Interactions of phenol and benzaldehyde in electrocatalytic upgrading process
Ln Ling ,Chunin Wng ,Xuki Lu ,Yunn Sun ,Beiei Yn ,Ning Li,c,* ,Gunyi Chen,c,*,Li’n Hou,d,*
a School of Environmental Science and Engineering,Tianjin Key Lab of Biomass/Wastes Utilization,Tianjin University,Tianjin 300350,China
b School of Mechanical Engineering,Tianjin University of Commerce,Tianjin 300134,China
c Georgia Tech Shenzhen Institute,Tianjin University,Shenzhen 518071,China
d High Tech Inst Beijing,Beijing 100085,China
Keywords: Interaction Bio-oil Electrocatalytic hydrogenation Phenol Benzaldehyde
ABSTRACT Biomass pyrolysis oil can be improved effectively by electrocatalytic hydrogenation (ECH).However,the unclear interactions among different components lead to low bio-oil upgrading efficiency in the conversion process.Herein,benzaldehyde and phenol,as common compounds in bio-oil,were chosen as model compounds.The interactions between the two components were explored in the ECH process by combining experiments and theoretical calculations.Results showed that phenol could accelerate the conversion of benzaldehyde in the ECH.The selectivity of benzyl alcohol was increased from 60.9% of unadded phenol to 99.1% with 30 mmol/L phenol concentration at 5 h.Benzaldehyde inhibited the ECH of phenol.In the presence of benzaldehyde,the conversion rate of phenol was below 10.0% with no cyclohexanone and cyclohexanol formation at 5 h.The density functional theory (DFT) calculations revealed that the phenol could promote the adsorption of benzaldehyde and facilitate the targeted conversion of benzaldehyde on the active site by lowering the reaction energy barrier.The research on the interaction between phenol and benzaldehyde in the ECH provides a theoretical basis for the application of ECH in practical bio-oil upgrading.
In recent decades,the dual pressure of energy shortage and environmental degradation has aroused interests in the field of renewable and clean energy [1].Biomass energy,as a renewable energy source,has attracted attention due to its abundance,environmental friendliness,low cost and carbon neutrality [2,3].In addition,biomass can be converted into bio-oil,biochar and combustible gas by thermochemical technology [4].Therein,bio-oil as a potential fuel is regarded to be a suitable alternative for future fuels [5].The raw bio-oil obtainedviathermochemical treatment cannot be directly used due to disadvantages of complex composition,instability,high viscosity,low calorific value and strong corrosion [5,6].Therefore,it is necessary to improve the viability of bio-oil as a liquid fuel by upgrading its quality.
Traditional upgrading technologies such as catalytic hydrogenation,steam reforming,catalytic cracking,supercritical water and emulsification can improve the properties of bio-oil [7].However,the reaction conditions of steam reforming,catalytic hydrogenation and catalytic cracking are harsh,and the catalyst is easily deactivated [8-10].Besides,the process of catalytic hydrogenation requires a large amount of external hydrogen (H2) [6].Supercritical water and emulsification require expensive organic solvents and emulsifiers [11,12].Comparatively,electrocatalytic hydrogenation(ECH),as an emerging bio-oil upgrading technology has received extensive attention [13,14].Bio-oil ECH is usually performed at reaction temperatures below 80°C and atmospheric pressure [15].Mild reaction conditions can reduce the occurrence of polymerization and catalyst deactivation caused by coking [16].Additionally,no external H2is required during the ECH process.The oxidation of water or organics at the anode produces hydrogen ions and electrons,which are transferred to the cathode for bio-oil refining[6,17].Ideally,the energy needed for bio-oil upgrading would be obtained through clean energy conversion such as solar and wind energy,making the whole process carbon-free [13].Therefore,ECH is considered as a green method for producing fuels and chemicals from pyrolyzed bio-oil [18].
The current research is focused on ECH of single bio-oil model compound.Reactive carbonyl groups in bio-oils could be converted into stable alcohols by ECH [19].Benzaldehyde could be selectively reduced to benzyl alcohol on C-supported Pt,Rh,Pd,and Ni electrodes in the ECH system [19].The Au cathode facilitated the conversion of benzaldehyde and glutaraldehyde to the corresponding alcohol products [20].The ECH conversion rate of furfural was 82.0% with 99.0% selectivity of furfuryl alcohol at room temperature on 3%Pt/activated carbon fibers (ACF) electrode (voltage:-0.5 V,electrolyte: 0.1 mol/L H2SO4) [21].Zhangetal.also synthesized Cu3P/carbon fiber cloth (CFC) electrocatalyst successfully for ECH of furfural,achieving about 100% selectivity of furfuryl alcohol [22].The three-dimensional Ru/TiO2electrode was employed for the phenol ECH with>99.5% conversion rate of phenol and up to 97.0% selectivity of cyclohexanol at pH 7 after 60 min [23].Liuetal.[24] designed a polyoxometalate (POM)-Pt/C dual catalyst system to achieve high-performance ECH and deoxygenation of phenol.More than 99.0% phenol was converted at 35°C within 11.3 min.The selectivity to cyclohexanol and cyclohexane reached 80.2% and 18.6%,respectively.However,the ECH of multiple bio-oil components and actual bio-oils has been less studied.Specifically,the interactions between components during the ECH upgrading of multi-component bio-oil are still unclear.In addition,conventional carbon carriers were considered neutral with defects of low wettability and poor reactivity [25].Doping nitrogen in the framework of carbon carriers can improve the compatibility and applicability of carbon carriers with metal catalysts,achieving interfacial interactions [26].Moreover,nitrogen-doped carbon materials can improve the electrical conductivity of the electrode,thereby boosting the catalytic activity [27].The use of nitrogen-containing biochar can realize resource utilization and avoid the addition of nitrogen sources.In our previous work,Pt-modified shrimp shell carbon (SSB) catalyst (Pt/SSB) electrodes have been prepared for the phenol ECH,achieving 100% conversion of phenol and 98% total selectivity of cyclohexanol and cyclohexanone at 5 h [28].Small molecule aldehydes and ketones are one of the main reasons for the instability of bio-oils [29].Besides,phenols and small molecule aldehydes are prone to polymerization [29].Hence,this work further studies the interactions of aldehydes and phenolic compounds at the Pt/SSB electrode in the ECH process.
In this study,phenol and benzaldehyde were selected as bio-oil model compounds.The effects of reaction conditions such as reactant concentration,Pt loading,reaction temperature,reaction current and electrode type on the conversion rate,and selectivity of products were explored in the ECH system.The interaction mechanism between the ECH processes of phenol and benzaldehyde was clarified by electrochemical performance test and DFT calculation.In addition,the composition changes of the actual bio-oil before and after electrocatalytic upgrading were further studied.The obtained results are beneficial to promote the application of electrocatalysis in upgrading of practical bio-oil.
The dried shrimp shells were obtained from commercially available ordinary fresh shrimp.The carbon felt and Nafion-117 membrane came from Inner Mongolia Wanxing Carbon Co.,Ltd.and Shanghai Hesen Co.,Ltd.,respectively.The main chemicals needed in this study were shown in Text S1 (Supporting information).
Platinum-based shrimp shell carbon electrodes were prepared according to previous studies [28].The platinum precursors were loaded onto the surface of the shrimp shell carbon by chemical reduction method.First,the pre-prepared shrimp shell charcoal(0.5 g) was immersed in PtCl4solution (0.4,0.8,1.2 and 1.6 mg/mL)and then sonicated for 2 h.The reaction was carried out by adding an appropriate amount of NaBH4solution (0.5 mol/L) under vigorous stirring.The reaction product was washed three times with deionized water and dried in a vacuum drying oven at 80°C for 12 h.Finally,the Pt/SSB catalyst was loaded onto the pretreated carbon felt.ECH of phenol and benzaldehyde were performed in a H-type reactor under the constant current.The 0.1 mol/L Na2SO4and 0.2 mol/L H2SO4solution were placed in the cathode and anode chambers,respectively.The cathode electrode is a carbon felt(~2×2 cm) loaded with Pt/SSB catalyst [28],and the anode electrode is a Pt sheet electrode (1 cm×1 cm,99.9%).Electrodes were activated at 40 mA for 10 min after the reaction platform was assembled.Phenol or benzaldehyde was mixed with 5 mL methanol in the cathode chamber to form a 50 mmol/L reaction solution.The electrocatalytic upgrading reaction of bio-oil model compounds was carried out by stirring with a magnetic stirrer for 5 h.In addition,0,15 and 30 mmol/L of phenol were added into the cathode compartment to study the effect of phenol on the ECH of benzaldehyde.Moreover,the ECH performance of benzaldehyde under different Pt loadings (0.0,0.6,1.2,1.8 and 2.4 mg/cm2),reaction currents (80,90,100,110 and 120 mA),temperatures (30,50 and 70°C) and electrodes (Pt/SSB,Pt/carbon black (CB) and Pt/SBA-15) were compared with and without phenol.The effect of benzaldehyde on the ECH of phenol was studied using the same method.The sample with a volume of 0.5 mL was withdrawn at 1 h intervals and extracted with 1 mL of dichloromethane.A gas chromatography-mass spectrometry (GC-MS) with a built-in Rtx-5 column (30 m×250 μm) was used to identify the products [28].Conversion rate and selectivity are calculated (Text S2 in Supporting information).Each experiment was carried out in triplicates.
The detail of actual bio-oil preparation was displayed in Text S3(Supporting information).
First-principle calculations were carried out using DFT with generalized gradient approximation (GGA) of Perdew-Burke-Ernzerhof (PBE) implemented in the Vienna Ab-Initio Simulation Package (VASP) [30,31].The adsorption energy of benzaldehyde on Pt sites can be defined as follows (Eq.1):
whereEtotal,EsubstrateandEmoleculerepresent the energy of substrate with adsorption,substrate and free molecule,respectively.
The mixture of phenol and benzaldehyde was hydrogenated by electrocatalysis.Fig.1 showed the conversion rate of benzaldehyde and product selectivity during ECH in the presence of different phenol concentrations.Obviously,the reaction rate of benzaldehyde ECH was accelerated from 1.57 h-1without phenol to 2.28 h-1with phenol concentration of 30 mmol/L (Fig.S1 in Supporting information).Up to 98.5% conversion rate of benzaldehyde was achieved within 2 h at a phenol concentration of 30 mmol/L(Fig.1a).In addition,the existence of phenol also had an important influence on the selectivity of benzyl alcohol (Fig.1b).In the absence of phenol,the selectivity to benzyl alcohol was only about 60.9% in 5 h,which increased to 94.7% when a small amount of phenol (15 mmol/L) was added.Evidently,phenol played an important role in promoting the directional transformation of benzaldehyde.As presented in Table S1 (Supporting information),the phenol conversion and product selectivity changed significantly with the addition of benzaldehyde.Compared with the ECH of phenol alone,the ECH process of phenol was inhibited after the addition of benzaldehyde.A little phenol was converted and no formation of cyclohexanone and cyclohexanol was found.Therefore,it can be speculated that benzaldehyde ECH inhibited phenol ECH,making benzaldehyde ECH superior to phenol ECH.

Fig.1.The effect of phenol concentration on the (a) benzaldehyde conversion and(b) selectivity of benzyl alcohol at Pt/SSB electrode.Pt loading: 1.8 mg/cm2,current:100 mA,reaction temperature: 50°C,reaction time: 5 h.
The changes in the conversion of benzaldehyde and the selectivity of benzyl alcohol during the ECH on Pt/SSB electrode with different Pt loadings in the presence of phenol were shown in Figs.2a and b.In particular,the reaction rate of benzaldehyde reached 3.45 h-1at a Pt loading of 0 mg/cm2,which was even higher than that of the Pt/SSB electrode (2.32 h-1) with a Pt loading of 2.4 mg/cm2(Fig.S2a in Supporting information).Moreover,100% benzaldehyde was converted without Pt loading (Fig.2a).However,the selectivity to benzyl alcohol was only 1.5% on the SSB electrode.Furthermore,the results of GC-MS analysis showed that most benzaldehyde was transformed into hydrobenzoin by intermolecular dehydration condensation reactions,which might be contributed by the carbon felt [32].The selectivity of benzyl alcohol increased from 1.5% to 87.2% in 5 h when the Pt loading increased from 0 to 0.6 mg/cm2(Fig.2b),indicating that benzaldehyde hydrogenation was the dominant reaction.As the Pt loading continued to increase,both the conversion rate and selectivity of benzaldehyde were improved.The addition of phenol enhanced the selectivity to benzyl alcohol significantly compared to the absence of phenol during ECH (Figs.S3a and b in Supporting information).The selectivity of benzyl alcohol increased from 19.2%,84.5%,90.0%,91.4% without phenol to 87.2%,90.6%,94.7%and 95.8% with phenol when the Pt loadings were 0.6,1.2,1.8,and 2.4 mg/cm2,respectively.The conversion rate of phenol and product selectivity under different Pt loadings in the presence of benzaldehyde are shown in Table S1.The ECH efficiency of phenol under different loadings of Pt were lower than 10.0% with no cyclohexanone and cyclohexanol detected.
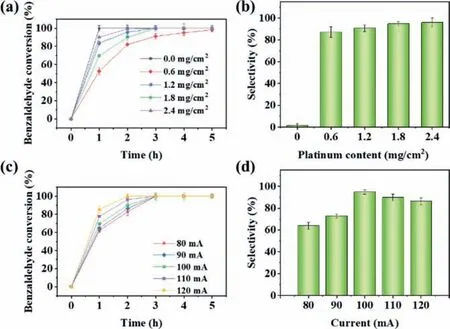
Fig.2.The effect of Pt loading on the (a) benzaldehyde conversion and (b) selectivity of benzyl alcohol at Pt/SSB electrode in the presence of phenol;the effect of current on the (c) benzaldehyde conversion and (d) selectivity of benzyl alcohol at Pt/SSB electrode in the presence of phenol.Except for the influencing factors,other reaction conditions including Pt loading: 1.8 mg/cm2,current: 100 mA,phenol concentration: 15 mmol/L,reaction temperature: 50°C,reaction time: 5 h.
Current could drive the continuous ECH of bio-oil model compounds.The change of current will influence the production rate of reducing hydrogen [33],thereby affecting reactant conversion and product selectivity.As indicated in Figs.2c and d,currents have a significant impact on the conversion of benzaldehyde and the selectivity of benzyl alcohol during ECH with Pt/SSB electrodes in the presence of phenol.It is obvious that the reaction rate of benzaldehyde was accelerated with the increase of reaction current.The rates of benzaldehyde ECH were 2.15,2.17,2.18,2.25 and 3.45 h-1at currents of 80,90,100,110 and 120 mA,respectively(Fig.S2b in Supporting information).Phenol enhanced benzaldehyde conversion effectively (Figs.S3c and d in Supporting information),reaching 100.0% benzaldehyde conversion in 2 h.In contrast to the product selectivity with benzaldehyde ECH alone (Fig.S3d),phenol improved the selectivity of benzyl alcohol remarkably (up to 94.7%) at a high current (100 mA).Similar to benzaldehyde ECH alone,excessively high or low currents were detrimental to the targeted conversion of benzaldehyde to benzyl alcohol.The conversion rate of phenol and product selectivity under different reaction currents with benzaldehyde are illustrated in Table S1.Analogous to the Pt loading,no significant conversion of phenol was found,further indicating that benzaldehyde ECH may occur preferentially.
The effect of reaction temperature on the ECH of benzaldehyde at the Pt/SSB electrode with phenol was shown in Figs.3a and b.Compared with the ECH reaction of benzaldehyde alone (Figs.S4a and b in Supporting information),the optimal reaction temperature for the ECH conversion of benzaldehyde changed from 50°C to 70°C in the presence of phenol,realizing the conversion rate of 100.0% with the higher reaction rate at 70°C (2.20 h-1,Fig.S2c in Supporting information).However,high temperature can promote the hydrogen evolution reactions,thereby inhibiting the formation of benzyl alcohol (Fig.3b) [6].Surprisingly,compared with the ECH of benzaldehyde alone (Figs.S4a and b),the benzaldehyde conversion and the selectivity of benzyl alcohol were greatly enhanced with phenol at low temperature (30°C).The reason could be attributed to the low reactivity of the low-temperature hydrogen evolution reaction and the greater adsorption energy of benzaldehyde on Pt site with phenol.The conversion rate of benzaldehyde and the selectivity of benzyl alcohol increased from 92.5%and 86.5% to 100.0% and 94.0% respectively,which provided a new idea for the practical application of benzaldehyde ECH.Besides,no obvious ECH conversion of phenol was found at different reaction temperatures (Table S1).

Fig.3.The effect of reaction temperature on the (a) benzaldehyde conversion and(b) selectivity of benzyl alcohol at Pt/SSB electrode in the presence of phenol;the effect of electrode on the (c) benzaldehyde conversion and (d) selectivity of benzyl alcohol in the presence of phenol in the ECH system.Except for the influencing factors,other reaction conditions including Pt loading: 1.8 mg/cm2,current: 100 mA,phenol concentration: 15 mmol/L,reaction temperature: 50°C,reaction time: 5 h.
The type of support can affect the electrocatalytic performance of catalysts [6].The ECH conversion and benzyl alcohol selectivity on Pt electrode with different supports in the presence of phenol were plotted in Figs.3c and d.Apparently,benzaldehyde exhibited excellent ECH reaction rate (2.18 h-1,Fig.S2d in Supporting information) and benzyl alcohol selectivity (94.7%,Fig.3d) on the Pt/SSB electrode with co-existing phenol.The selectivity of benzyl alcohol was enhanced by 4.6% on the Pt/SSB electrode with phenol existenceversusthe ECH of benzaldehyde alone (Figs.S4c and d in Supporting information).Surprisingly,the phenol also improved the performance of Pt/SBA-15 and Pt/CB electrodes for the ECH of benzaldehyde,which may be ascribed to greater adsorption capacity of benzaldehyde on the Pt sites in the presence of phenol.The conversion rate of benzaldehyde and the benzyl alcohol selectivity were also improved significantly on the Pt/SBA-15 and Pt/CB electrodes.To some extent,the existence of phenol is working on the electrode surface.In addition,no obvious ECH conversion of phenol and product formation were found under the action of Pt electrodes with different supports (Table S1),thereby the benzaldehyde ECH occurs preferentially over phenol ECH on Pt electrodes with different carriers.
Based on the experimental investigation of multi-component influencing factors,the preferential occurrence of benzaldehyde ECH reaction over phenol ECH reaction was inferred (Table S1).To explore the phenol conversion after the complete conversion of benzaldehyde,the reaction time was appropriately extended to 10 h to observe the change of the final product.The content changes of phenol,benzaldehyde and their ECH products were shown in Fig.4.Almost no phenol ECH occurred within the first 4 h.A slight decrease of phenol may be ascribed to its attachment to the electrode surface and participation in the benzaldehyde ECH.In addition,benzaldehyde was almost converted into benzyl alcohol completely at 4 h.Meanwhile,phenol declined slowly with the gradual formation of cyclohexanone and cyclohexanol.Moreover,the conversion of phenol and the product selectivity within 5-10 h in the mixed ECH system were lower than those of phenol ECH alone [28],attributing to the reduction of the active sites on the electrode surface.Therefore,benzaldehyde might inhibit the phenol ECH.Notably,a few benzyl alcohols started to be further converted into cyclohexyl methanol after all the benzaldehyde was converted into benzyl alcohol.In detail,about 60.0% of benzyl alcohol was diminished.Nonetheless,only about 5.0% of cyclohexyl methanol was produced,suggesting that further ECH of benzyl alcohol was more difficult.
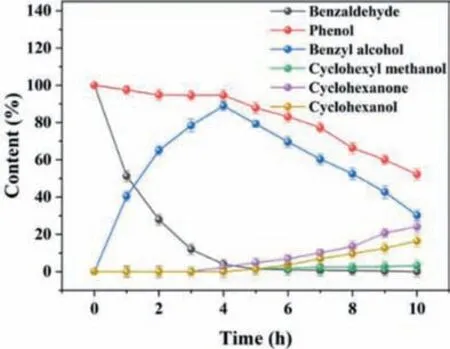
Fig.4.Content changes of phenol,benzaldehyde and their ECH products.Electrode:Pt/SSB,Pt loading: 1.8 mg/cm2,current: 100 mA,reaction temperature: 50°C,reaction time: 10 h.
The electrocatalytic upgrading performance of actual bio-oil on Pt/SSB electrode was also shown in Fig.S5 (Supporting information).
Fig.5 showed the linear sweep voltammetry curves of Pt/SSB catalysts in 0.1 mol/L Na2SO4solutions with different concentrations of phenol and benzaldehyde.Apparently,the current value of 90 mA could be driven by a voltage of -0.76 V on the Pt/SSB electrode in the absence of phenol and benzaldehyde.The potential of Pt/SSB electrode shifted significantly to -0.48 V with the addition of 15 mmol/L phenol,which was 0.28 V lower than that without phenol and benzaldehyde.When 15 mmol/L benzaldehyde was added,the potential of Pt/SSB electrode was shifted to -0.64 V to the left with an offset of 0.12 V,which was less than 1/2 of the potential left shift by adding an equal concentration of phenol.A potential shifted to the left by 0.26 V with the simultaneous addition of 15 mmol/L phenol and 15 mmol/L benzaldehyde,indicating phenol could reduce the reaction potential of benzaldehyde ECH.Therefore,phenol can promote the ECH of benzaldehyde.

Fig.5.Linear scanning voltammetry curves of Pt/SSB catalyst with different concentrations of phenol and benzaldehyde at a scanning rate of 10 mV/s.
The energy for the stepwise ECH of benzaldehyde on Pt site was calculated in the presence and absence of phenol to further explore the interaction mechanism of ECH of bio-oil model compounds(Fig.6).The adsorption energy of benzaldehyde was -0.56 eV during ECH of benzaldehyde alone.The adsorption energy expanded to-0.98 eV after phenol was added.Hence,the phenol could enhance the adsorption capacity of benzaldehyde at the active sites dramatically,facilitating the subsequent gradual reduction of benzaldehyde.The energy barriers to be overcome for the stepwise ECH of benzaldehyde with or without phenol on Pt sites were shown in Table 1.Clearly,the energy barrier for the stepwise ECH of benzaldehyde was decreased significantly in the presence of phenol.In detail,the energy required for TS1 and TS2 transition was less than 1/2 of the energy barrier needed for the ECH of benzaldehyde alone.Therefore,phenol can not only promote the adsorption of benzaldehyde,but also facilitate the directional conversion of benzaldehyde on the active site by lowering the reaction energy barrier.
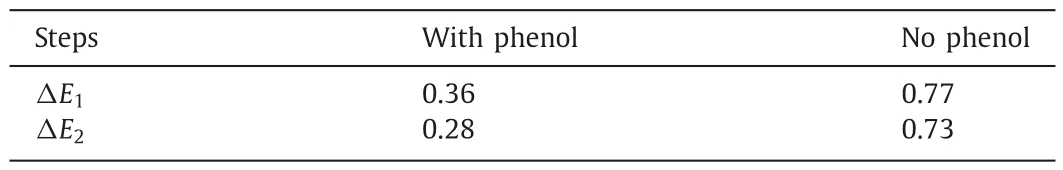
Table 1Energy barriers (eV) to be overcome for the stepwise hydrogenation of benzaldehyde at Pt active sites in the presence or absence of phenol.

Fig.6.Energy for stepwise ECH of benzaldehyde at Pt active sites in the presence or absence of phenol.
In this work,the electrocatalytic upgrading rate and product selectivity of single and mixed bio-oil model compounds were compared.The interaction mechanism of phenol and benzaldehyde during ECH was clarified.Phenol facilitated the directed transformation of benzaldehyde,while benzaldehyde inhibited phenol ECH.The ECH of benzaldehyde occurs preferentially over that of phenol.The ECH of phenol began as the conversion of benzaldehyde completed.Pt loading of 1.8 mg/cm2,reaction current of 100 mA,reaction temperature of 50°C and carrier of SSB were more favorable for the conversion of benzaldehyde to benzyl alcohol in the ECH of mixed phenol and benzaldehyde.DFT calculation results showed that phenol increased the energy difference between free and adsorbed benzaldehyde from -0.56 eV to -0.98 eV.Besides,the energy barrier for the stepwise ECH of benzaldehyde was reduced with the presence of phenol.Therefore,phenol promoted the targeted conversion of benzaldehyde on the Pt active site by enhancing the adsorption of benzaldehyde and lowering the reaction energy barrier.In addition,the relative content of unsaturated oxygenated compounds such as aldehydes,acids and phenols in pyrolytic bio-oil decreased after electrocatalytic upgrading,while the relative content of alcohols,ketones and esters increased.The obtained results provided an experimental basis for the criticality of interactions between bio-oil model compounds for bio-oil ECH.In addition,catalysts played an important role in the ECH of bio-oil.Therefore,further studies on the interaction between complex components of bio-oil and the design of catalysts with multiple types of active sites are beneficial to improve the quality of biooil in electrocatalytic processes.However,noble metals hinder the large-scale applications of bio-oil ECH due to high cost and barren reserves.Hence,the development of non-precious metals can promote industrial applications of bio-oil electrocatalytic upgrading [34-39].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to express gratitude to Shenzhen Science and Technology Program (No.JCYJ20200109150210400).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108581.
 Chinese Chemical Letters2024年2期
Chinese Chemical Letters2024年2期
- Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
