Fabrication and Thermal Conductivity Improvement of Novel Composite Adsorbents adding with Nanoparticles
WU Qibai, YU Xiaofen, ZHANG Haiyan, , CHEN Yiming, LIU Liying, XIE Xialin, TANG Ke, LU Yiji, WANG Yaodong, , and ROSKILLY Anthony Paul
?
Fabrication and Thermal Conductivity Improvement of Novel Composite Adsorbents adding with Nanoparticles
WU Qibai1, 2, YU Xiaofen1, ZHANG Haiyan1,*, CHEN Yiming1, LIU Liying1, XIE Xialin2, TANG Ke2, LU Yiji2, WANG Yaodong2,*, and ROSKILLY Anthony Paul2
1 School of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China;2 Sir Joseph Swan Centre for Energy Research, Newcastle University, Newcastle NE1 7RU, UK
Thermal conductivity is one of key parameters of adsorbents, which will affect the overall system performance of adsorption chiller. To improve adsorbent’s thermal conductivity is always one of research focuses in chemisorption field. A new chemical composite adsorbent is fabricated by adding carbon coated metal(Aluminum and Nickel) nanoparticles with three different addition amounts into the mixture of chloride salts and natural expanded graphite aiming to improve the thermal conductivity. The preparation processes and its thermal conductivity of this novel composite adsorbent are reported and summarized. Experimental results indicate that the nanoparticles are homogenously dispersed in the composite adsorbent by applying the reported preparation processes. The thermal conductivity of the composite adsorbent can averagely enlarge by 20% when the weight ratio of the added nanoparticles is 10 wt%. Moreover, carbon coated aluminum nanoparticles exhibit more effective enlargement in thermal conductivity than nickel nanoparticles. As for the composite adsorbent of CaCl2-NEG, there is a big reinforcement from 30% to 50% for Al@C nanoparticles, however only 10% in maximum caused by Ni@C nanoparticles. The proposed research provides a methodology to design and prepare thermal conductive chemical composite adsorbent.
thermal conductivity, carbon coated metal nanoparticles, chemical composite adsorbent
1 Introduction
As an effective means of saving energy, utilization of low grade thermal energy such as solar energy, industrial waste heat and geothermal energy attracts more and more attentions because of concerns on climate change and global warming. Chemisorption is one of attractive chemical processes, which can be widely used in different areas such as heat pump, energy storage, adsorption refrigeration, and combined power and cooling systems[1–6]. Inorganic salts adsorbing ammonia by solid-gas reaction has obtained ever-increasing attentions because of the high flexibility in the application of different temperature heat sources, especially low-grade thermal energy[7–9]. However, during adsorption and desorption process, the salts tend to swelling and caking due to the chemical reaction which hinder mass and heat transfer, leading to low system working efficiency. Introducing porous matrix materials in chloride salts to make the composite adsorbent is an effective method. TOKAREV, et al studied three porous matrices including expanded vermiculite, γ-alumina and carbon sibunit with barium chloride, and Active Carbon Fiber and calcium chloride composite as new type of composite ammonia sorbents[9–11]. A composite adsorbent composed of BaCl2impregnated into expanded vermiculite could provide effective operation of the chiller using a low potential heat source(80–90℃) giving COP(coefficient of performance) as high as around 0.54. The cooling performance of a consolidated composite reactive bed consist of expanded graphite and CaCl2was experimentally assessed by WANG, et al, and CaCl2/activated carbon compound adsorbent was studied as well[12–13]. BU, et al reported that the mass transfer is enhanced by carbonizing and activating the sawdust and heat transfer is increased with adding of expanded graphite[14].
The heat and mass transfer performance play essential roles in the adsorption/desorption process. Faster the heat and mass transfer rate, shorter the cycle time, higher the system efficiency. Normally, the thermal conductivity could represent the heat transfer performance. The thermal conductivities of some carbon materials were measured by WANG, et al, which were compacted expanded natural graphite with different bulk density, activated carbon and expanded natural graphite or graphite after sulphuric acid treatment with different composite ratio[15–17]. The thermal conductivity measurement results for different compound adsorbents including seven kinds of chloride salts and sodium bromide with expanded natural graphite as the matrix indicate that the salt ratio and the consolidated density of the compound adsorbent affect the thermal conductivity of ammoniated salts strongly[18–19].
Recently many researches focused on improving heat transfer performance of composites with carbon nanomaterials embedded in because of their excellent thermal property. Some reports reported on the enhancement of thermal conductivity of polymer composites, such as multi-walled carbon nanotubes/EPDM rubber, carbon nanotubes/reinforced syntactic foam, graphite nanoplatelets/silicone resin, reduced graphene oxide/PVDF-HFP polymer, and carbon coated copper nanoparticles/silica gel composites[20–24]. However, a few studies on improvement of heat transfer characteristic of composite sorbents using carbon nanomaterials. The ammonia adsorption and thermal transfer performance of pure multi-walled carbon nanotubes and the composite adsorbent made of CaCl2and carbon nanotubes have been studied. The research results show that although the pure MWCNT is not appropriate to be adsorbent for the solid-gas adsorption refrigeration due to its low adsorption capacity, it can be used as additive to some other chemical adsorbents to improve their heat transfer characteristics[25–26]. While carbon nanotubes are usually curved, aggregated and entangled together, it is not easy to be dispersed homogeneously. It seems carbon coated metal/alloy nanoparticles with core-shell structure have more advantages relatively. Carbon coatings can protect metal core in ambient conditions, maintaining excellent thermal properties, and offering an economically attractive route to broader use of metal nanoparticles[24, 27–29]. The granule shape in nanometer size can be more easily to spread and occupy a little space in the adsorbent, therefore should void causing greater obstruction of mass transfer. From this point, carbon coated metal nanoparticles are expected to become a potential effective additive for adsorbent.
On the other hand, materials are the basis for all industries. With industrial development, especially the rise of Industry 4.0, manufacturing technologies for manufacturing innovation and new materials to improve the performance and efficiency of existing machine become more and more important. In this paper, a new composite adsorbent was designed and fabricated to enhance thermal transfer. Natural expanded graphite(NEG)was selected to be the host matrix for adsorbents as NEG has been known as one of the important materials for improvement of heat and mass transfer performance and reduction of agglomeration and swelling[15, 17–19]. Core-shell-structured nanoparticles with metal core and carbon shell, as additive, were mixed with metal chloride salts and natural expanded graphite to make a new composite adsorbent. The distribution of nanoparticles in the adsorbents was characterized and the enhancement of the thermal conductivity of the composite adsorbents mixing with nanoparticles was studied in detail.
2 Experimental Tests
Normally copper is one of the best heat conductive metals. However, copper could react with ammonia, it is not suitable to use in the sorbents for ammonia adsorption and desorption process. Sotwo kinds of core-shell-structured nanoparticles were selected, one was carbon-coated aluminum(Al@C) nanoparticle and the other was with a nickel core encapsulated in a carbon shell(Ni@C). To prepare composite adsorbent, as shown in Fig. 1, first a small certain amount of nanoparticles was dispersed in ethanol solution using ultrasonic bath for 30 min in order to obtain homogenous suspension. Then natural expanded graphite and metal chloride(Calcium chloride or Strontium chloride) with weight ratio 1:2 was added in the suspension for further mixing under ultrasonic treatment for another 30 minutes. The whole mixture suspension was heated to remove the solvent and dried in oven at 120℃ for over 24 h to 48 h to generate mixture powders. Stirring regularly during dry process is necessary in order to avoid caking caused by chloride salt precipitation. The microstructure of nanoparticles and their distribution in the composite adsorbent powders were examined by Transmission electron microscopy (TEM, JEOL JEM-2010HR), Energy Dispersive X-Ray Spectrometer(EDX, Bruker Nano) and Scanning Electron Microscope(SEM, Hitachi TM3030).
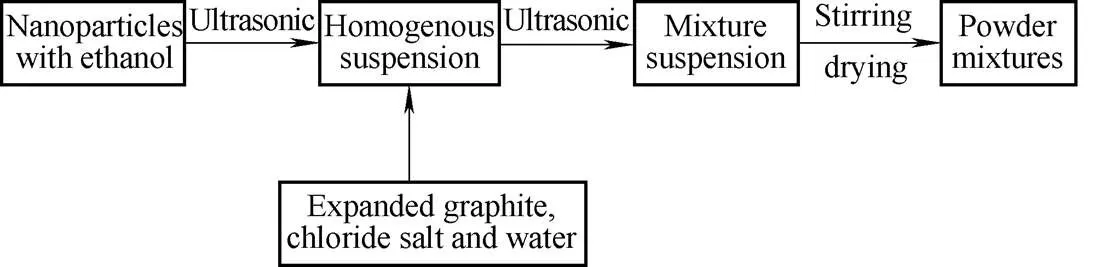
Fig. 1. Schematic diagram of fabrication process of composite adsorbents
The thermal conductivity() of the composites was measured using thermal constants Analyzer (Hotdisk TPS 500S), based on the Transient Plane Source(TPS) technique. Two disks made of the mixture powders for measurement were prepared by compressing with 12.9 mm in diameter and about 5mm in height. The bulk density of the disk is around 2200 kg/m3by calculation using its weight and volume.
3 Results and Discussion
The morphologies and chemical composition of Ni@C and Al@C nanoparticles are shown in Fig. 2, which present the core-shell structure clearly. As observed in the TEM and high-resolution TEM image of Ni@C(Figs. 2(a)–(b)), the core-shell structure particles are quasi-spherical with diameter ranging from 30 nm to 100 nm. Fig. 2(b) also reveals that the core is crystalline, having a calculated lattice spacing of 0.175 nm, which consistent with the interlayer spacing corresponding the (2 0 0) plane of nickel. The ordered layers surrounding the Ni core with the calculated inter-planar distance about 0.357 nm, which corresponds to the d-spacing of the (0 0 2) basal planes in graphite, showing the outer layer is carbon. However, the distance is quite broad compare to the value of (0 0 2) plane of bulk graphite from PDF card No. 46-0945, which is 0.335 nm, indicating that the degree of graphitization is low. It is suggested that the carbon shell of the nanoparticles consisted of both ordered graphitic layers and amorphous carbon. EDX result shows strong Ni element peaks, further indicating the Ni@C nanoparticles contain Nickel element (Fig. 2(c)). Copper signal is come from the copper mesh as sample holder in TEM observation. The encapsulated structures of Al@C nanoparticles are clearly seen in Figs. 2(d)–(e), where cores with spherical shape and diameter between 50nm and 100 nm are dominant. The TEM images reveal that the aluminum cores are completely surrounded by carbon shells with thickness of only 2 nm to 4 nm, including approximately 6 to 11 ordered graphitic layers. EDX result of Al@C nanoparticles shows a strong Al element peak indicating aluminum element as well(seen Fig. 2(f)). Core-shell structure of the nanoparticles with metallic core and carbon shell could shield metal core from environmental degradation during ammonia adsorption/ desorption process because carbon materials are stable even in harsh conditions, providing effective enhancement of thermal conductivity in a long-term.
In order to evaluate the mixing uniformity of the composite adsorbent powders, SEM and EDX characterization was used with elemental mapping technique. Figs. 3(b) and 3(c) are the nickel and carbon elemental mapping results respectively, corresponding to the observed area of the mixture powder shown in Fig. 3(a). It could be seen clearly that the distribution of nickel element is quite uniform as light spot representing the specific element spreads in the whole area with mainly the same brightness. Comparing Figs. 3(b) and 3(e) can be found that the spot density of nickel element become much intensive while the addition concentration of nanoparticles increasing from 2 wt% to 10 wt%. The nanoparticles distribute more sparsely at lower adding concentration and show dense distribution at higher concentration. While the density of bright spots, representative of carbon element, exhibit almost the same brightness. As shown in Fig. 3(d), there are some brighter spot on the surface of the expanded natural graphite. And higher concentration of Ni element could be viewed at the same position in Fig. 3(e) (white circle); however, there is no obvious difference of distribution for carbon element, as shown in Fig.3(f), indicating the agglomeration of nanoparticles still exists at certain degree, especially at higher adding concentration. These observation results reveal the relative homogeneously distribution of the nanoparticles in the composite adsorbent powders, which also means the preparation method used is effective. As nanoparticles are easily tending to agglomerate, it is essential to be dispersed thoroughly as far as possible during the fabrication process. Thence the first step of fabrication process is to make nanoparticles dispersed uniformly into the ethanol solution by ultrasonic treatment, as it is an efficient method fordispersion of nanoparticles. Then the ethanol suspension was mixed with other materials. The characterization experimental results indicate a new chemical composite adsorbent within uniformly dispersed nanoparticles could be prepared successfully through the fabrication process described earlier(see Fig. 1).

Fig. 2. TEM observation results of Ni@C and Al@C nanoparticles
(a) SEM image (b) Ni element (c) C element
(d) SEM image (e) Ni element (f) C element
Fig. 3. Morphology and elemental mapping images of powders mixing with 2 and 10 wt. % of Ni@C nanoparticles respectively
As during adsorption/desorption process, the heat and mass transfer performance are two key and contradictory factors. High density of adsorbents may improve heat transmission, but could block adsorbate molecules pass through. So in this case, a small amount of carbon coated metal nanoparticles was considered to add in the composite sorbents, that is, the weight fraction concentrations of nanoparticles corresponding to the Natural Expanded Graphite(NEG) were selected as 0 (without adding nanoparticles), 2%, 5%, and 10% respectively. Thermal conductivity measurement results show clearly that adding carbon coated metal nanoparticles in the adsorbents could improve its heat transfer performance to a certain extent, as shown in Figs. 4(a) and 4(b). Both Ni@C and Al@C nanoparticles can enhance the thermal conductivity of the composite adsorbents consisting of NEG and calcium chloride or strontium chloride. The thermal conductivity is 6.84 W/(mK) for SrCl2-NEG-5 wt% Ni@C, and it reaches 7.39 W/(mK) for the sample of SrCl2-NEG-5wt% Al@C while the sample without adding nanoparticles is 6.23 W/(mK). It is could be seen clearly that the value of thermal conductivity increases along with the ratio of nanoparticle’s increasing. For example, the thermal conductivity enhancement is about 4% when Ni@C nanoparticles concentration is 2 wt% and it rise to slightly more than 10% when Ni@C nanoparticles concentration reaches to 10 wt% for the adsorbent mixtures of SrCl2and NEG. Measurement results of other samples are in the similar way. The enhancement is about 8% for adding Al@C nanoparticles at 2 wt. % and it over 20% at 10 wt% of the amount. The enhancement caused by adding of Ni@C is quite close in both kinds of sorbents, i.e. NEG mixed with CaCl2and with SrCl2as well. It is also obvious that Al@C nanoparticles play a greater role than Ni@C in the thermal conductivity enhancement. Comparing with the measurement results obtained from the composite of SrCl2- NEGmixing with two kinds of nanoparticles, the thermal conductivity enhancement of Ni@C is in the range of 4%–12%, while the Al@C nanoparticles can achieve 8 to 22% growth in thermal conductivity. As for the composite adsorbent of CaCl2-NEG, there is a big reinforcement from 30% to 50% for Al@C nanoparticles, however only 10% in maximum caused by Ni@C nanoparticles. These experimental results reveal that adding carbon coated metal nanoparticles can improve the thermal conductivity of the composite adsorbents effectively. The enhancement could reach 10%–20% in average with addition amount of 5–10wt%. It also indicates clearly that Al@C nanoparticles could enlarge the thermal conductivity more significantly. The maximum value of enhancement could reach 50% when Al@C nanoparticles were added in the mixture of calcium chloride and NEG with 10 wt% of amount. It seems reasonable because the thermal conductivity of aluminum is more than twice as of nickel, which resulting in different enhancement at the same addition amount of nanoparticles. On the other hand, with the increase of the amount of nanoparticles added, more and more nanoparticles are distributed uniformly on the surface of the expanded graphite granules, or maybe between the graphite sheets, mixed with chloride salt particles. It may increase the number of heat transfer channels compared with the mixture of graphite and chloride salt only, therefore improve the thermal conductivity.

Fig. 4. Thermal conductivity properties of the composite adsorbents vs. nanoparticles weight fraction concentrations
According to the above experiment results, a new type of chemical composite adsorbents with uniformly dispersed nanoparticles (chloride salt-NEG-carbon coated metal nanoparticles) are fabricated. Introducing carbon coated metal nanoparticles into adsorbents has been proved to be a successfully approach to improve its thermal conductivity. Manufacturing technologies play an important role in the new era of Industrial 4.0 and the application of nanotechnology is one of its important direction of development. The enhancement of thermal conductivities of the novel composite adsorbents by adding nanoparticles will lead to performance improvement of the machine using this kind of adsorbents, and then will result in increasing the overall efficiency of the system.
4 Conclusions
(1) A novel chemical composite adsorbent has been fabricated with carbon coated metal nanoparticles dispersinghomogenously in the mixture of CaCl2-NEG and SrCl2-NEG respectively.
(2)The enhancement of thermal conductivity of this novel chemical composite adsorbent could reach about 10%–20% with addition amount of nanoparticles between 5–10 wt%.
(3) Carbon coated aluminum nanoparticles exhibit more effective enlargement in thermal conductivity than carbon coated nickel nanoparticles. The maximum value of enhancement could reach 50% with the composite adsorbent of CaCl2-NEG-10 wt% Al@C.
(4) The novel developed chemical composite adsorbents adding with carbon coated metal nanoparticles could potentially be used in adsorption systems or chemical thermal energy systems to improve the overall system performance.
[1] PESARAN A, LEE H, HWANG Y, et al. Review article: Numerical simulation of adsorption heat pumps[J]., 2016, 100: 310–320.
[2] LU Y, WANG Y, BAO H, et al. Analysis of an optimal resorption cogeneration using mass and heat recovery processes[J]., 2015, 160: 892–901.
[3] CHOUDHURY B, SAHA B B, CHATTERJEE P K, et al. An overview of developments in adsorption refrigeration systems towards a sustainable way of cooling[J]., 2013, 104: 554–567.
[4] XU S Z, WANG L Y, WANG R U. Thermodynamic analysis of single-stage and multi-stage adsorption refrigeration cycles with activated carbon-ammonia working pair[J]., 2016, 117: 31–42.
[5] WANG L, ZIEGLER F, ROSKILLY A P, et al. A resorption cycle for the cogeneration of electricity and refrigeration[J]., 2013, 106: 56–64.
[6] BOUZEFFOUR F, KHELIDJ B, ABBES M T, et al. Experimental investigation of a solar adsorption refrigeration system working with silicagel/water pair: A case study for Bou-Ismail solar data[J]., 2016, 131: 165–175.
[7] GAO P, WANG L Y, WANG R Z, et al. Experimental investigation of a MnCl2/CaCl2-NH3two-stage solid sorption freezing system for a refrigerated truck[J]., 2016, 103: 16–26.
[8] AMMITZBLL A L, LYSGAARD S, KLUKOWSKA A, et al. Surface adsorption in strontium chloride ammines[J].. 2013, 138:164701.
[9] VESELOVSKAYA J V, TOKAREV M M, ARISTOV Y I. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation 1. Barium chloride in various matrices[J]., 2010, 30: 584–589.
[10] VESELOVSKAYA J V, CRITOPH R E, THORPE R N, et al. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation: 3. Testing of “BaCl2/vermiculite” composite in a lab-scale adsorption chiller[J]., 2010, 30: 1188–1192.
[11] TOKAREV M M, VESELOVSKAYA J V, YANAGYI H, et al. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation 2. Calcium chloride in ACF felt[J]., 2010, 30: 845–849.
[12] OLIVEIRA R G, WANG R Z, WANG C. Evaluation of the cooling performance of a consolidated expanded graphite calcium chloride reactive bed for chemisorption icemaker[J]., 2007, 30: 103–112.
[13] LU Z S, WANG R Z, LI T X, et al. Experimental investigation of a novel multifunction heat pipe solid sorption icemaker for fishing boats using CaCl2/activated carbon compound-ammonia[J]., 2007, 30: 76–85.
[14] BU X B, LU Z N, WANG L B. Preparation of composite adsorbent with high performance of heat and mass transfer[J]., 2013, 58(30): 3709–3714.
[15] WANG L W, TAMAINOT-TELTO Z, METCALF S J, et al. Anisotropic thermal conductivity and permeability of compacted expanded natural graphite[J]., 2010, 30: 1805–1811.
[16] WANG L W, TAMAINOT-TELTO Z R, THORPE R, et al. Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration[J]., 2011, 36: 2062–2066.
[17] WANG L W, METCALF S J, CRITOPH R E, et al. Two types of natural graphite host matrix for composite activated carbon adsorbents[J]., 2013, 50: 1652–1657.
[18] TIAN B, JIN Z Q, WANG L W, et al. Permeability and thermal conductivity of compact chemical and physical adsorbents with expanded natural graphite as host matrix[J]., 2012, 55: 4453–4459.
[19] JIANG L, WANG L W, JIN Z Q, et al. Effective thermal conductivity and permeability of compact compound ammoniated salts in the adsorption/desorption process[J]., 2013, 71: 103–110.
[20] ZHANG X, ZHANG H, LIN J, et al. Thermal conductivity and thermal stability enhancement of ethylene propylene diene methylene with carbon nanotube[J]., 2014, 33(8): 767–774.
[21] BHAT P, ZEGEYE E, GHAMSARI A K, et al. Improved thermal conductivity in carbon nanotubes-reinforced syntactic foam achieved by a new dispersing technique[J]., 2015, 67(12): 2848–2854.
[22] LIN J, ZHANG H, TANG M, et al. Improved thermal property of a multilayered graphite nanoplatelets filled silicone resin composite[J].2015, 24: 920– 929.
[23] KUMAR P, YU S, SHAHZAD F, et al. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides[J].2016, 101: 120–128.
[24] LIN J, ZHANG H, HONG H, et al. A Thermally conductive composite with a silica gel matrix and carbon-encapsulated copper nanoparticles as filler[J]., 2014, 43: 2759–2769.
[25] YAN T, LI T X, LI H, et al. Experimental study of the ammonia adsorption characteristics on the composite sorbent of CaCl2and multi-walled carbon nanotubes[J]., 2014, 46: 165–172.
[26] YAN T, LI T X, WANG R Z, et al. Experimental investigation on the ammonia adsorption and heat transfer characteristics of the packed multi-walled carbon nanotubes[J]., 2015, 77: 20–29.
[27] ZHANG H, WU Q, LIN J, et al. Thermal conductivity of polyethylene glycol nanofluids containing carbon coated metal nanoparticles[J]., 2010, 108: 124304.
[28] VALES-PINZON C, MEDINA-ESQUIVEL R A, ORDONEZ- MIRANDA J, et al. Thermal transfer in mixtures of ethylene glycol with carbon coated iron nanoparticles under the influence of a uniform magnetic field[J]., 2015, 643: S71–S74.
[29] WU Q, ZHANG H, CHEN M, et al. Preparation of carbon-coated iron nanofluid and its application in radiofrequency ablation[J]., 2015, 103(4): 908–914.
Biographical notes
WU Qibai, born in 1968, is currently an associate professor at. Her research interests include nanomaterials and its application.
E-mail: wuqb@gdut.edu.cn
YU Xiaofen, born in 1993, is currently a master candidate at.
E-mail: 2536956558@qq.com
ZHANG Haiyan, born in 1957, is currently a professor and a PhD candidate supervisor at. Her research interests include novel carbon nanomaterials and their application in energy field.
E-mail: hyzhang@gdut.edu.cn
CHEN Yiming, born in 1977, is currently a lecturer at. His research interests include carbon nanomaterials and super capacitor.
E-mail: chenym@gdut.edu.cn
LIU Liying, born in 1975, is currently a lecturer at. Her research interests include materials and properties for lithium ion batteries.
E-mail: liyingliusy@l63.com
XIE Xialin, born in 1990, is currently a master of philosophy candidate at.
E-mail: X.Xie9@newcastle.ac.uk
TANG Ke, born in 1994, is currently a PhD candidate at. Her research interests include chemisorption and energy storage.
E-mail: K.Tang4@newcastle.ac.uk
LU Yiji, born in 1989, is currently a research associate at. His research interests include chemisorption and energy storage.
E-mail: y.j.lu@ncl.ac.uk
WANG Yaodong, born in 1956, is currently a senior lecturer and a PhD candidate supervisor at.His research interests include renewable energy systems, Low-grade heat driven absorption refrigeration and Low-grade heat driven cogeneration of power and cooling.
Tel: +44-191-2084934; E-mail: yaodong.wang@newcastle.ac.uk
ROSKILLY Anthony Paul, born in 1962, is currently a professor and a PhD candidate supervisor atHis main research interests include CHP, Trigeneration & Energy Storage and Renewable thermal energy system design.
E-mail: tony.roskilly@ncl.ac.uk
Supported by National Natural Science Foundation of China(No. 51276044), Science and Technology Planning Project of Guangdong Province, China(Grant Nos. 2015A050502047, 2015B010135011), Science and Technology Planning Project of Guangzhou City, China (Grant Nos. 201508030018, 2016201604030040), Youth Foundation of Guangdong University of Technology, China(Grant No. 252151038), EPSRC Grants(Grant Nos. EP/I027904/1, EP/K004689/1, EP/M008088/1), and IChemE Global Awards 2015: MCSA for FP&VA
? Chinese Mechanical Engineering Society and Springer-Verlag Berlin Heidelberg 2016
Received May 20, 2016; revised August 8, 2016; accepted August 10, 2016
10.3901/CJME.2016.0810.091, available online at www.springerlink.com; www.cjmenet.com
E-mail: hyzhang@gdut.edu.cn; yaodong.wang@newcastle.ac.uk
 Chinese Journal of Mechanical Engineering2016年6期
Chinese Journal of Mechanical Engineering2016年6期
- Chinese Journal of Mechanical Engineering的其它文章
- Surface Topography and Roughness of High-speed Milled AlMn1Cu
- Integrated Simulation Method for Interaction between Manufacturing Process and Machine Tool
- Engineering the Smart Factory
- Exploring Barriers and Opportunities in Adopting Crowdsourcing Based New Product Development in Manufacturing SMEs
- Development of the Supply Chain Oriented Quality Assurance System for Aerospace Manufacturing SMEs and Its Implementation Perspectives
- Collaborative Simulation Method with Spatiotemporal Synchronization Process Control
