in the same eye implies a common environmental or genetic susceptibility,or that these white dot syndrome (WDS) have a common etiology.However, the probability of incidental concurrence of 2 rare diseases is very small.It is difficult to determine whether the clinical manifestations of MEWDS precede AZOOR or appear simultaneously.Although the prognosis of MEWDS is better than that of AZOOR, the subsequent onset of AZOOR succeeding MEWDS may predict a worse outcome[16].The overlapping manifestations in different WDS have also been reported.AZOOR also occurred in patients with a prior diagnosis of other types of WDS, including PIC[60]and MFC[61], suggesting a common genetic association with the pathogenesis[62].
In some patients, MEWDS and AZOOR may share common features, including gender (mostly female), unilaterality, low-tomoderate myopia, punctate or confluent hyperautofluorescence in the active phase on FAF, hypofluorescent lesions on late-ICGA.However, as being 2 distinct entities, there are some differences in different examinations as follows helping to differentiate.First, MEWDS is mainly seen in young women aged 15 to 30 years old, whereas AZOOR occurs mostly in their 30s to 40s.Second, MEWDS shows obvious white dots mostly located in the posterior pole on CFP, also seen in the midperiphery.However, when it comes to AZOOR,there is no visible abnormality in the early phase of CFP, and atrophic changes are only seen around the optic disc in the late phase.Third, in MEWDS, ERG commonly shows no abnormalities, with the abnormality of a wave occasionally,while in AZOOR, ERG could show abnormality in some cases.Most importantly, FAF plays an important role in differentiating these 2 entities.FAF is a noninvasive means of assessing RPE function[63].Inflammation in the acute phase tends to cause the accumulation of autofluorescence so that hyper-autofluorescence is seen in active lesions or at the edges of the chorioretinal scar.In chronic disease, the apoptosis of RPE may result in hypo-autofluorescence.In MEWDS, it has a self-limiting course and a good prognosis, and the lesions on FAF could restore spontaneously in MEWDS.Yet, in AZOOR,the prognosis varies depending on the site of lesions, and the lesions could not recover on FAF.AZOOR usually has a more severe outer retinal impairment and may be associated with thinning of the inner nuclear layer.IS/OS was restored during the recovery phase of MEWDS, which is uncommon in AZOOR.
Possible pathogenesis of MEWDS with white dot syndromesThe patients initially diagnosed with MEWDS finally develop into other types of WDS, further complicating our understanding of MEWDS (Table 2)[15-16,46-47].Investigating atypical cases of MEWDS may provide profound insights into understanding the underlying mechanisms of MEWDS.For example, according to the different manifestations of varying severity of inflammatory diseases, these overlapping entities probably have two completely distinct progressions(e.g., photoreceptor inflammation secondary to choroiditis or a primary choriocapillaris).It is suggested that these diseases may share a common genetic susceptibility and/or pathological factors.All these patients have relatively common nonspecific gene clusters at specific loci that predispose patients to immune dysregulation and autoimmune diseases.In addition, common susceptibilities (including immune dysregulation, interactions between specific environmental triggers, and other genes) lead to the overlapping occurrence of one or more diseases.What’s more, environmental triggers and major histocompatibility antigens explain some changes in clinical course.Finally,retinal specialists must study the differentiation between MEWDS and other entities of WDS, because appropriate evaluation allows clinicians to estimate whether patients with MEWDS will develop into other overlapping entities of WDS and helps to determine the treatment and evaluate the prognosis.
Secondary MEWDS with PXE, BVMD, History of Surgery, Trauma, or PhotocoagulationIn the traditional recognition of MEWDS, it is an inflammatory disease with a self-limiting course.Although the characterized presentation,reports have described MEWDS occurring in conjunction with other, apparently unrelated, ocular diseases, either concurrently or over the course of follow-up.We comprehensively reviewed the related literatures of secondary MEWDS with other ocular diseases and previous therapeutic history probably inducing retinal insults, and then summarize the features to help differentiate and discuss the underlying mechanisms of MEWDS occurring in the setting of posterior segment abnormalities mentioned above.
Secondary MEWDS with PXEPXE is a rare autosomal recessive multisystem disease characterized by skinlesions, premature calcification, occlusive vascular disease and complications such as angina pectoris, claudication,hypertension, fundus angioid streaks changes, especially occurring in young women.Mutations inABCC6gene are responsible for PXE, although no clear genotype-phenotype relationship has been elucidated yet.Retinal features of PXE include several alterations as a peaud’orange-like appearance,comet lesions, angioid streaks, and peripapillary drusen.In particular, the calcification of BrM may be in association with the alternation of the fundus and finally lead to retinal atrophy with vision loss.Of note, the disruption of the calcified BrM corresponds to the angioid streaks in the ophthalmoscope and gives rise to the development of CNV and exposure of the retinal, RPE and choroidal antigens.Although the pathogenesis is elusive, there are several literatures suggesting that the underlying mechanism is associated with exposure to retinal antigens and the subsequent immune response to these antigens.Gliemet al[24], who conducted a study enrolling 9 patients with acute retinopathy (frequency of 5%) in a cohort with PXE, hypothesized that PXE may be due to serum antiretinal autoantibodies and anti-RPE autoantibodies, and found that these features of acute outer retinopathy and the frequency of 5% suggested a true association between PXE and MEWDS,rather than a coincidental coexistence.In addition, Marcheseet al[64]also reported two young PXE patients presented with features MEWDS and MFC.In the PXE-associated acute outer retinopathy, the lesions (BrM rupture) along vessels are most evidently seen, where antigens of the retina, RPE, and BrM to the immune system may trigger autoimmune processes.Meanwhile, acute outer retinopathy could also explain the previously reported undetected ERG response in PXE patients.The literature reported that autoantibodies against the anti-RPE protein (anti-62-kDa protein) were found in 4 of the 6 cases(67%).Only two cases were tested during the acute phase of retinopathy and the antibodies were tested up to 4y after the onset of acute outer retinopathy.However, if the antigens stimulate the immune system, autoantibodies can persist in the circulation for a long time[24].Therefore, further investigation on the association between RPE as well as retinal antibodies and etiopathology of MEWDS should be conducted.

Table 2 Literature review of MEWDS with other types of WDS
Secondary MEWDS with BVMDWith regard to BVMD,there is a case report documenting the presentation of MEWDS in a patient with BVMD, yet without persistent lesions other than the foveal lesion of BVMD[16].We conclude that BrM disruption, RPE changes, and angioid streaks (disruption of calcified BrM) are likely causes of acute outer retinopathy.Additionally, although fibrotic proliferation is a common complication of angioid streaks, it may also play a pathogenic role in the development of inflammatory-like reactions.
MEWDS with toxoplasmosisGass[62]first described deep retinal involvement for toxoplasmosis, then, Friedmann and Knox[65]described more typical presentations, including punctate inner retinopathy and deep punctate lesions with subretinal fluid, mostly involving the macula or peripapillary area[65].Furthermore, Doft and Gass[66]documented the features in detail and named this condition as ‘punctate outer retinal toxoplasmosis.In reviewing literatures, 3 cases of ocular toxoplasmosis followed by MEWDS, proposing the assumption of the possibility that infection triggers an immune response[67].The authors deemed that whether the succedent MEWDS was related to the patient’s ocular toxoplasmosis infection remained elusive.Alternatively, it may also be due to impaired immune defenses associated with steroid treatment or the immunosuppression related to toxoplasmosis (toxoplasma could also suppress immune function).The state of immunosuppression induced by the former 2 causes could lead patients to be susceptible to an inflammatory condition.Ocular toxoplasmosis can trigger fleeting disruption of the outer retina with photoreceptor loss in the absence of choroidal ischemia.This disruption has been also described in MEWDS and may occur in the setting of inflammatory conditions typically affecting young and healthy patients.However, further studies are needed to better understand the link between the transient disruption of the outer retina triggered by MEWDS and ocular toxoplasmosis[67].In all, toxoplasma gondii is a possible pathogen or may be a pathogen of immunoreaction in some cases of inflammatory diseases.Animal studies also verified that toxoplasmosis can cause defect and migration of RPE cells[68].Taken together, hypothetically, toxoplasmosis is possibly a triggering factor of immune response which ultimately leads to the MEWDS.
MEWDS after surgery, trauma, laser photocoagulationPatients with MEWDS who have an ocular history positive for previous or concurrent ocular events have been described in recent literatures.These ocular events in the published reports include ocular surgery (most being repairment for retinal detachment), trauma, and laser photocoagulation.In our review of the literatures, we identified patients diagnosed with overlapping features of MEWDS and MFC after retinal detachment in the same eye[69]or in the contralateral eye[46].One of two cases of retinal detachment preceding the manifestation of MFC was attributed to a hypersensitive response to the scleral buckling material[70].According to the authors’ hypothesis, it is possible that the retinal damage of retinal detachment and subsequent surgical repair commonly serve to trigger a local inflammatory response.Other reports in the literature also support the theory, and it seems that MEWDS-like reactions are also associated with ocular trauma,including laser photocoagulation treatment of peripheral retinal tears[71], choroidal rupture[22], penetrating trauma[21], and history of orbital lymphangioma resections[25].Funget al[22]reported a case of a 24-year-old female with blunt trauma to the right eye combined with choroidal rupture, who presented 10wk after the initial trauma with gray deep retinal spots, foveal granularity, mild vitreous inflammation, optic disc congestion and edema, and retinal vasculitis.FFA, ICGA, and OCT showed overlapping features consistent with MEWDS.Given the duration after damage and the anatomical location of the injury, retinal damage at the time of detachment and subsequent surgical repair may have played a role in triggering the local inflammatory response.Therefore, the authors concluded that exposure to choroidal antigens or dehemoglobinized blood was the trigger for the fundus manifestation.Overall, in light of the findings reported previously, it is speculated that the coexistence of ocular events and inflammatory retinal diseases may not be coincidental, but rather due to susceptibility or trigger for the local inflammatory response and immunemediated process, including immunogenic triggers and host genetic susceptibility.
Possible pathogenesis of secondary MEWDS with PXE,BVMD, history of surgery, trauma, or photocoagulationAll in all, the ocular diseases or ocular events mentioned above have a greater or lesser role in triggering the concurrent or following MEWDS.Although etiopathology remains to be determined, we have summarized several aspects of triggers according to the previous literature, including infection,genetic susceptibilities to autoimmune diseases, and traumatic events.Taken together, all the triggers seem to contribute to impairing the outer retina or inner choroid and disruption of BrM leading to more exposure to retina and RPE antigens.In macular diseases, although the simultaneous presence of macular diseases (e.g., CNV or BVMD) and MEWDS in the same eye may be coincidental, it is hypothesized that macular RPE or BrM damage is associated with acute inflammatory events.The presence of MEWDS-like features attributed to prior or concurrent ocular injury events may trigger a local nonspecific inflammatory response.Concerning PXEassociated acute retinopathy, the lesions are usually along angioid streaks (disruption of calcified BrM), also supporting the theory of antigen exposure.What’s more, traumatic subretinal hemorrhage, choroidal rupture, penetrating trauma of the fellow eye, or choroid insults also imply the role of antigen exposure as a possible cause.At the site of BrM disruption, more antigens of the outer retina or RPE are exposed to the immune system, which may contribute to the development of secondary MEWDS.Additionally,individuals with outer retinopathy are prone to MEWDS, and the overlapping manifestations indicate a common pathogenic pathway, too[71].Although the underlying mechanism remains speculative, it is found to be strongly relevant to the immunoreaction and pathological progress of inner choroid,RPE, and photoreceptor, suggesting that antigen exposure is a possible trigger[62].Literature about MEWDS concurrently with PXE, BVMD, and MEWDS-like change after surgery,trauma, photocoagulation, and OT were summarized in Table 3.
Conclusion and Future ProspectsIn conclusion, typical fundus manifestations of MEWDS can be diagnosed by MMI (CFP, OCT, FFA, ICGA, OCTA, FAF), and the main diagnostic criteria contain multiple, small, white spots in the posterior pole and deep layers of the peripheral retina, yellowwhite foveal granularity; wreath-like hyperfluorescence on early-FFA and SHRM in RPE, EZ, and outer nuclear layer on OCT; and mild anterior chamber and vitreous cells.Secondary MEWDS was diagnosed by the aforementioned criteria of primary MEWDS, meanwhile with a cause/disease resulting in RPE-BrM-CC disruption.Due to its self-limiting course,MEWDS patients aren’t often given specific treatment.However, in recent years, reports of secondary MEWDS combined with other diseases increased, especially CNV and FCE secondary to MEWDS with juxtafoveal isolated yellowwhite lesion, MEWDS with BVMD and PXE, and secondary MEWDS after surgery, trauma or photocoagulation.Those diseases involving macula have a poor prognosis and cause irreversible damage to vision.Some studies suggested that early steroid treatment is effective for the diseases, suggesting ophthalmologists perceive the occurrence of secondary MEWDS with other diseases in the early stage.Although the manifestations are still evanescent, the prognosis and treatment of secondary MEWDS are determined by the severity of the complications.The etiology and pathogenesis of secondary MEWDS remain undetermined.Current studies support the association of secondary MEWDS pathogenesis with immune factors, including a history of viral infection, inflammation of the choroidal vessels and BrM, traumatic subretinal hemorrhage, choroidal rupture, contralateral eye penetrating injury, or combined choroidal insults, resulting in antigen exposure.More etiologic and pathogenic studies are urgently needed in the future such as the research on retinal antigens and antibodies.Accurate diagnosis could guide clinical management and prognostic assessment to avoid more serious complications.
ACKNOWLEDGEMENTS
Authors’ contributions:Chen CL: Conceptualization, drafting the original manuscript.Cheng YZ: Drafting the original manuscript, editing, visualization.Zhang ZH: Editing.Peng XY: Visualization, reviewing.All authors read and approved the final manuscript.
Foundations:Supported by the National Natural Science Foundation of China (No.82171073; No.82101147).

Table 3 Literature review of MEWDS concurrently with PXE, BVMD and MEWDS-like change after surgery, trauma, photocoagulation and OT
Conflicts of Interest: Chen CL,None;Cheng YZ,None;Zhang ZH,None;Wang G,None;Peng XY,None.
 International Journal of Ophthalmology2024年3期
International Journal of Ophthalmology2024年3期
- International Journal of Ophthalmology的其它文章
- Meibomian glands segmentation in infrared images with limited annotation
- Artificial intelligence for the detection of glaucoma with SD-OCT images: a systematic review and Meta-analysis
- Overexpression of TRPV1 activates autophagy in human lens epithelial cells under hyperosmotic stress through Ca2+-dependent AMPK/mTOR pathway
- Dry environment on the expression of lacrimal gland S100A9, Anxa1, and Clu in rats via proteomics
- Semaphorin 7A impairs barrier function in cultured human corneal epithelial cells in a manner dependent on nuclear factor-kappa B
- Novel MIP gene mutation causes autosomal-dominant congenital cataract
