Bone tissue engineering scaffold materials: Fundamentals,advances,and challenges
Chng Xu ,Zhize Liu ,Xi Chen ,Yng Go ,Wenjun Wng ,Xijing Zhung,* ,Ho Zhng,Xufeng Dong
a Department of Cardiovascular Surgery,Central Hospital of Dalian University of Technology,Dalian 116089,China
b School of Materials Science and Engineering,Dalian University of Technology,Dalian 116024,China
c Department of Orthopaedics,Central Hospital of Dalian University of Technology,Dalian 116089,China
d Department of Dermatology,Allergology,and Venereology,University of Lübeck,Lübeck 23562,Germany
Keywords: Biomaterials Bone defects Tissue engineering Scaffolds Osteogenesis
ABSTRACT Bone damage caused by trauma and tumors is a serious problem for human health,therefore,threedimensional (3D) scaffolding materials that stimulate and promote the regeneration of broken bone tissues have become the focus of current research in the field of bone damage repair.To this regard,a preferential combination of materials and preparation techniques is considered crucial for the preparation of advanced bone tissue engineering scaffolds to better facilitate the regeneration of broken bone.In this review,current research advances and challenges in bone tissue engineering scaffolds are discussed and analyzed in detail.First,we elucidated the structure and self-healing mechanism of bone tissue.Subsequently,the main applications of different materials,including inorganic and organic materials,in bone tissue engineering scaffolds are summarized.Moreover,we overview the latest research progress of the mainstream preparation strategies of bone tissue engineering scaffolds,and provide an in-depth analysis of the different advantages of each method.Finally,promising future directions and challenges of bone tissue engineering scaffolds are systematically discussed.
1.Introduction
Bone defects caused by trauma,infection,tumor,or inherent genetic disease are among the most common clinical injuries [1-4].Bone tissue has ability to repair and regenerate by itself,small defects usually heal without additional treatment [5,6].However,when bone defects exceed a critical size threshold (approximately>2 cm),the healing process is extremely poor and often cannot repair itself [7,8].Globally,4 million people require graft or bone replacement surgery each year [9].Therefore,effective treatment of bone defects is of great clinical importance.Bone grafting is mainstay in clinic [10,11],and the defect site can regenerate effectively under certain conditions by selecting different grafts [12,13].However,the current grafting strategies all have their own different drawbacks,e.g.,autologous bone grafts are often limited by limited amount of bone supply [14,15].Hence,it is an urgent need for more reliable,stable and efficient treatment modalities to avoid these problems,which is also a top challenge in the field of bone defect repair.
In recent years,bone tissue engineering (BTE) has attracted much attention in the field of bone defect repair and regeneration[16,17].The theory of BTE is to isolate and culture seed cellsin vitro,plant on scaffolds to construct tissue engineering repair materials,and then implant them into the bone defect site for repair[18,19].In the field of tissue repair engineering,scaffold,seed cells and bioactive growth factors are the main factors,and the synergy of these factors offers innovative possibilities for regenerative medicine (e.g.,Fig.1) [20-23].Among them,scaffold is the core part [24].It is not only a temporary site for cell adhesion,proliferation and differentiation,but supports the generation of new tissues[25,26].In addition,scaffold should interact with cells and multiple bioactive molecules that regulate tissue remodeling [23,27].Therefore,scaffolds usually need to mimic the structure and composition of extracellular matrix (ECM) to establish and restore the communication between cells and ECM,which in turn promotes tissue remodeling [28,29].Recently,with the advancement of synthetic technology and deepening understanding of the microenvironment at the defect site,researchers have focused on the design and development of scaffold materials that mimic the composition of ECM [30],including its complex structure and composition.Although these scaffold materials can perform certain repair functions,their applications are further limited by their single performance,poor osteoconductivity and limited angiogenesis,which in turn lead to unsatisfactory repair results [31].Therefore,reasonable design of scaffold materials,combining with various biopotency in the process of bone defect repair and tissue engineering,and endow with special functions and excellent osteogenic properties,is a hot topic in this field.

Fig.1.Three elements and construction methods of bone tissue engineering.
In this review,we introduced the structures of bone tissue and the main processes of bone repair,and importantly,the construction materials and strategies of bone tissue engineering scaffolds(BTES) are summarized in detail.Based on these analyses,the challenges and prospects in the preparation of BTES are discussed in detail.In addition,future directions and new design strategies are also proposed.This review will help to illustrate the importance of BTES in the applications of bone defect repair,and promote the development of BTES in a more effective and convenient direction.
2.Overview of bone tissues
2.1. Bone tissue structure
The hierarchical structure of bone tissue starts from the nanolevel,spanning several magnitude levels,and reaching the macroscopic bone (Fig.2a) [32,33].Compact bone plays an important role in maintaining the mechanical properties of bone tissue,and has a large number of central tube structures composed of blood vessels,that is,Haversian system (Fig.2b) [34].The hard and dense structure can protect the fragile spongy tissue inside.Spongy bone constitutes the interior of the bone and shows a loose and porous structure,consisting mainly of trabeculae located at the ends of the long and flat bones,providing space for the intercommunication between bone tissues [35].Besides,the high porosity of spongy bone allows the transport of nutrients and metabolites [36].The organic component of the bone tissue consists mainly of type I collagen and a small proportion of type III and IV collagen,and collagen fibers make up about 25% to 30% of the bone matrix.The remaining bone (about 65% to 70% of the total mass) is occupied by substances such as calcium and phosphate [37].
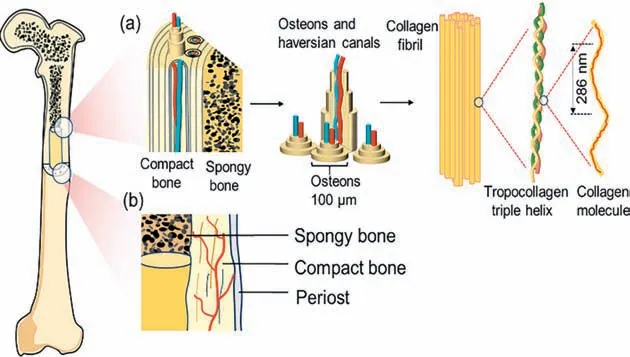
Fig.2.(a) Schematic diagram of bone tissue structure from macro to nano level.(b) Macrostructure of bone.
Microscopically,bone tissue is mainly divided into osteocytes,which produce inorganic components;osteoblasts,which produce organic components;osteoclasts,which act on bone resorption;and the corresponding extracellular matrix [38].Among them,osteocytes and osteoblasts are the basis for the formation of bones and responsible for bone tissue conversion and regeneration [39].The main components of the extracellular matrix are collagen fibers and hydroxyapatite,combining a complex network of cells to participate in cell growth and metabolism,which is also a basic component for the formation and maintenance of bone tissue [40].
2.2. Repair of bone tissue
Bone tissue repair and healing is a complex process involving multiple stages,mainly including inflammation and hematoma formation,recruitment and proliferation of stem cells,formation of blood vessels,differentiation of mesenchymal stem cells (MSCs),and the final stage of tissue remodeling (Fig.3a).The occurrence of a fracture results in localized disruption of the vascular network and surrounding tissue,leading to the formation of a hematoma and subsequent inflammatory phase [41].Within 24 h after the fracture,the soft matrix at the hematoma site recruits immune cells to mediate an inflammatory response.The hematoma and inflammatory response are cleared after one week and the hematoma site is replaced by granulation tissue.After the inflammatory and hematoma phase,a variety of cells such as osteoblasts and endothelial cells at the defect site are activated.Along with stem cell proliferation,angiogenesis occurs in the process of bone tissue repair.The abundance of blood vessels in bone is essential for bone regeneration (Fig.3b) [42,43].The final bone remodeling is also a physiological process,in which the interaction between osteoblasts and osteoclasts plays an important influence.It is an important goal to combine the characteristics and keys of each process in the bone tissue repair with scaffolds,endowing the scaffold materials with multifunctionality through specific modifications to achieve better and more efficient results in the treatment of bone defects.
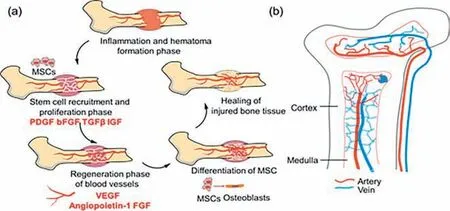
Fig.3.(a) The process of bone tissue repair.(b) The distribution of blood vessels in the bone.
3.Development and materials for BTES
Bone tissue is a complex system with unique biochemical and biomechanical properties [44,45].Although bone tissue has the ability to repair itself,its complex structure and slow rate of re-generation often lead to failed or delayed repair of various types of bone defects [46].In recent years,with the development of tissue engineering,many artificial biological substitutes have been used for the repair and regeneration of bone tissue [47].In order to make them work better in defective tissues,the basic requirements of BTES materials,material types and preparation processes should be understood first,and then the scaffold materials with better repair functions should be designed and prepared by combining the characteristics of each stage of bone healing [48].
Among them,scaffold materials play an irreplaceable role in the reconstruction of new organs and tissues by serving as a substrate for cell adhesion,proliferation and differentiation.The following properties must be met by the ideal BTES materials.First,biocompatibility is a fundamental requirement for any type of BTES,which must avoid health hazards to the patient after implantation,such as the occurrence of tumors [80].Biocompatibility refers to the ability of a biomaterial to perform its intended function without causing any adverse local or systemic reactions [81].The ideal scaffold must be biocompatible and nontoxic,non-immune rejection,and allow for cell attachment,migration,proliferation and differentiation on the scaffold [82,83].Biodegradability is also important for BTES.Ideally,scaffold materials can degrade slowly after a certain period of time and at a rate that matches the rate of mineralization deposition of the tissue [84].The appropriate mechanical strength to prevent collapse of the new tissue is essential.The mechanical strength of the material should match the native bone tissue and need not far exceed the strength of the bone tissue,minimizing the risk of “stress shielding”,reduction of bone mass due to reduced bone density,and re-fracture [85-87].Furthermore,the scaffold material should have a certain porosity to ensure sufficient permeability to transport nutrients and biomolecules,which also directly affects cell attachment,growth,and metabolism.So,an interconnected porous structure is another essential requirement for bone tissue engineering scaffolds [88].Different porosity significantly affects bone healing behavior,with higher porosity providing greater surface area and helping to promote cell attachment,yet also weakening the mechanical properties of the scaffold material [89].Lower porosity,on the other hand,significantly worsens the interaction of the scaffold material with the ECM [90].
The popular BTES materials can be classified into two categories organic and inorganic materials.Organic materials include natural polymers widely found in nature and partially biodegradable synthetic polymers,while bioactive glass and bioceramic materials are representatives of the many inorganic materials.Some typical BTESs that have been reported are summarized in Table 1.This section will discuss the development history of BTES in detail from a materials perspective,including their structures and respective unique advantages.

Table 1Currently popular raw material for the preparation of BTES.
3.1. Metals and biological ceramics
The superior mechanical properties of metallic materials,including high strength and modulus,are driving them to become an important scaffold material for bone tissue engineering [91,92].Representatively,Xuetal.prepared Ti-Zr-Nb scaffolds with lower corrosion rates and higher mechanical toughness by adding Nb and Zr to Ti alloys.Moreover,the mixture of Nb and Zr drove an increase in ALP activity and a decrease in cytotoxicity [93].Magnesium (Mg)-based materials have better biodegradability compared to Ti alloys and can be biodegraded through corrosion,making them more suitable in BTE [94,95],which means that there is no need for second surgery to remove the implant after the healing of bone tissue [96].Harris hip score (HHS) indicates that the treatment group using Mg-based screws for fixation achieved a bone tissue recovery rate of over 95% at 12 months,while the blank control group only achieved 84% [97].Similarly,Chen’s study also confirmed that magnesium metal screws can improve the treatment effect of osteonecrosis of the femoral head [98].In addition,zinc is an essential element in BTES [99].Zinc plays a crucial role in the mineralization of osteoblasts and bone metabolism[100].Relevant studies have shown that the hemolysis rate of Mg-Zn alloys is only 3.4%,which is below the safety limit of 5%,implying that they do not lead to the rupture of erythrocytes [101].He’s team [102] implanted Mg-Zn alloy into New Zealand rabbits to evaluate its biocompatibility andinvivodegradation.The results demonstrated that the implant was completely disappeared after 24 weeks,and most of the material was absorbed by the organism within 14 weeks.In a study by Yuetal.[103],zinc-doped calcium silicate (ZnCS) was used as a coating on the surface of titanium alloy to enhance the osseointegration ability of the implant(Fig.4a).The results showed that the titanium scaffold with ZnCS coating promoted osteoblast differentiation and new bone formation.Metal oxides have likewise been used in the preparation of scaffold materials.Panetal.designed a porous scaffold doped with magnesium oxide (MgO) (Fig.4b),and the released Mg2+can induce osteogenic differentiation and accelerate bone tissue regeneration [104].
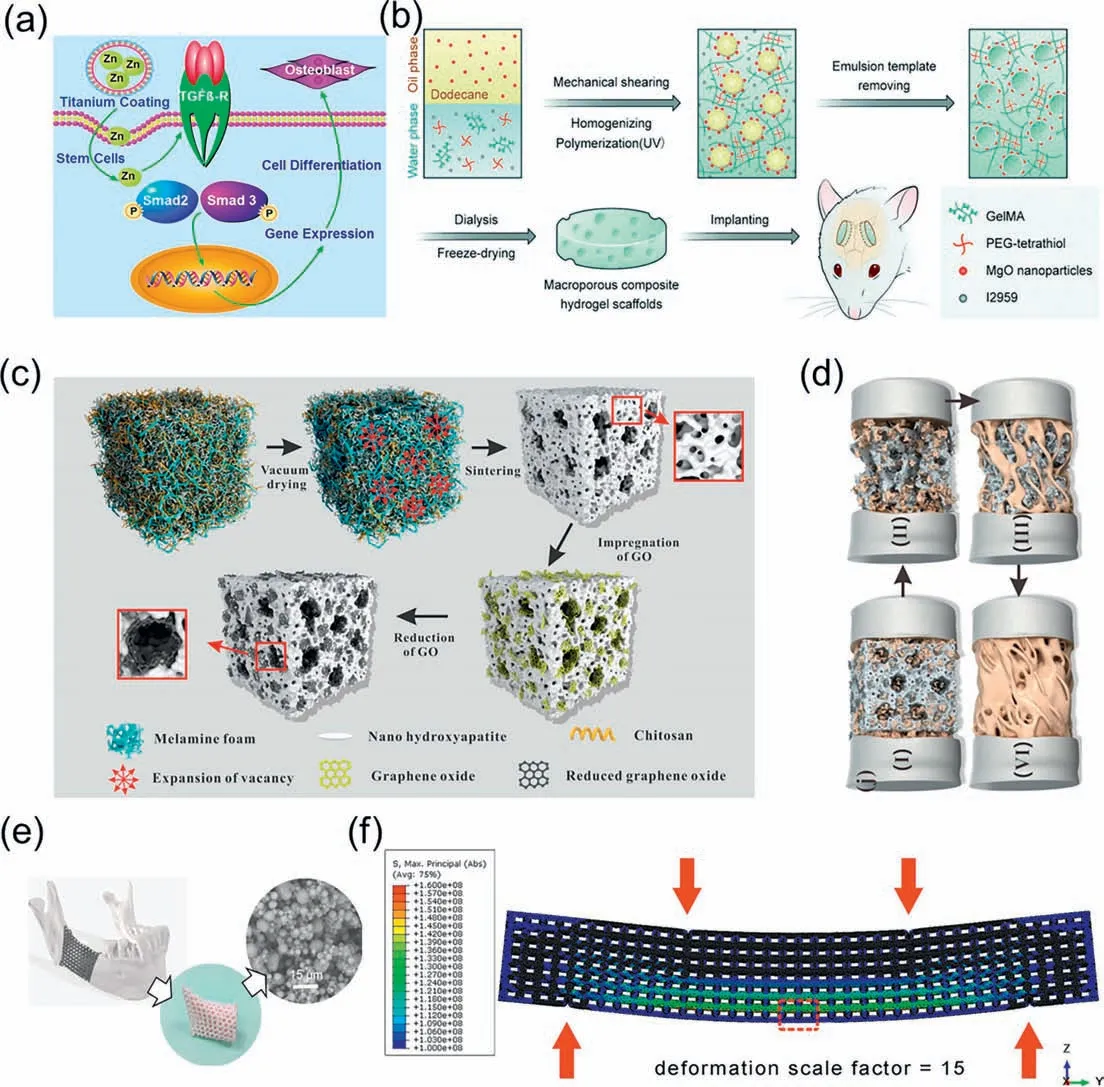
Fig.4.(a) Mechanism of promoting bone differentiation process by zinc-modified calcium silicate coating.Copied with permission [103].Copyright 2017,Nature.(b)Schematic diagram of the preparation process of porous scaffolds doped with MgO.Copied with permission [104].Copyright 2020,Royal Society of Chemistry.(c) Diagram of the formation mechanism of the porous HA/rGO composite scaffold.(d) Diagram of the HA/rGO composite scaffold substitution and new bone formation mechanism.Copied with permission [110].Copyright 2019,American Chemical Society.
Bioceramics,including ceramic composites,crystalline ceramics and amorphous glasses,are a class of inorganic biomaterials with an important role in BTE [105].An increasing number of studies have been devoted to investigate how to exploit the biological effects,such as osteogenesis or angiogenesis,produced by the bioactive metal ions released by these materials [106].These metal ions can replace some biomolecules (e.g.,growth factors) in a less costly and more stable form [107].Calcium phosphate is the most common bioactive ceramic material with excellent osteoconductivity and osteoinductivity [108].A series of calcium phosphate-based bone regeneration biomaterials have been developed,such as hydroxyapatite (HAp),β-tricalcium phosphate (β-TCP) and composites of the two.HAp,a core inorganic component of bone structure,promotes the expression of osteogenic growth factors such as BMP and enhances alkaline phosphatase (ALP) activity [109].Zhouetal.[110] developed a porous HAp/chitosan/graphene oxide scaffold by using a soft template method (Fig.4c).The hybrid combination of different materials provided a hierarchical pore structure(Fig.4d).In contrast,β-TCP is an absorbable bioceramic material with a suitable macro-and microporous structure and good mechanical properties [111].For instance,Kasperetal.successfully developed a class of porous scaffolds by introducingβ-TCP into poly(trimethylene carbonate) (PTMC) [112].Although the content of TCP exceeds 50%,it is still biologically friendly and resorbable.Bioactive glass,composed of calcium-containing silicates,is another important class of bioceramics.Ions released during the interfacial reactions,such as Ca2+,PO43-and Si,can enhance the osteogenic capacity of the scaffold by activating the surrounding cells[113,114].The development of scaffold materials with such ionreleasing behavior has attracted great interest from researchers,but the fragile mechanical properties of bioactive glasses pose certain difficulties for practical applications.Targeting this problem,Weietal.used PLA coated on the surface of a porous bioactive glass to improve the flexibility of the scaffold [115].This skincore structure increases the fracture work of the scaffold material by more than 10,000% and results in a non-brittle mechanical response.
3.2. Degradable polymers
3.2.1.Naturalpolymer
The natural polymers can be divided into two types,animalbased natural polymers and plant-based natural polymers [116].Animal-based natural polymers mainly include gelatin,collagen,chitosan and silk proteins,while plant-based natural polymers include sodium alginate,cellulose and starch [117-120].Moreover,the molecular chains of these polymers contain a large number of hydroxyl and amino groups and other reactive groups,which are very favorable for the adhesion and fusion between the material and biological tissues (Fig.5).These polymers often have the advantage of excellent biocompatibility,wide range of sources,good plasticity and low immunogenicity [121].In addition,natural polymers have better performance in simulating ECM and interacting with tissues compared to semi-synthetic or synthetic polymers,mainly due to their high similarity to the tissue surrounding environment [122].
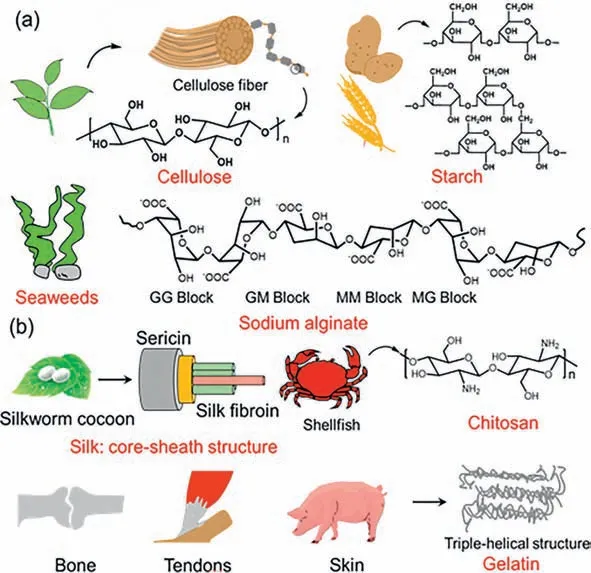
Fig.5.(a) Representative plant-based natural polymers and their molecular formulas.(b) Representative animal-based natural polymers and their structures.
Among them,collagen,mainly found in bone (type I),cartilage (type II) and blood vessel walls (type III),is the main protein component of natural bone and has an amino acid sequence that facilitates cell adhesion [123,124].Besides,due to its animal origin,this protein is non-cytotoxic,biocompatible and biodegradable,and these advantages make it a promising candidate for BTES[125].Moreover,it also has abundant mineral deposition sites in its structure,which are well induced for calcium deposition in bone tissues [126].Attractively,collagen can be processed into a variety of forms such as membranes,fibers or foams and applied to various tissues such as bone,cartilage,heart,ligaments and nerves depending on the needs,which are necessary and important for clinical applications [127-131].However,its disadvantages such as low elasticity and mechanical strength and poor stabilityinvivomake it difficult to prepare pure collagen as a scaffold material alone,and it is usually compounded with other materials to further modulate mechanical and degradation properties.Qian’s group designed and prepared a composite hydrogel composed of collagen and nano-HA (Fig.6a) [132].This composite gel tissue engineering scaffold material showed good biocompatibility and induced regeneration and healing of bone tissue well after implantation into the body (Figs.6b and c).SF is a natural polymeric fibrous protein extracted from silk [133].SF can self-assemble to form a fibrous structure with excellent biocompatibility and certain mechanical properties,which is very conducive to cell attachment and growth,and is a suitable biomaterial for bone repair and regeneration [134-136].Currently,SF has been prepared as thin films,hydrogels,porous materials,sponges or composite scaffolds and used in different tissue engineering fields [137-140].For instance,Joet al.evaluated the possibility of SF/HAp/sodium alginate composites as bone fillers [141].The results confirmed that the rate of defective bone healing was significantly higher in the SF-based scaffoldfilled than in the blank group.Moreover,the degraded scaffold was not surrounded by immunogenic reactions or giant cells,suggesting that the scaffold can successfully complete bone tissue repair without causing inflammation or rejection reactions (Fig.6d).CS,a linear polysaccharide derived from chitin by deacetylation,has a semi-crystalline structure and is the main component of the exoskeleton of crustaceans [142].CS is a cationic polymer that can interact electrostatically with negatively charged biomolecules and cell membranes,having a strong adsorption capacity to biological tissues [143].In addition,CS can hydrolyze the bacterial cell wall by binding to phosphopeptidic acid in the bacterial peptidoglycan layer through non-covalent bonding,thus achieving satisfactory bactericidal and antibacterial properties [144-146].Moreover,its good solubility has led to the development of various forms of bone and cartilage tissue engineering materials such as films,particles and hydrogels [147].Bikendraetal.[148] fabricated high-strength hydrogel scaffolds by hybridizing regenerated cellulose (rCL) nanofibers with CS hydrogels.The hybrid materials significantly improved the mechanical properties of the gel(Fig.6e).The porous and high-strength rCL/CS scaffold enhanced the process of accelerated proliferation and biomineralization of pro-osteoblasts (Fig.6f).Hyaluronic acid (Ha) is widely distributed in the tissue interstitium of vertebrates,and has good application prospects as an excellent tissue engineering material.For example,Wuetal.[149] prepared a new scaffold by introducing polyphosphate (PolyP) as an osteoinductive effect signaling molecule into cross-linked Ha hydrogels.The scaffold can enhance the osteogenic effect of preosteoblasts.Taken together,natural polymers have excellent biological properties and demonstrate innate advantages in the field of bone tissue engineering.However,these polymers have inherent drawbacks,such as poor mechanical properties and unregulated degradation rates.
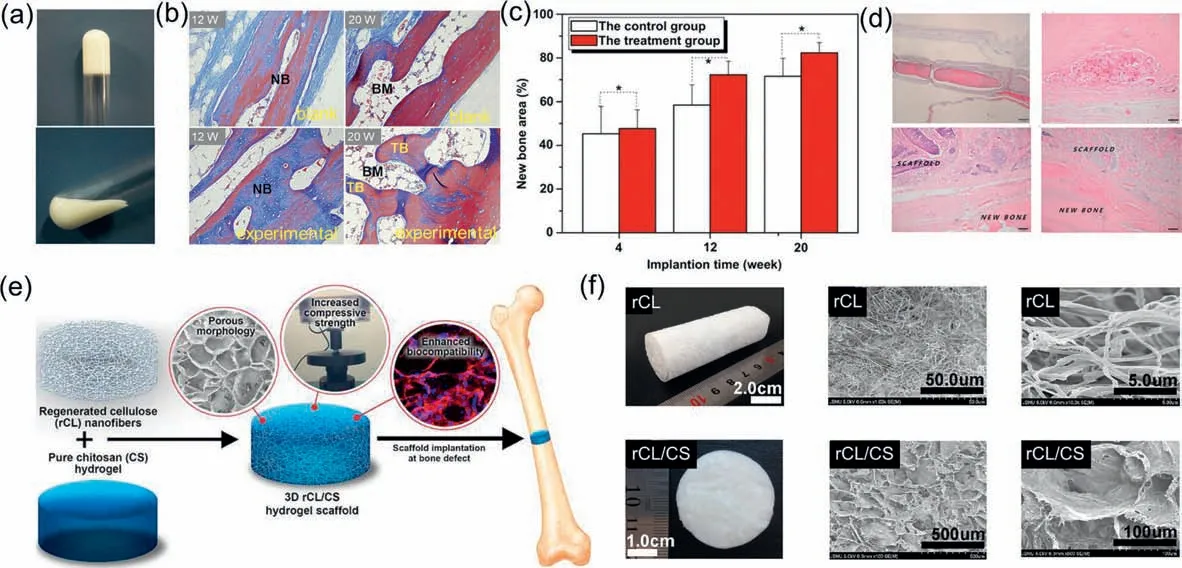
Fig.6.(a) Digital photo of the collagen-nano-HA hydrogel.(b) Masson staining images of bone tissue sections in the blank and experimental groups at different times after implantation.(c) Changes in the area of repaired bone defects in the blank and experimental groups with time (*P <0.05).Copied with permission [132].Copyright 2012,Elsevier.(d) Histological images extremely low inflammatory response and regenerated bone tissue.Copied with permission [141].Copyright 2017,MDPI.(e) Preparation process of rCL/CS hydrogel scaffold.(f) Porous structure of the rCL/CS hydrogel scaffold.Copied with permission [148].Copyright 2021,Elsevier.
3.2.2.Syntheticdegradablepolymers
Compared to natural polymers,synthetic polymers offer more possibilities for chemical modification [150,151].This section focuses on materials that have been approved by the U.S.Food and Drug Administration (FDA),including polylactic acid (PLA)[152,153],polycaprolactone (PCL) [153],and poly(lactic-co-glycolic acid) (PLGA) [154].PLA is polymerized from lactic acid,which can be obtained from renewable resources including sugar and starch,and has good thermal stability and nontoxic degradation products[155].Due to the chirality of lactic acid,PLA usually exists in different forms,such as poly(L-lactic acid) (PLLA) and poly(D-lactic acid)(PDLA).Among them,PLLA has been widely used in drug delivery carriers,skin regeneration scaffolds,vascular grafts,bone screws,etc.[156].PCL is synthesized by ring-opening polymerization ofεcaprolactone [157].PCL has outstanding mechanical strength,good biodegradability,high chemical,thermal stability,and satisfactory histocompatibility [158-160].However,its own hydrophobicity and slow degradation rate also limit its brighter development to some extent.Currently,copolymerization or blending with other materials is the dominant modification that can significantly modulate the main physicochemical properties of PCL.For example,Wuetal.[157] blended PCL with bioactive glass and ceramic-like materials,which could improve the hydrophilicity and bioactivity of the scaffold.The results showed that the composite scaffold had interconnected pores with porosity up to 73% and controlled degradability(Fig.7a).In contrast to the two self-polymers PLA and PCL,PLGA is prepared by random copolymerization of lactic acid (LA) and glycolic acid (GA) [161].As a biodegradable material,the mechanical and degradation properties of PLGA can be achieved by regulating the ratio of LA and GA,which is the most attractive advantage of PLGA [162].By adjusting the ratio of LA to GA,its degradation time can be controlled within a few months to match the bone regeneration cycle.With the gradual degradation of PLGA,the stress stimulation of the scaffold on the new bone is gradually enhanced,which can further promote bone regeneration and bone tissue remodeling [163,164].In addition,the abundance of active hydroxyl groups on the side chains allows it to be easily endowed with certain biological functionality by chemical modification [165].In a study by Takeokaetal.,various PLGA/hydroxyapatite scaffolds were prepared throughinsitupolymerization.By adjusting the ratio of two components,their mechanical strength could be achieved to 54.3-78.8 MPa,3-4 times higher than that of pure hydroxyapatite scaffolds.Invitroexperiments revealed that the composite scaffold has a certain induction effect on osteoblast differentiation (Figs.7b and c) [166].In Liuetal.’s work,PLGA with high mechanical strength was used as a backbone and carrier for functional peptide proteins.The compressive strength of the composite is more than 16 MPa,the pore structure is controllable,and the composite has good biocompatibility,which can provide effective support for defective bone and create a microenvironment conducive to cell growth [167].Synthetic polymers can compensate for the poor mechanical properties and uncontrolled degradation rate of natural polymers,and the functionalities of these materials are easy to achieve and more suitable for chemical modifications to facilitate better biological properties.Although these materials have promising biocompatibility,they lack certain osteoinductive properties when used in the field of bone tissue engineering scaffolds and cannot effectively regulate the behaviors of cell proliferation and differentiation.

Fig.7.(a) SEM images of the cross-sectional morphology of MP/PCL-40 scaffold.Copied with permission [157].Copyright 2012,Elsevier.(b) Bending strength and fracture energy of PLGA composites.(c) Relationship between porosity and bending strength.Copied with permission [166].Copyright 2015,Nature.(d) Mechanism of mesoporous silica coated iron oxide nanoparticles (M-MSNs) to promote bone repair.Copied with permission [174].Copyright 2019,Elsevier.(e) Representative X-ray photographs of tibial bone defects in rats 2 weeks after implantation of GO scaffolds.Copied with permission [184].Copyright 2017,Elsevier.
3.3. Nano and nanocomposites
Nanobiomaterials for BTES are a new class of synthetic bone repair materials with a variety of excellent physicochemical and biological properties [168].Mainstream nanomaterials include inorganic nanomaterials (nanoceramics,inorganic metal nanomaterials,etc.) and nanopolymers.Among them,inorganic nanomaterials have a broad application prospect due to their advantages such as good strength,easy processing,and better biological properties [169-171].Representative inorganic nanomaterials that have been applied to BTES mainly include some metal-based nanomaterials,silicon-based nanomaterials,carbon nanomaterials,and many other inorganic metallic nanomaterials [172].
Moderate amounts of iron oxide (Fe3O4) nanoparticles can promote the formation mesenchymal stem cell (MSC) exosome and increase osteogenic and angiogenic effects in the presence of a fixed magnetic field [173].Jia and co-workers [174] prepared a mesoporous silica-coated Fe3O4,which successfully promoted the differentiation of MSCs to osteoblasts and accelerated the regeneration of bone tissue (Fig.7d).In addition,Huetal.[175] prepared nano-hybrid BTES by compositing superparamagnetic iron oxide nanoparticles (SPIONs) and gel sponges.The scaffold could show good biocompatibility and degradation rate consistent with new bone formation,and could induce regeneration of bone tissue [176].Silver nanoparticles can effectively reduce the toxicity and enhance the anti-infective properties.Chenetal.[177] synthesized an antimicrobial silver nanoparticle-loaded TiO2nanotubes (Ag/TiO2-NT),a hybrid nanomaterial that can induce macrophage polarization towards the M2 phenotype by controlled release of ultra-low doses of silver ions and create a bone immune microenvironment suitable for bone tissue regenerationin vitro.Gold nanoparticles,which have the property of mediating bone tissue regeneration,are also commonly used to build bone bioengineering scaffolds.Typically,Leeetal.[178] constructed a gelatin/N-acetylcysteine (NAC) injectable hydrogel attached with gold nanoparticles.The composite gel demonstrated ideal mechanical strength and high biocompatibility to promote the growth and osteogenic differentiation of human lipid-derived mesenchymal stem cells (ADSC).
Carbon-based nanomaterials,including carbon nanotubes,graphene,and fullerenes,show promising prospects in the field of bone tissue engineering [179].Single-walled carbon nanotubes(SWCNTs) and multi-walled carbon nanotubes (MWCNTs) have similar basic structures and both have excellent mechanical properties,ultra-high specific surface area,which make carbon nanomaterials one of the important candidates in the field of BTES preparation [180,181].For example,Lietal.[182] chose adipose-derived MSCs as a model to investigate the effects of MWNTs on cell proliferation and growth.The results showed that MWNTs not only facilitated the osteogenic differentiation of MSCs,but also induced the formation of ectopic bone in the back muscles of mice.Graphene family is a representative class of two-dimensional carbon nanomaterials,mainly including graphene and graphene oxide (GO).GO has the effect of promoting vascular regeneration,and vascular regeneration has been the hotspot and difficulty of bone tissue engineering [183].Recently,Saravananetal.[184] established a bone defect model and implanted GO/chitosan/gelatin composite scaffold.This scaffold promoted the regenerative repair of tibial bone defects (Fig.7e).In addition,Zhao’s team [185] prepared a quartz substrate with GO coating.The results confirmed that this composite substrate was able to promote osteogenic differentiation of MC3T3-E1 cells and increase the secretion of alkaline phosphatase and osteocalcin.
Silica-based mesoporous nanomaterials have become another research hotspot for bone tissue repair materials.Recently,Zhang and coworkers [186] prepared three mesoporous silica-activated glass scaffolds (MBGs) and modified two of them with amination and carboxylation,respectively.Notably,after 12 weeks of implantation of amino-modified MBG scaffolds,38% of new bone was formed,which further confirms the outstanding bone repair ability of MBG materials.Typically,Chengetal.prepared collagen/calcium silicate composite nanofibers with diameters of 200-400 nm by electrostatic spinning.The composite nanofibers significantly induced osteogenic differentiation of bone mesenchymal stem cells[187].All in all,inorganic nano-biomaterials are irreplaceable in the field of BTES construction with their unique biological and mechanical properties,and have a broad application prospect.
4.Preparation strategy of BTES
Due to the pore structure has an important influence on the mechanical and biological response of bone tissue,the ideal manufacturing technology must be able to prepare a controllable layered porous structure to obtain suitable mechanical properties and improve the adhesion and proliferation of cells.At present,the technologies for preparing BTES mainly include melting method,solution casting forming,freeze drying technology,phase separation technology,electrospinning technology and 3D printing technology (Fig.8).The following is a detailed description of the common preparation methods.

Fig.8.Current main construction strategies for BTES.(a) melting method,(b) solution casting forming,(c) freeze-drying,(d) phase separation technology,(e) electrospinning technology,(f) 3D printing technology.
4.1. Melting method and solution casting forming
The melting method is similar to the principle of solution casting in flowing raw materials,mostly polymers,is transferred to the mold to prepare BTES with a specific macroscopic morphology.There is no organic solvent involved in the melt molding process,so the prepared scaffolds can be used for the controlled release of bioactive molecules,and it is possible to construct scaffolds with complex 3D structures.Studies have confirmed that porous scaffold prepared using this technique do not have any adverse effects on the formation of new tissue [188].Chengetal.[189] developed a PLGA/CNT porous scaffold with appropriate mechanical strength and controlled surface roughness using NaCl as poreforming agents (Fig.9a).The scaffold exhibited good cell adhesion and could significantly promote the differentiation and proliferation of MC3T3-E1 osteoblasts.

Fig.9.(a) AFM images of PLGA and CNT incorporated PLGA films.Copied with permission [189].Copyright 2023,Springer.(b) Effect of calcium ion and GO introduction on the macroscopic morphology of scaffold materials.Copied with permission [194].Copyright 2018,American Chemical Society.(c) Schematic diagram of the process of preparing PEG/PLGA scaffold by phase separation.Copied with permission [197].Copyright 2023,Nature.(d) Electrospun fiber for dual delivery of BMP-2 and dexamethasone.Copied with permission [207].Copyright 2015,Elsevier.(e) Assembly of 3D printed SrHA/PCL scaffolds and electrospun nanofibers.(f) Effect of nanofibers on the macroscopic morphology of SrHA@PCL scaffolds.Copied with permission [213].Copyright 2023,American Chemical Society.
4.2. Freeze-drying and phase separation
The essence of freeze-drying is the sublimation of water in the material,which leads to the creation of porous structures [190].The method is divided into three main steps,namely preparation of the solution,pre-freezing of the solution,and freezing and drying at low pressure [191].The scaffolds prepared by this method have a porosity of more than 90% and an adjustable pore size in the range of 20-200 μm [192].The size of the pores is usually controlled by the freezing rate,the type of material and the temperature.For example,Wangetal.[193] prepared a calcium silicate/chitosan (SIS/CS) composite scaffold with a continuous three-dimensional structure.The change of CS content significantly affects the pore size of the scaffold,implying that the properties of the material can be adjusted according to the various application.Similarly,Elayarajaetal.reported a sodium alginate/chitosan/collagen composite scaffold by relying on freezedrying (Fig.9b) [194].The maximum porosity of the scaffold was close to 250 μm,and both ionic cross-linking and GO introduction changed the porosity and mechanical properties of the scaffold.The phase separation technique is based on the thermodynamic instability of polymer solutions at a certain temperature,by which a homogeneous multi-component mixed solution system can be separated into two phases [195].In the work of Qiuetal.nanofiber scaffolds were prepared by phase separation technique and used as templates for depositing drug-loaded nanoparticles.The results ofinvitroandinvivoexperiments showed that the scaffold could effectively promote osteogenic differentiation [196].Zhangetal.achieved the preparation of structurally tunable porous novel Janus bionic membranes using PLA as the main substrate relying on phase separation technique (Fig.9c) [197].This scaffold can accelerate or stimulate the angiogenesis around the defective tissue.
4.3. Electrospinning and 3D printing
Electrospinning can prepare a variety of biodegradable polymer materials into nanofibers,with the advantages of simple processing,controllable performance,and easy industrial scale-up[198-200].Electrospinning device typically consist of high-voltage power supply,syringe,syringe pump,needle connected to the syringe,and a metal collector [201].As the potential changes,the polymer solution is stretched at the tip of the needle to form a cone called a Taylor cone [202].Once the repulsive force exceeds the critical threshold of surface tension,the charged solution stream of polymer is ejected and quickly collected by the metal substrate [203].The diameter and shape of the fibers as well as the porosity of the fiber membrane can be controlled by changing various processing parameters [204,205].For example,Xuetal.successfully prepared a PCL-based electrospun fiber scaffold material by adjusting the spinning conditions.The scaffold has a continuous hierarchical structure with a pore diameter of more than 300 μm [206].Li’s team developed a novel nanoparticle-loaded electrospun fiber scaffold for the delivery of two functional drugs,BMP-2 and dexamethasone (Fig.9d) [207].The results showed that the unique combination of this drug and fibrous scaffold produced superb osteogenic induction.The coaxial spinning allows the combination of two polymer materials to create composite or hybrid fiber scaffold with better performance.For example,Zhangetal.reported a CS/PLA fibrous scaffold with a skin-core structure that is wrapped with biotin-modified exosomes on its surface [208].Modification of the substance improves the loading stability of the exosome and enables slow local release.3D printing is a technology that converts computer 3D data models into solids,also known as “additive manufacturing” [209].Designability is the most prominent advantage of 3D printing technology.This technology can be used to produce unprecedented material structures containing complex topological features,allowing for the one-step construction of scaffold materials that combine desirable macrostructure,good biocompatibility,and satisfactory mechanical strength[210,211].Thermoplastic polymer materials with good rheological behavior such as PLA,PCL,polycarbonate and some thermosetting polymer materials such as epoxy resins can be chosen as raw materials for 3D printing [212].Typically,Zhouetal.[213] designed a strontium-containing hydroxyapatite/polycaprolactone (SrHA@PCL)3D printed scaffold for accelerated vascularized bone regeneration(Fig.9e).To enhance the therapeutic effect,short nanofibers containing dimethyl oxalyl glycine were selected for assembly with the SrHA@PCL scaffold,and the pore structure of the composite scaffold could be controlled by changing the density of the nanofibers(Fig.9f).Notably,the different degradation rates of nanofibers and 3D printed scaffolds make possible the programmed release of active ingredients,which effectively accelerates the regeneration of blood vessels and bone tissue.
5.Conclusion and outlook
BTES play an irreplaceable role in bone injury repair,including providing physical support,meeting cell adhesion,promoting angiogenesis and osteogenesis.More refined and adjustable structure,appropriate mechanical properties,outstanding resorbability and better osteogenic effect are important development trends of BTES,which put forward higher requirements for the selection and preparation of future scaffold materials.This review takes the repair process of bone tissue as a systematic entry point,summarizes recent advances in BTES,systematically describes the selection of raw materials and main preparation strategies for the preparation of BTES,and analyzes the advantages of different materials and construction methods.Despite the obvious progress and significant achievements,there are still some challenging issues to be faced in the future development of BTES (Fig.10).

Fig.10.Challenges for the future development of BTES.
First,the preparation of the reported BTES is complex and highcost,which limited the construction of related materials to laboratory scale.And the effects of most newly developed BTES have only been validated in animals,including Sprague-Dawley rats and rabbits,have not been applied clinically,making it difficult for experimental results to be truly accepted.Thus,the actual clinical effects of most bone engineering scaffold materials should be explored in order to promote their application in biomedical fields and truly contribute to human health.According to the literature,drug loading,porosity,and mechanical properties affect the therapeutic effi-cacy of BTES;however,there is a lack of any statistical results confirming a functional relationship between these material properties and actual clinical outcomes.Therefore,subsequent studies should focus on the association between a particular structure of the stent and clinical effects,which could provide important guidance for the development of new scaffolds and avoid excessive duplication of experiments.Second,the exploration of new materials and technologies is indispensable in the design and construction of future bone engineering scaffolds.The currently hot nanomaterials,including one-dimensional carbon nanotubes,two-dimensional MXene and graphene,and three-dimensional organometallic framework materials,can provide the materials with appropriate bioactivity,allowing them to be considered as promising functional materials in the field of bone tissue engineering.Last but not least,new drug development should also be focused on,which is in line with,but not limited to,the needs of the bone tissue engineering field.Drugs are important bodyguards of human health and effi-cient drugs are essential to ensure the repair of bone defects.Such drugs must have good compatibility with BTES and achieve slow release during bone regeneration.
Although surprising progress has been made in the design and preparation of BTES materials,there are still drawbacks such as harsh preparation conditions and difficulty in clinical application.In the face of such problems,the development of new BTES materials still requires continuous efforts in order to improve the treatment options for bone tissue in the clinic.Perhaps the solidification of BTES materials in the body can be achieved by minimally invasive injection,thus avoiding the large wounds caused by surgery on the patient.In summary,with the continuous efforts of researchers,we believe that better BTES preparation methods and strategies will certainly be created and continue to serve the healthy life of human beings.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by the Fundamental Research Funds for the Central Universities of China (Nos.DUT22QN203 and DUT22YG201).
 Chinese Chemical Letters2024年2期
Chinese Chemical Letters2024年2期
- Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
