Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation
Pakkath Narayanan Arya,Iyyappan Saranya,Nagarajan Selvamurugan
Abstract Mesenchymal stem cells (MSCs) originate from many sources,including the bone marrow and adipose tissue,and differentiate into various cell types,such as osteoblasts and adipocytes.Recent studies on MSCs have revealed that many transcription factors and signaling pathways control osteogenic development.Osteogenesis is the process by which new bones are formed;it also aids in bone remodeling.Wnt/β-catenin and bone morphogenetic protein (BMP) signaling pathways are involved in many cellular processes and considered to be essential for life.Wnt/β-catenin and BMPs are important for bone formation in mammalian development and various regulatory activities in the body.Recent studies have indicated that these two signaling pathways contribute to osteogenic differentiation.Active Wnt signaling pathway promotes osteogenesis by activating the downstream targets of the BMP signaling pathway.Here,we briefly review the molecular processes underlying the crosstalk between these two pathways and explain their participation in osteogenic differentiation,emphasizing the canonical pathways.This review also discusses the crosstalk mechanisms of Wnt/BMP signaling with Notch-and extracellular-regulated kinases in osteogenic differentiation and bone development.
Key Words: Bone;Mesenchymal stem cells;Osteogenic differentiation;Wnt/β-catenin;Bone morphogenetic proteins
INTRODUCTION
Bone is a mineralized connective tissue composed of four cell types: Osteoblasts,osteocytes,bone-lining cells,and osteoclasts.Despite its apparent immobility,bone is an extremely dynamic organ that is constantly resorbed by osteoclasts and neoformed by osteoblasts.The functions of bone-lining cells are key for balancing bone resorption and formation[1].Bone remodeling is essential for the bone to adapt to the constant mechanical modifications necessary for skeletal functions under various environmental conditions[2].Bone develops during early fetal development depending on the interactions between the two cell lineages.The primary cells involved in remodeling are osteoblasts and osteoclasts,which are responsible for bone formation and resorption,respectively[3].Osteoblasts arise from mesenchymal stem cells (MSCs) and aid in bone formation.Osteoclasts are responsible for bone resorption and are derived from a hematopoietic lineage that comprises various osteogenically differentiated cell types in the bone marrow[4].Modifications may occur during the commitment or differentiation of MSCs to the osteogenic lineage,resulting in calcification or bone loss under various conditions[5].Osteogenesis or bone formation is a process by which the preexisting mesenchymal tissue is transformed into bone tissue[6].
“I only ask one thing,” she replied; “let no one know that you have a little bird who tells you everything. It will be best to conceal it.” So saying, the nightingale flew away.
Several signaling mechanisms stimulate osteogenesis in stem cells.For example,Wnt signaling is a well-established pathway of osteogenic differentiation[7].Wnt proteins are crucial for several biological functions,including organogenesis,tissue regeneration,and cancer.The Wnt system comprises two independent intracellular cascades: Canonical (βcatenin-mediated) and non-canonical Wnt pathways.Neither of these factors induce osteogenic differentiation.Wnt/βcatenin signaling is the conventional (or classic) Wnt pathway[8].Canonical Wnt signaling pathway results in the nuclear translocation of β-catenin protein and regulation of its target genes.In the absence of Wnt ligands,β-catenin is destroyed by an intracellular complex composed of glycogen synthase kinase 3 (GSK-3).Canonical Wnt activation induces the expression of disheveled 1 protein and suppresses GSK-3[9].Although non-canonical Wnt signaling is less understood compared to the β-catenin-mediated Wnt pathway,it may also contribute to bone tissue development[10].In Wnt signaling,a set of co-receptors,known as low-density lipoprotein receptor-related proteins 5 or 6 (LRP5/6),enhance the binding of Wnt ligands to frizzled proteins (FZDs).The binding of LRP5/6 inhibitors [known as dickkopf (DKK)] to LRP5/6 coreceptors disrupts Wnt ligand-FZD binding and suppresses Wnt signaling[11].
Another key osteogenic signaling pathway is the bone morphogenetic protein (BMP)/small mothers against decapentaplegic (Smad) signaling pathway,which is also a pro-osteogenic and pro-adipogenic signaling system[12].This pathway becomes active when transforming growth factor-beta (TGF-β) and superfamily member BMPs bind to the heterodimeric type I/II BMP transmembrane serine/threonine kinase receptors[13].A Smad-dependent or-independent intracellular cascade is activated depending on the context.The Smad-dependent pathway becomes active when the BMP ligand binds to its specific receptor,inducing the phosphorylation and binding of receptor Smad (Smad1/5/8) to common Smad (Smad4)[14].The translocation of this complex to the nucleus subsequently modulates the BMP target genes and osteogenic differentiation.
Wnt and BMP pathways are essential signaling pathways in osteogenesis[15].Activation of Smad proteins initiates the BMP signaling pathway,stimulating the Runt-related transcription factor 2 (Runx2) gene.Runx2 is a transcription factor that promotes the differentiation of MSCs toward the osteogenic lineage[16].It functions upstream of the osteoblastspecific transcription factor,osterix,and other specialized osteoblastic genes,including Sparc (osteonectin),SPP1(osteopontin),and collagen type I alpha 1[17].Wnt signaling,which is important for bone formation,regulates Runx2 expression.
This review outlines the importance of the Wnt and BMP signaling pathways and their individual impact and crosstalk in osteogenic differentiation.We also discuss the currently available techniques and clinical studies on the regulation of Wnt and BMP signaling to promote bone osteogenesis and the limitations of these strategies.In addition,we highlight the potential therapeutic effects of targeting the Wnt-BMP interactions in bone and bone-related ailments.
OSTEOGENIC DIFFERENTIATION
In contrast,MSCs initially develop into cartilage and are eventually replaced by boneviaendochondral ossification(indirect ossification) in the long bones,vertebrae,and base and posterior regions of the skull.Endochondral ossification is divided into five phases.First,mesenchymal cells commit to becoming cartilage cells[25].Second,cartilage calcification occurs when chondrocytes move toward the center of the cartilage model and change their ECM content.Following the establishment of the main ossification center,the cartilage model gradually became vascularizedviavascular bud penetration.Through the vascular buds,osteoprogenitor cells enter the cartilage model and develop into osteoblasts[26].The creation of the main ossification center in the diaphysis (central section of the bone) occurs during this process.Following the establishment of a secondary ossification center,the bone gradually replaces the cartilage in the diaphysisviaosteoblast proliferation,while the cartilage continues to grow at the ends of the bone (epiphysis),increasing the bone length[27].This is where the secondary ossification center developed.Finally,mature and compact bones are formed in the cartilage model (Figure 1).
The Fairy loved her with all her heart, for she was at once original and gentle, and she had nearly reached the age at which the gifts were generally bestowed4
Intramembranous and endochondral ossification
Wnt signaling is an immensely conserved pathway that is crucial for the development of several tissues and organs.Wnt signaling regulates cellular activities,including cell fate,differentiation,migration,and proliferation,are regulated by Wnt signaling[28].A subset of extracellular Wnt ligands,including 19 secreted glycoproteins,activate the canonical Wnt pathway.Wnt ligands bind to the dual receptor complex of FZD and either LRP5 or LRP6 to initiate this signaling.To avoid β-catenin phosphorylation and subsequent proteasomal breakdown,the multiprotein “destruction complex” for βcatenin is deactivated.After that,β-catenin builds up in the cytoplasm and translocates into the nucleus,where it acts with transcription factors to control the transcription of the target gene.Numerous studies have emphasized the crucial role that canonical Wnt signaling plays in maintaining bone homeostasis;when this pathway is activated,bone mass and bone production strength increase[29-31].
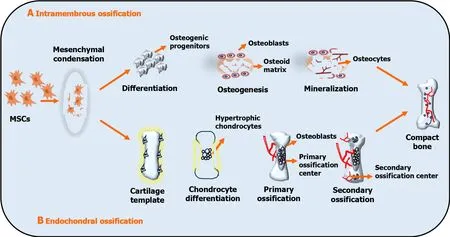
Figure 1 Schematic representation of intramembranous and endochondral ossification. A: Undifferentiated mesenchymal cells develop into osteoprogenitor cells (osteoblasts),which lay down the osteoid matrix and mineralize to form ossification centers.Osteoblasts die due to apoptosis or become trapped in the matrix,developing into osteocytes;B: Condensed mesenchymal cells commit to become the cartilage and undergo chondrogenic differentiation.Chondrocytes at the primordium core establish a growth plate and undergo hypertrophy.Hypertrophic chondrocytes calcify and are penetrated by microvessels,resulting in primary ossification.Vessels infiltrate the epiphyses and produce secondary ossification centers in conjunction with osteoblasts and bone marrow.The growth plate aids in long bone formation.MSCs: Mesenchymal stem cells.
Osteogenesis is a sophisticated multistep mechanism that involves the differentiation of MSCs into osteoblast precursor cells,pre-osteoblasts,osteoblasts,and osteocytes,along with the various ways in which these cells communicate with each other for the formation and remodeling of bones.Most studies have focused on the basic characteristics of MSCs,signaling pathways,and variables promoting the osteogenic differentiation of MSCs[18].The first phase of the osteogenic differentiation of MSCs,termed the proliferative phase,includes the acquisition and proliferation of osteoprogenitor cells,the second phase involves cell maturation in pre-osteoblasts after extracellular matrix (ECM) development,and the final phase involves matrix mineralization.Various signaling pathways influence each of these actions.TGF-β/BMP and Wnt/β-catenin cascades play key roles in bone healing and have been extensively explored for therapeutic applications[19].Although distinct factors control them,both cascades involve Runx2,the principal transcription factor involved in osteogenesis[20].
Roles and regulation of Wnt signaling in osteogenesis
Bone is a dynamic and robust connective tissue that constantly remodels throughout life.There are two basic types of bone formation,called osteogenesis[21],which entails the transformation of pre-existing mesenchymal tissue into bone tissue (Figure 1).Intramembranous ossification refers to the direct conversion of mesenchymal tissue into bone.This process occurs mainly in the skull bones,flat bones of the neurocranium and viscerocranium,and a portion of the clavicle,which is characterized by the condensation of MSCs into osteoblasts upon commitment as osteoprogenitors[22].Osteoblasts build a collagen-proteoglycan matrix that binds to calcium salts.This interaction causes calcification of the pre-bone (osteoid) matrix.Generally,a layer of the osteoid matrix constituted by osteoblasts is separated from the calcification region.However,osteoblasts become entangled in the calcified matrix and transform into bone cells,called osteocytes.As calcification advances,bony spicules extend from the location where ossification begins[23].Furthermore,dense mesenchymal cells comprising the periosteum (protective barrier of bone) surround the entire calcified spicule region.Osteoblastic cells on the inner surface of the periosteum lay down the osteoid matrix parallel to the previously existing spicules.Multiple layers of bone develop during this process.Mature osteoblasts differentiate into bone-lining cells and osteocytes or cause cell death.Osteocytes can act as mechanical sensors,modify the perilacunar environment,and contribute to bone function by interacting with organic and inorganic compounds in response to mechanical stimuli[24].
Non-canonical Wnt signaling routes function without the assistance of β-catenin,whereas the canonical pathway facilitates signaling through the stability of β-catenin[32].Wnt signaling promotes MSC development toward the osteoblast lineage and inhibits differentiation toward the adipocyte and chondrocyte lineages[33].Abnormal changes in the Wnt signaling pathway markers are linked to changes in bone metabolism and MSCs’ osteogenic ability of MSCs[34].Activation of the Wnt signaling pathway can boost Wnt-related gene expression,restore the osteogenic capacity of bone marrow stem cells (BMSCs),and reduce bone loss.DKK-1 diminishes the osteogenic capability of BMSCs,resulting in bone loss.Earlier studies stated that the reduced expression and osteogenic ability of β-catenin,phospho-GSK-3β,and lymphoid enhancing factor 1 (LEF1) in BMSCs obtained during osteoporosis (OP-BMSCs) were upregulated by transducing OP-BMSCs with methyltransferase-like 3 (METTL3).Overexpression of METTL3 partially restored the osteogenic ability of OP-BMSCs,activated the Wnt signaling pathway,and elevated the expression of osteopontin,Runx2,phospho-GSK-3β,β-catenin,and LEF1[35].
Lysine-specific demethylase 4A (KDM4A) inhibited the canonical Wnt signaling pathway.Overexpression of KDM4A increased the expression of secreted FZD-related protein 4 (SFRP4) and CCAAT/enhancer-binding protein α,resulting in the promotion of adipogenesis by inhibiting canonical Wnt signaling and decreasing osteogenesis from marrow stromal progenitor cells.These results demonstrated the existence of a network between KDM4A,SFRP4,and Wnt/β-catenin signaling that balances osteogenic and adipogenic development.These findings show that KDM4A may be an appealing prospective target for novel therapeutics targeting metabolic diseases,such as osteoporosis[36].Recent studies have reported the involvement of various molecules,such as transcription factors and protein-coding genes,in the regulation of osteogenesisviathe Wnt signaling pathway (Table 1).
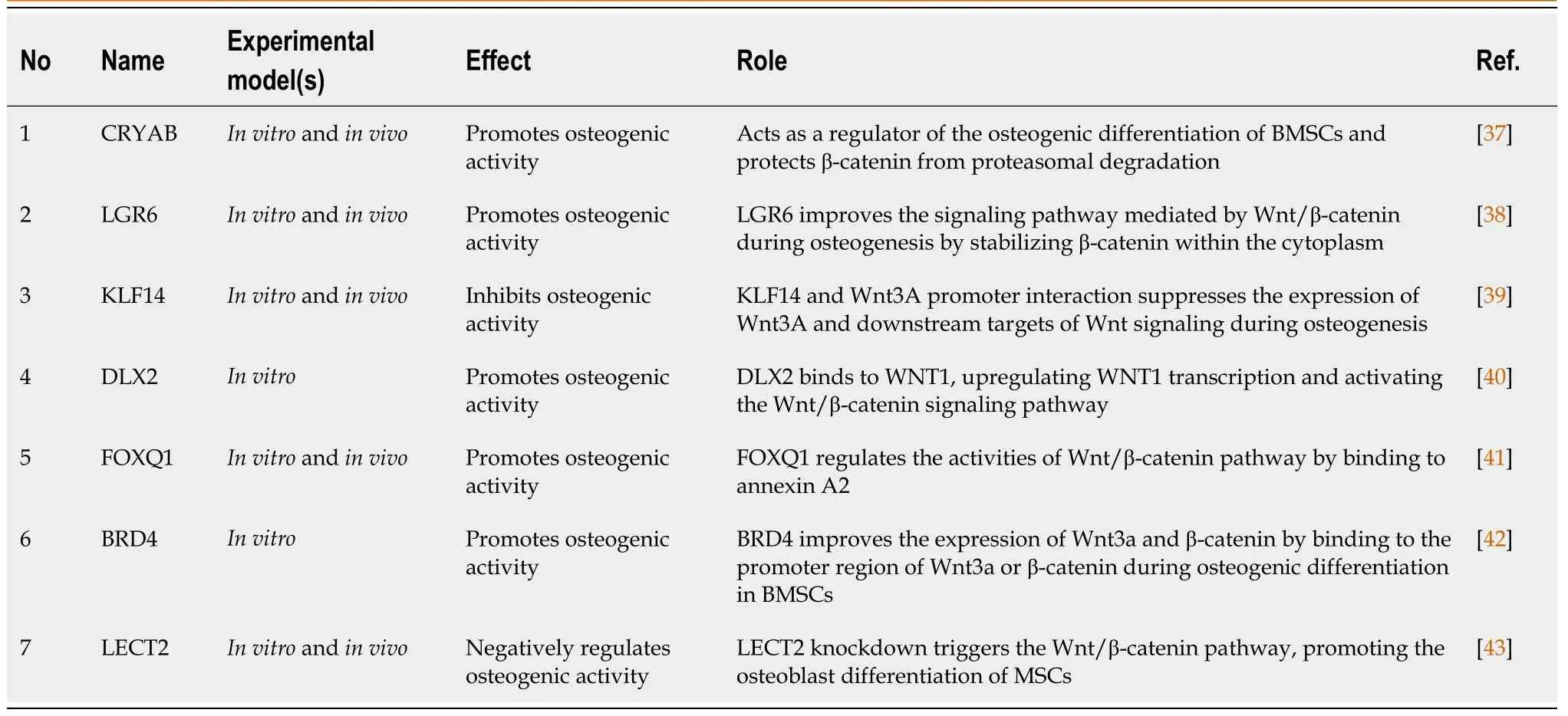
Table 1 Molecules regulating osteogenesis via the Wnt/β-catenin signaling pathway
Roles and regulation of BMP signaling in osteogenesis
Wnt and BMP signaling pathways are known for their roles in bone development and homeostasis regulation.In this review,we discuss the interplay between both canonical Wnt and BMP pathways in stimulating the expression of downstream osteogenic target genes during osteogenic differentiationviaaccumulation and translocation of β-catenin to the nucleus and formation of the β-catenin-TCF/LEF complex (Figure 3).This functional interaction between the BMP and Wnt pathways is vital for combining the anabolic actions of both pathways in bone formation.The crosstalk between the Wnt/β-catenin/BMP and other signaling pathways,such as ERK and Notch pathways,implies that these pathways play critical regulatory roles in osteogenic differentiation and bone formation.However,further investigation is necessary to determine the interplay between Wnt/BMP signaling and other signaling pathways,such as the Hedgehog,fibroblast growth factor,Hippo,and TGF-β pathways,in the regulation of osteogenic development.Recent studies have highlighted the potential of small molecules for the treatment of common bone-related illnesses by targeting the Wnt and BMP signaling cascades.Future studies should focus on determining the specific mechanisms of small molecules and Wnt/BMP signaling in osteogenic differentiation.
The primary downstream components of BMPs that induce the osteogenic differentiation of BMSCs are Smad 1/5/8 and the transcription factors,Runx2 and Sp7[46].Studies have shown that Runx2 is not only a downstream target of the BMP pathway but may also regulate BMP expression.The underlying mechanism between Runx2 and the BMP pathway in primary BMSCs from patients with craniofacial deformities patients was investigated in a recent study,which demonstrated that Runx2 controls the BMP4 pathway by repressing Chordin-like 1 (CHRDL1) transcription.Researchers have discovered an intriguing RUNX2/CHRDL1/BMP4 axis that promotes osteogenic differentiation,and hypothesized that BMP4 could serve as a possible treatment for bone disorders[47].Previous studies demonstrated that BMP2 is a potent activator of osteoblastogenesis.Runx2 and SP7 are prominent BMP2-target genes,whereas DLX5 is crucial for SP7 expression during BMP signaling.Interaction of osteomodulin with BMP2 positively regulated osteogenesis[48].
What if the old man should die and leave her here alone in the solitary6 cottage deep in the heart of the wood! She would be as terribly lonely as he had formerly7 been
Liet al[49] identified a novel mechanism for BMP2 in boosting osteogenic differentiation of MSCs;particularly,BMP2 modulated mitochondrial activityviaregulating peroxisome proliferator-activated receptor gamma coactivator 1-alpha(PGC-1) to enhance osteogenic differentiation of MSCs.Collectively,these results suggested that BMP2 and mitochondrial activity influenced osteogenic differentiation and bone development.This study reported that overexpression of BMP2 increased osteogenic differentiation and addressed the correlation between BMP2,mitochondrial activity,and PGC-1[49].In recent studies,various molecules,such as protein-coding genes,cytokines,growth factors,and phytocompounds,have been shown to regulate osteogenesisviathe BMP signaling pathway (Table 2).
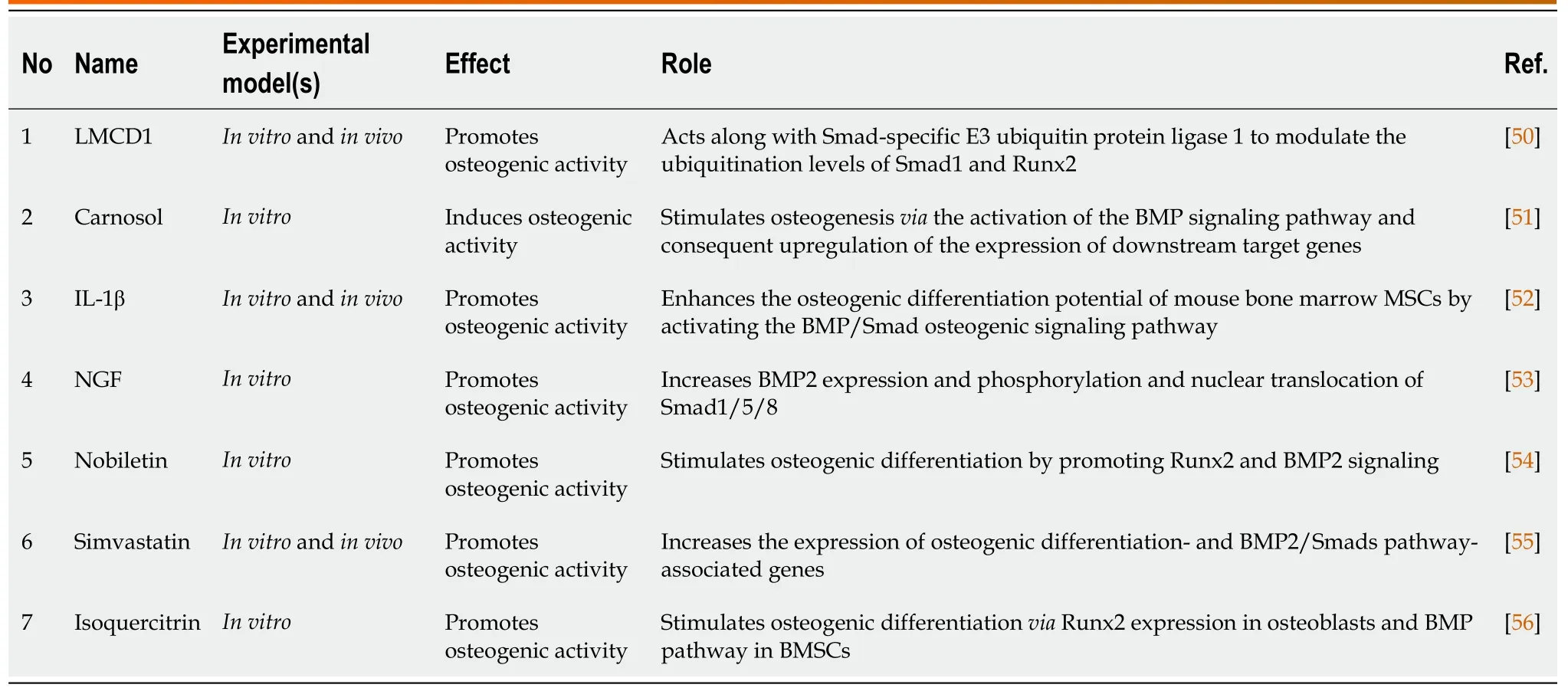
Table 2 Molecules regulating osteogenesis via the bone morphogenetic protein/Smad-dependent pathway
CROSSTALK BETWEEN THE WNT AND BMP SIGNALING PATHWAYS IN OSTEOGENESIS
The Wnt and BMP signaling pathways play significant roles in osteogenesis.They can act independently of one anotherviaseparate ligands,receptors,and cytoplasmic and nuclear signal transducers without sharing any significant pathway aspects.However,in many biological circumstances,Wnt and BMP ligands happen spatially or across time in overlapping or complementary ways,as if they are “crosstalking” with one another[57].Indeed,recent research has identified several instances in which these two pathways interact with or attenuate each other,resulting in outcomes that either alone cannot attain[58].Recently,numerous studies have explored small-molecule compounds as osteogenic inducers[59] in cell-based therapies for bone regeneration,as shown in Figure 2[60].The intense osteogenic effect of GDA,a Ganoderma lucidum-derived tetracyclic triterpenoid molecule,on human amniotic MSCs has been previously reported.Key elements,including β-catenin,Wnt3,FZD4,BMP3,BMP4,Smad4,and Smad5,have significantly upregulated in association with Wnt/β-catenin and BMP/SMAD signaling,which in turn stimulated human adipose-derived MSC differentiation into osteoblasts[61].Recent studies have reported that albiflorin,an active component inPaeonia lactiflora,increased BMP-2/Smad and Wnt/β-catenin signaling and promoted Runx2 expression,a critical transcription factor for osteoblast differentiation to produce osteogenic genes.Furthermore,the potential of albiflorin to stimulate osteoblast differentiation improved fracture healing in a rat femoral fracture model[16].The conditioned exosomes originated from human-exfoliated deciduous tooth stem cells (SHED) significantly promoted the osteogenic differentiation of periodontal ligament stem cellsviathe Wnt/β-catenin and BMP/Smad signaling pathways.Furthermore,crosstalk between the Wnt and BMP signaling pathways regulates the activity of dental stem cells.Increased phosphorylation of Smad1/5/8 and nuclear β-catenin expression improved both BMP/Smad signaling and Wnt/β-catenin signaling.SHED-exosomes exosomes have elevated levels of Wnt3a and BMP2.Silencing Wnt3a and BMP2 in SHED-exosomes slightly restored the increased osteogenic differentiation.These results provide new insights into the application of SHED exosomes in treating periodontitis-induced bone abnormalities[62].Polydatin (PD),a natural resveratrol glucoside obtained from the roots ofPolygonum cuspidatum,has been found to enhance the levels of the β-catenin and enable its nucleus translocation,resulting in increased expression of downstream target genes.PD also aided human bone marrow-derived MSCs (hBMSCs) osteogenesis by activating the BMP2-induced Wnt signaling pathway and increasing the accumulation and nuclear translocation of β-catenin[63].Sclerostin small-molecule inhibitors(SMIs) significantly promoted Wnt/β-catenin and BMP signalingin vitroandin vivo,enhancing osteogenesis while significantly reducing bone resorption activity.Further findings imply that sclerostin inhibition by SMIs might occur in a multifrontal drive onto osteogenesis by blocking the receptor activator of nuclear factor-kappaB ligand-mediated osteoclastogenic response to endogenous BMP-2 despite increasing both Wnt and BMP signaling[64].The extract ofJuglans regia L(JRL) enhanced the expression level of osteogenic genes in hBMSCs.Meanwhile,JRL extract stimulated the differentiation of osteogenic cells and cell autophagy by activating the BMP2/Smad/Runx2 and Wnt/β-catenin signaling pathways[65].

Figure 2 Schematic illustration of small molecule compounds inducing the osteogenic differentiation of mesenchymal stem cells. Small molecule compounds stimulate the bone morphogenetic protein signaling pathway and promote β-catenin accumulation.Then,β-catenin migrates to the nucleus and forms a β-catenin-T-cell factor/lymphoid enhancer-binding factor complex to initiate the transcription of genes associated with osteogenesis.GSK-3β: Glycogen synthase kinase 3 beta;APC: Adenomatosis polyposis coli;CK1-α: Casein kinase 1α;DSH: Disheveled;TCF/LEF: T-cell factor/lymphoid enhancer-binding factor;DKK1: Dickkopf 1;LRP5/6: Lipoprotein receptor-related proteins 5 or 6;BMP: Bone morphogenetic protein.
Wanget al[66] reported that a cycloxygenase-2 specific inhibitor significantly decreased BMP9-induced osteogenic markers in MSCs,and this adverse effect was substantially ameliorated by the inclusion of all-trans-retinoic acid (ATRA),an
 World Journal of Stem Cells2024年2期
World Journal of Stem Cells2024年2期
- World Journal of Stem Cells的其它文章
- Multiple pretreatments can effectively improve the functionality of mesenchymal stem cells
- Cellular preconditioning and mesenchymal stem cell ferroptosis
- Therapeutic utility of human umbilical cord-derived mesenchymal stem cells-based approaches in pulmonary diseases: Recent advancements and prospects
- Unlocking the versatile potential: Adipose-derived mesenchymal stem cells in ocular surface reconstruction and oculoplastics
- Human pluripotent stem cell-derived kidney organoids: Current progress and challenges
- Recent progress in hair follicle stem cell markers and their regulatory roles
