SARS-CoV-2 variants are associated with different clinical courses in children with MlS-C
Andres F. Moreno Rojas·Emelia Bainto·Helen Harvey·Adriana H. Tremoulet·Jane C. Burns·Kirsten B. Dummer
Abstract Background Recent infection with SARS-CoV-2 in children has been associated with multisystem inf lammatory syndrome in children (MIS-C).SARS-CoV-2 has undergone different mutations.Few publications exist about specif ic variants and their correlation with the severity of MIS-C.Methods This was a single-center,retrospective study including all patients admitted with MIS-C at Rady Children’s Hospital-San Diego between May 2020 and March 2022.Local epidemiologic data,including viral genomic information,were obtained from public records.Demographics,clinical presentation,laboratory values,and outcomes were obtained from electronic medical records.Results The analysis included 104 pediatric patients.Four MIS-C waves were identif ied.Circulating variants in San Diego during the f irst wave included clades 20A to C.During the second wave,there were variants from clades 20A to C,20G,21C (Epsilon),20I (Alpha),and 20J (Gamma).The third wave had Delta strains (clades 21A,21I,and 21J),and the fourth had Omicron variants (clades 21K,21L,and 22C).MIS-C presented with similar symptoms and laboratory f indings across all waves.More patients were admitted to the pediatric intensive care unit (PICU) (74%) and required inotropic support(63%) during the second wave.None of the patients required mechanical circulatory support,and only two required invasive ventilatory support.There was no mortality.Conclusions The various strains of SARS-CoV-2 triggered MIS-C with differing severities,with the second wave having a more severe clinical course.Whether the differences in disease severity across variants were due to changes in the virus or other factors remains unknown.
Keywords Complications·COVID-19·MIS-C·SARS-CoV-2 variants
Introduction
In April 2020,a new syndrome characterized by severe inf lammation,multiorgan dysfunction,and features resembling Kawasaki disease and toxic shock syndrome in previously healthy children was described.These patients shared a recent infection with SARS-CoV-2.This condition is now recognized as multisystem inf lammatory syndrome in children (MIS-C) [1,2].
Throughout the pandemic,the coronavirus has undergone mutations that have changed its infectivity,transmission,and severity of the clinical presentation.Studies have reported that acute infection with the Omicron variant had less severe clinical outcomes,while Delta caused more severe disease[3,4].When looking at various time periods and linking the cases to the prevalent circulating variants,some studies suggested an effect on the presentation and severity of MISC,while others have not shown differences [5— 14].However,no reports have described specif ic local circulating variants and their correlation with MIS-C severity.We evaluated how the SARS-CoV-2 variants affected the clinical presentation and severity of MIS-C in San Diego County.
Methods
This retrospective study was conducted at Rady Children’s Hospital,San Diego,a single tertiary care pediatric center that serves a population base of approximately 3.5 million residents.The Institutional Review Board at the University of California,San Diego,approved prospective data collection,and parents and participants gave signed informed consent or assent as appropriate.
All patients diagnosed with MIS-C between April 2020 and March 2022 were included in the evaluation.MIS-C was diagnosed using the 2020 CDC MIS-C case def inition,as all the patients were diagnosed prior to the updated CDC def inition for MIS-C (November 2022) [15].The MIS-C/Kawasaki disease (KD) team developed a clinical pathway to standardize the approach and management of the patients.Two physicians (AHT and JCB) conf irmed the history and clinical presentation,adjudicated each diagnosis and were primarily responsible for treatment in collaboration with pediatric intensivists when necessary.Our center participated in the MIS-C Comparative Effectiveness Study(MISTIC) trial (NCT04898231) that started on December 22,2020.The trial randomized the patients who received IVIG but clinically warranted further anti-inf lammatory therapy to one of three treatment arms (inf liximab,steroids,or anakinra) and allowed for rerandomization to one of the two remaining arms if clinically warranted [16].
Four MIS-C waves were identified during the study period.We def ined the “f irst wave” from April 2020 to August 2020,the “second wave” from September 2020 to April 2021,the “third wave” from May 2021 to December 2021,and the “fourth wave” from January 2022 to March 2022.The separation of the waves was based on analyzing the variation in the number of MIS-C cases at our institution,with at least 4 weeks with no cases between the waves.
COVID-19 case data for San Diego County were obtained from https:// searc hcovid.info/ dashb oards/ epide miolo gy/ and https:// outbr eak.info [17].The CDC established national surveillance for SARS-CoV-2 genomic sequencing in November 2020 [18].The f irst reports appeared in December 2020,and previous genomic information in the US about circulating variants is limited.The SARS-CoV-2 genomic data of the sequences isolated in San Diego were obtained from The Scripps Research Institute and included samples obtained before December 2020 [19,20].The genomic data were analyzed for variant classif ication (World Health Organization (WHO) and Phylogenetic Assignment of Named Global Outbreak (PANGO) lineages) and clade assignment(Nextstrain clade) using Nextclade CLI v2.9.1.Since the WHO classif ication includes mostly variants of interest and concern and the PANGO lineage system is extensive and detailed,Nextstrain clades were used to facilitate the analysis of the circulating SARS-CoV-2 variants during the waves.Detailed information about Nextstrain clades can be found at https:// clades.nexts train.org/ [21].Monthly frequency was analyzed,and variants with a frequency below 2% were excluded from the graphics.The results and discussion included only variants with a frequency above 5%.
Demographic characteristics (age,sex,race,weight,height),comorbidities (obesity,hypertension,asthma,diabetes mellitus type 1 and type 2,congenital heart disease,chronic kidney disease,autoimmunity),presenting clinical signs (fever,rash,conjunctival injection,erythema of the lips,oral mucosa or pharynx,cervical lymphadenopathy,erythema of the hands,abdominal pain,emesis,and diarrhea),laboratory results at the time of admission and worst value during hospitalization [white blood cell count,hemoglobin,platelet count,erythrocyte sedimentation rate,C reactive protein,ALT,AST,GGT,sodium,albumin,creatinine,ferritin,D dimer,f ibrinogen,brain natriuretic peptide (BNP),and troponin I],volume of intravenous f luid boluses (with normal saline,ringer’s lactate,plasmalyte,3%hypertonic saline or 5% albumin) administered in the f irst 24 hours,use of intravenous immunoglobulin (IVIG),time from initial evaluation to IVIG administration,additional anti-inf lammatory therapies (steroids,anakinra,inf liximab),need for PICU admission,length of stay in the PICU,use of inotropic and respiratory support,and echocardiogram results were obtained from the electronic medical records.The IVIG protocol was a single 2 g/kg dose over 8—12 hours.Additional anti-inf lammatory therapies were used per the MISTIC trial.
Because the severe cases of MIS-C present in shock,the main criteria for admission to the PICU were hemodynamic instability,cardiac dysfunction requiring a continuous infusion of vasoactive medications,or respiratory insufficiency requiring more than a high-f low nasal cannula.Overweight/obese was def ined as BMI >85 percentile for age and gender for patients over two years of age and weight for length more than two standard deviations above the median for patients under two years of age.Severe obesity was defined as BMI >99 percentile for age and gender.CDC growth charts were used.Laboratory results were analyzed as continuous variables and converted into categorical values with the following def initions: leukopenia: WBC <5 × 10 9/L.Lymphopenia: lymphocyte count <4.5 × 10 9/L if aged <8 months or <1.5 × 10 9/L if aged ≥ 8 months.Thrombocytopenia: Platelet count <150 × 10 9/L.Hypoalbuminemia:Albumin <3.5 g/dL.Hyponatremia: Sodium <135 mmol/L.Elevated ferritin: ≥ 300 ng/mL.Elevated NLR: Neutrophil to lymphocyte ratio >3.5.Elevated troponin I >0.05 ng/mL.Elevated BNP ≥ 1000 pg/mL.For the analysis,cardiac dysfunction was def ined as a left ventricular ejection fraction(LVEF) <55% on echocardiogram.
The primary analysis was focused on f inding differences between the different waves.Data analysis was performed in R (https:// www.r-proje ct.org/).Normal distribution was not assumed;the Mann-WhitneyUtest was used to compare continuous variables among two groups,while the Kruskal-Wallis test was used when comparing more than two groups.Pearson’s Chi-square or Fisher’s exact test was performed to compare nominal variables.If a test was signif icant,a post hoc analysis with Bonferroni correction was performed.
Results
From April 2020 to March 2022,109 patients were diagnosed with MIS-C.Five patients were excluded because the f inal diagnosis did not meet the CDC criteria for MIS-C upon adjudication.All cases occurred in patients who had never received a vaccine against SARS-CoV-2.All patients had serologic evidence of previous infection with SARS-CoV-2,mostly by a positive IgG nucleocapsid antibody.Only one case was negative for the nucleocapsid but positive for the anti-spike protein antibody.All patients underwent PCR testing for SARS-CoV-2 on admission,but only 12/104 (12%) had a positive result.
The data from https:// github.com/ ander sen-lab/ HCo-V-19-Genom ics [19,20] included 79,421 SARS-CoV-2 sequences from San Diego County obtained between March 2020 and November 2022.The Nextclade CLI could not align and analyze 559 sequences,which were excluded.Figure 1 shows the proportion of circulating SARS-CoV-2 variants in San Diego County over time with the number of MIS-C cases at RCHSD.Circulating viruses during the f irst MIS-C wave included clades 20A,20B,and 20C.The second wave began with the same viruses but also when clade 20G started circulating,its peak coincided with the circulation of 21C (Epsilon) and faded with the appearance of clades 20I (Alpha,V1) and 20J (Gamma,V3).The third and fourth waves coincided with a predominant circulation of clades 21A,21I and 21J (Delta strains) and clades 21K,21L and 22C (Omicron variants),respectively.MIS-C usually presents 2—6 weeks after COVID infection,and as expected,the peak of waves of MIS-C occurred after the peak of the waves of COVID cases reported in San Diego (Fig.2).Clades 20D,20E (EU1),20H (Beta,V2),21B (Kappa),21D(Eta),and 21G (Lambda) had low circulation (frequency less than 2% per month) during the period of study.

Fig.1 Circulating SARS-CoV-2 variants in San Diego (stacked area) and frequency of MIS-C cases at RCHSD (dark blue columns)

Fig.2 Number of cases of COVID in San Diego (area in light blue) and frequency of MIS-C cases at RCHSD (dark blue columns)
Analysis of MIS-C waves
The demographics,presenting clinical signs,and laboratory values prior to treatment did not differ among the different waves (Table 1).None of the patients had hypertension,diabetes mellitus,congenital heart disease,chronic kidney disease,or autoimmune conditions.Only 6/104 (6%) patients had a history of asthma.The worst values during hospitalization for troponin I,BNP,ferritin,platelet count,or CRP were also not signif icantly different across the waves (Table 2).
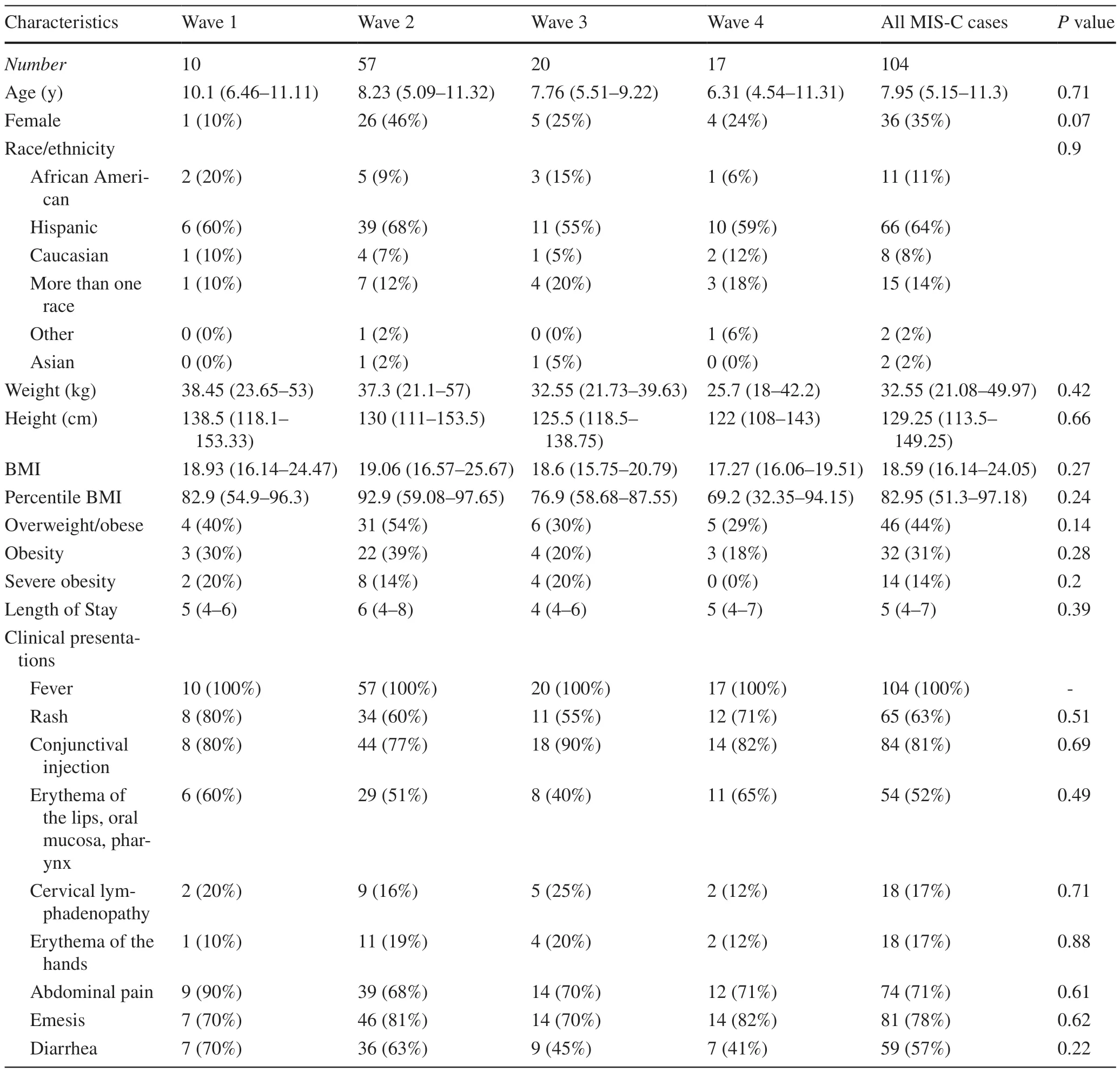
Table 1 Demographic and clinical characteristics of MIS-C patients across the waves

Table 2 Comparison of laboratory data across the four waves
Overall,79/104 (76%) presented with hyponatremia,63/104 (61%) with thrombocytopenia,49/103 (47%) with hypoalbuminemia,and 88/104 (85%) with lymphopenia on admission.Elevated troponin levels were not signif icantly different among the groups on admission or during hospitalization.For all the waves,28/100 (28%) had elevated troponin on admission,but the number increased to 54/104(52%) during hospitalization.Half of the patients (52/102,51%) presented with an elevated BNP (≥ 100 pg/mL),which increased to 85/104 (82%) during the hospital course.A markedly elevated BNP value of ≥ 1000 pg/mL was noted in 11/102 (11%) of patients on admission and increased to 33/104 (32%) during the hospital stay,although the percentage of patients was not different across variant groups.
As a measure of clinical severity,the need for intensive care differed across the waves,with more patients admitted to the PICU and more patients requiring inotropic support during the second wave (Table 3).The median length of stay in the PICU and the need for respiratory support were not signif icantly different among the waves.Only two patients required mechanical ventilation,and none required extracorporeal circulatory support.The median volume of IV f luid boluses administered during the f irst 24 hours of admission was about 20 mL/kg for all waves.There were also no signif icant differences in the use of diuretics.All waves had a similar proportion of patients with cardiac dysfunction at the time of admission.Throughout hospitalization,the second wave had more patients with cardiac dysfunction (61%) than the other waves (35% to 47%).However,no statistical signif icance was reached (P=0.19).The medianZscore for the worst coronary artery measurement was similar among all waves.Most patients presented with either normal coronary artery dimensions (Zscore <2.0,71%) or mild coronary artery dilation (Zscore 2.0—3.0,18%).There were no patients with large or giant coronary aneurysms.The median time from admission to initiation of the IVIG infusion was 9.95 hours,with no signif icant differences across the wave groups.There were no differences in the number of anti-inf lammatory therapies received (Table 3).
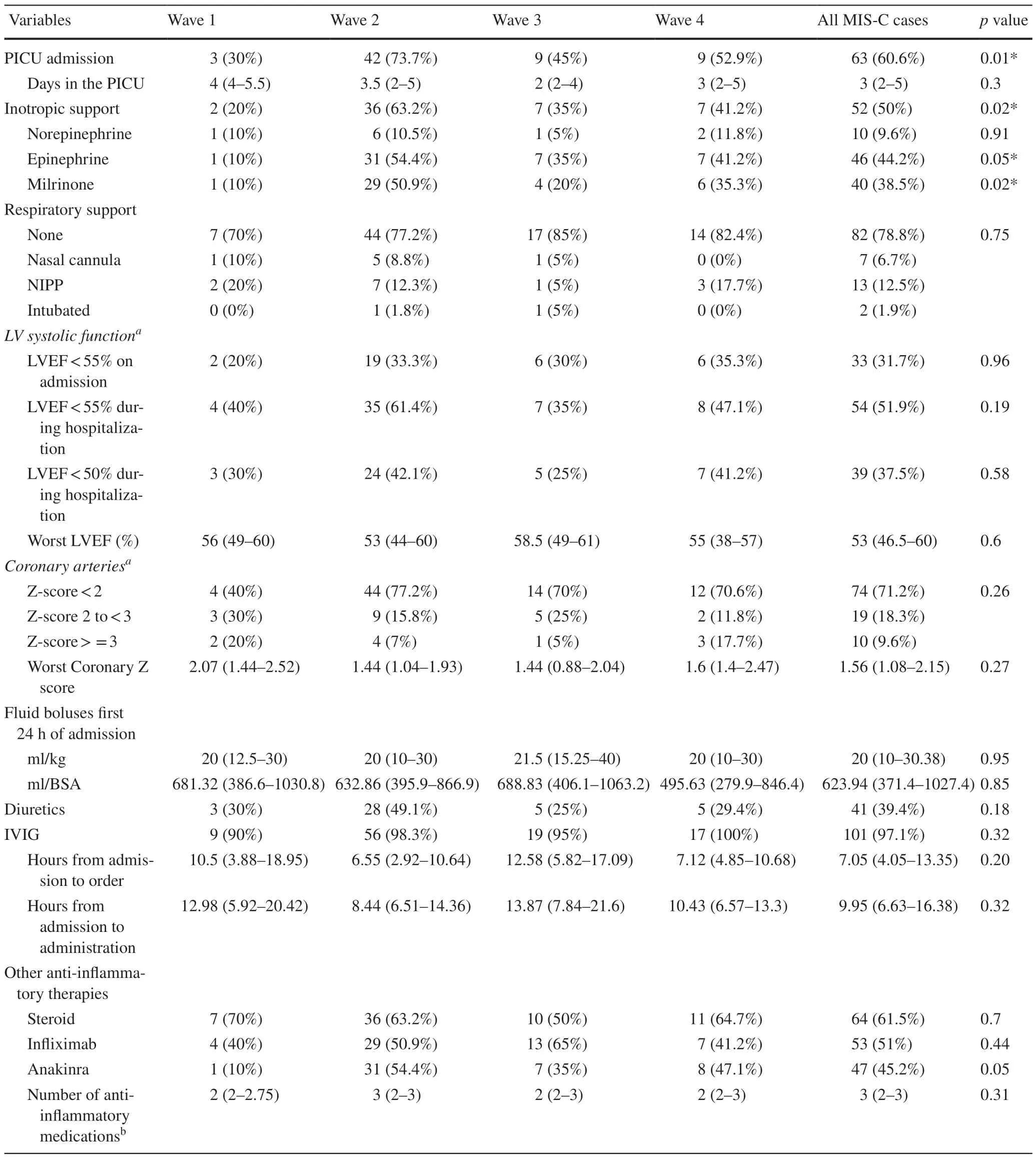
Table 3 Comparison of clinical support,echocardiographic f indings,and management across the four waves
Discussion
SARS-CoV-2 has mutated throughout the pandemic,changing its infectivity and clinical presentation.Little is known about how these mutations affect delayed COVID complications such as MIS-C.We divided the waves based on the local rate of MIS-C cases and utilized local SARS-CoV-2 sequence data to compare MIS-C cases with contemporaneous circulating variants.The San Diego experience showed that patients affected with MIS-C presented initially with similar symptoms and laboratory f indings across all the variant waves.However,the severity of MIS-C differed,with more severe cases presenting when a mixture of variants (clades 20A-C,20G,21C Epsilon,20I Alpha,and 20J Gamma) was circulating.These patients required more admission to the PICU and inotropic support.Different from what has been reported in other studies,the second wave was a combination of several variants.
Raju Abraham et al.reported their experiences in Cape Town,South Africa,with similar wave dates to our cohort[9].They found no signif icant differences across groups in the clinical presentation,laboratory features,or disease course.However,the management was heterogeneous.Levy et al.described the experience in Israel during the most recent three waves [11].They performed a prospective study in 12 Israeli hospitals.The group admitted during the Omicron wave had a shorter length of stay,required fewer vasopressors,had lower NT-proBNP levels,reduced need for mechanical ventilation,and fewer patients with an LVEF of less than 40%.There was also a lower MIS-C incidence rate during the Omicron wave.Ganguly et al.published their experience with the “f irst and second waves” in Eastern India,with the Delta variant causing a more severe presentation with poorer outcomes [10].The management between those waves differed,with fewer patients receiving IVIG therapy during the Delta wave and more receiving steroids.The reported experience in all hospitals in Catalonia,Spain,by Pino et al.,did not demonstrate differences in phenotype and severity throughout the pandemic [13].At the University Children’s Hospital of Cracow in Poland,Ptak et al.did not demonstrate changes in the clinical course of MIS-C [14].However,the authors analyzed the patients with only the“Original/Alpha” and “Delta/Omicron” variants groups.
In the United States,Harahsheh et al.also described their experience with the f irst two waves in Washington,DC,with a more standardized treatment [7].For patients in wave 2,they found a higher proportion of children older than 15 years,higher median troponin I and BNP values,greater need for vasopressors and anti-inf lammatory therapies,and a higher rate of admission to the PICU.Despite these differences,systolic function,coronaryZscores,and length of stay were similar.All patients were discharged home at a median of 11 days.Jain et al.reported the experience in
Houston,Texas [8].They merged the f irst two waves (original and alpha variant cohorts) and compared them against the third wave (delta variant cohort).In the original/alpha cohort,there were more males,more respiratory and musculoskeletal symptoms on presentation,longer PICU stays,and a greater need for mechanical ventilation/ECMO/LV assist devices.The groups also differed in median values for INR,PT,WBC,sodium,phosphorus,and potassium.Miller et al.analyzed data from local,state,and territorial health departments reporting cases across the United States [5,6].The classif ication of the waves was based on the trends in MIS-C cases but not on the circulating variants.They noted a trend toward decreased MIS-C severity over time,including less cardiac dysfunction,shorter hospital and PICU stays,and decreased case fatality.Last,Laird-Gion et al.published data from Boston,Massachusetts,sorting the patients into three cohorts (“Alpha”,“Delta”,and “Omicron”) based on national and regional data of variant prevalence [12].More patients had a documented history of COVID-19 infection in the two months before presenting with MIS-C in the “Omicron” cohort than in the “Alpha” cohort.Except for the lowest counts of platelets and absolute neutrophils during“Omicron”,there were no differences in other laboratories and markers of clinical severity (ICU admission,length of stay,use of inotropes,LV dysfunction) across the different variants.
SARS-CoV-2 spread worldwide,and acute infection occurred at different times in different locations.Some variants were more prevalent only in certain regions.These factors might explain part of the differences noted in the reported studies.However,the def inition and analysis of waves,groups,and cohorts have not been standardized among those studies (Table 4).

Table 4 Comparison of periods used in studies evaluating differences across MIS-C waves
Compared with the experience in other centers,none of our patients required ECMO or ventricular assist devices, and only two needed mechanical ventilation.One required intubation for a sedated procedure;the other was transferred from another institution after receiving extensive volume resuscitation and presented with pulmonary edema and cardiogenic shock.Our approach was to initiate IVIG therapy as quickly as possible and avoid excessive f luid resuscitation in the f irst 24 h.We also report a shorter hospital length of stay than other single-center studies and more aligned with the US national surveillance data.After the wave related to Omicron clades 21K and L,we saw only rare sporadic cases of MIS-C.This observation suggests that as the virus becomes endemic and variants cause milder symptoms with initial infection,coupled with the increasing vaccination rate among children and large percentage of previously infected children,perhaps MIS-C will become less prevalent.
Strengths and limitations
A single team with extensive experience in Kawasaki disease guided the management of MIS-C patients,allowing rapid diagnosis and standardized management of children early in the pandemic.The analysis was based on sequencing of variants in the same community as the patients,which allowed a detailed analysis of the clinical impact of each variant wave.All patients had serologic evidence of previous COVID-19 infection.However,we could not attribute specif ic SARS-CoV-2 genomic information to each patient.As the samples were not obtained randomly,there is a possibility of bias.This is a single-center study,with data collected from the electronic medical record and all the limitations associated with a retrospective study.The low number of patients in some of the waves limited the power of the statistical analysis.
In conclusion,this study suggests that various strains of SARS-CoV-2 trigger MIS-C with differing severities.The second wave had several circulating clades in San Diego: 20A to C,20G,21C (Epsilon),20I (Alpha),and 20J (Gamma).Those patients had a more severe clinical course than patients in other waves.Overall,patients in this series did well.Judicious f luid management and prompt initiation of IVIG therapy might have contributed to the shorter length of stay and less need for mechanical cardiovascular and invasive respiratory support reported in this study.Whether the differences in disease severity across variants were due to changes in the virus or other factors,including previous infection,remains unknown.
Author contributionsKD: conceptualization,methodology,investigation,Writing—review and editing,and supervision.JB,AT: conceptualization,investigation,and writing—review and editing.AMR: methodology,investigation,formal analysis,writing—original draft,review and editing,and visualization.EB: data curation.HH: investigation.All authors approved the f inal manuscript as submitted and agreed to be accountable for all aspects of the work.
FundingNo funding was secured for this study.
Data availabilityThe COVID-19 epidemiologic data for San Diego County is publicly available from https:// searc hcovid.info/ dashb oards/epide miolo gy/ and https:// outbr eak.info .The local genomic data is also publicly available at https:// github.com/ ander sen-lab/ HCo-V-19-Genom ics.These databases are updated regularly.The datasets containing specif ic patient information are in a secured data storage system(Research Electronic Data Capture,REDCap) managed by the Kawasaki Disease Research Center,La Jolla,CA,USA.They are not openly available as they contain protected health information.De-identif ied records might be available from the corresponding author upon reasonable request.
Declarations
Conflict of interestNo f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethics approvalThe Institutional Review Board at the University of California,San Diego,approved prospective data collection,and parents and participants gave signed informed consent or assent as appropriate.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article's Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
 World Journal of Pediatrics2024年2期
World Journal of Pediatrics2024年2期
- World Journal of Pediatrics的其它文章
- Growth and development of children in China: achievements,problems and prospects
- Childhood sleep: assessments,risk factors,and potential mechanisms
- Childhood sleep: physical,cognitive,and behavioral consequences and implications
- Risk factors for long COVID in children and adolescents: a systematic review and meta-analysis
- NLRP3 activation in macrophages promotes acute intestinal injury in neonatal necrotizing enterocolitis
- Exploration of pathogenic microorganism within the small intestine of necrotizing enterocolitis
