The extraction of aromatics using N-methylpyrrolidone: Liquid-liquid equilibrium determination and mechanism exploration
Yanjing Li, Shilong Dong, Lili Wang, Xiaoyan Sun,, Wenying Zhao, Shuguang Xiang
1 College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
2 College of Chemistry and Chemical Engineering, Qilu Normal University, Jinan 250200, China
Keywords:Extraction Liquid-liquid equilibrium Thermodynamic models Quantum chemistry calculation
ABSTRACT LLE data of cyclooctane/3-methylpentane + benzene/toluene + N-methylpyrrolidone (NMP) at 298.15 K and 313.15 K under a pressure of 101.3 kPa were measured in this work.The Othmer-Tobias and Hand equations were adopted to validate the reliability of LLE data,where the correlation coefficients(R2)were close to unity, indicating the high reliability of the experimental data.The experimental data were analyzed using the distribution coefficient(D)and separation factor(S),and the effect of NMP extracting benzene and toluene from aromatics was explored.Meanwhile, the reason for the different extraction efficiencies of benzene and toluene using NMP was analyzed by quantum chemical calculations.The NRTL and UNIQUAC thermodynamic models were used to correlate the liquid-liquid equilibrium data,and the relevant binary interaction parameters were obtained.The calculated root mean square deviation(RMSD)were all less than 0.0063,indicating that the obtained binary interaction parameters can be used to simulate and calculate the extraction of aromatics using NMP.
1.Introduction
Aromatics are extensively used in synthetic fiber,synthetic rubber, dyes, medicine, spices, pesticides, and many other organic chemicals.They play an important role in developing the national economy and enhancing people’s lives [1,2].However, aromatics often contain many non-aromatic components, and it is difficult to separate using normal distillation.Liquid-liquid extraction technology has been widely applied in aromatic extraction, since the process has large processing capacity and good separation effect[3-7].Sulfolane has high selectivity and it is widely used in the aromatic extraction process, but sulfolane is toxic and harmful to the human body and environment [1,8-11].N-methylpyrrolidone(NMP) can be used as a substitute for sulfolane because of its low toxicity and low cost [12].
Liquid-liquid equilibrium (LLE) data are critical for the simulation and optimization.However, the LLE data of alkanes + aromat ics + NMP only involve n-hexane and n-heptane [13,14].LLE data for 3-methylpentane/cyclooctane + benzene/toluene + NMP are not publicly available.During the aromatics extraction process,these alkanes account for a significant portion of aromatic extraction, and the absence of these LLE data will result in inaccurate simulation results.At the same time,different aromatics structures have different effects on aromatics extraction.Multiwfn is a potential application for conducting electron wave function analysis,which is a key component of quantum chemistry[15].VMD(visual molecular dynamics) is a molecular visualization program [16].The AIM (atoms-in-molecules) topology analysis has been widely used in interaction analysis because of its ability to describe the bonding paths and interactions between molecules.
The LLE data for cyclooctane/3-methylpentane + benzene /toluene + NMP were measured at 313.15 K and 298.15 K under 101.3 kPa.The distribution coefficient (D) and separation factor(S) were calculated to evaluate the extraction of benzene and toluene using NMP.The experimental LLE data were correlated by the NRTL and UNIQUAC models, and the binary interaction parameters were obtained.The Gaussian program was used to optimize molecules, and the AIM (atoms-in-molecules) topology analysis diagram was drawn using Multiwfn and VMD.The effect of aromatics structure on NMP extraction was discussed along with the computation of the topological parameters, molecular dipole moment, induced dipole moment, and MPI (molecular polarity index) value.
2.Materials and Methods
2.1.Chemicals
The names, CAS, purity, and suppliers of the chemicals were listed in Table 1.

Table 1 Experimental chemical reagents details
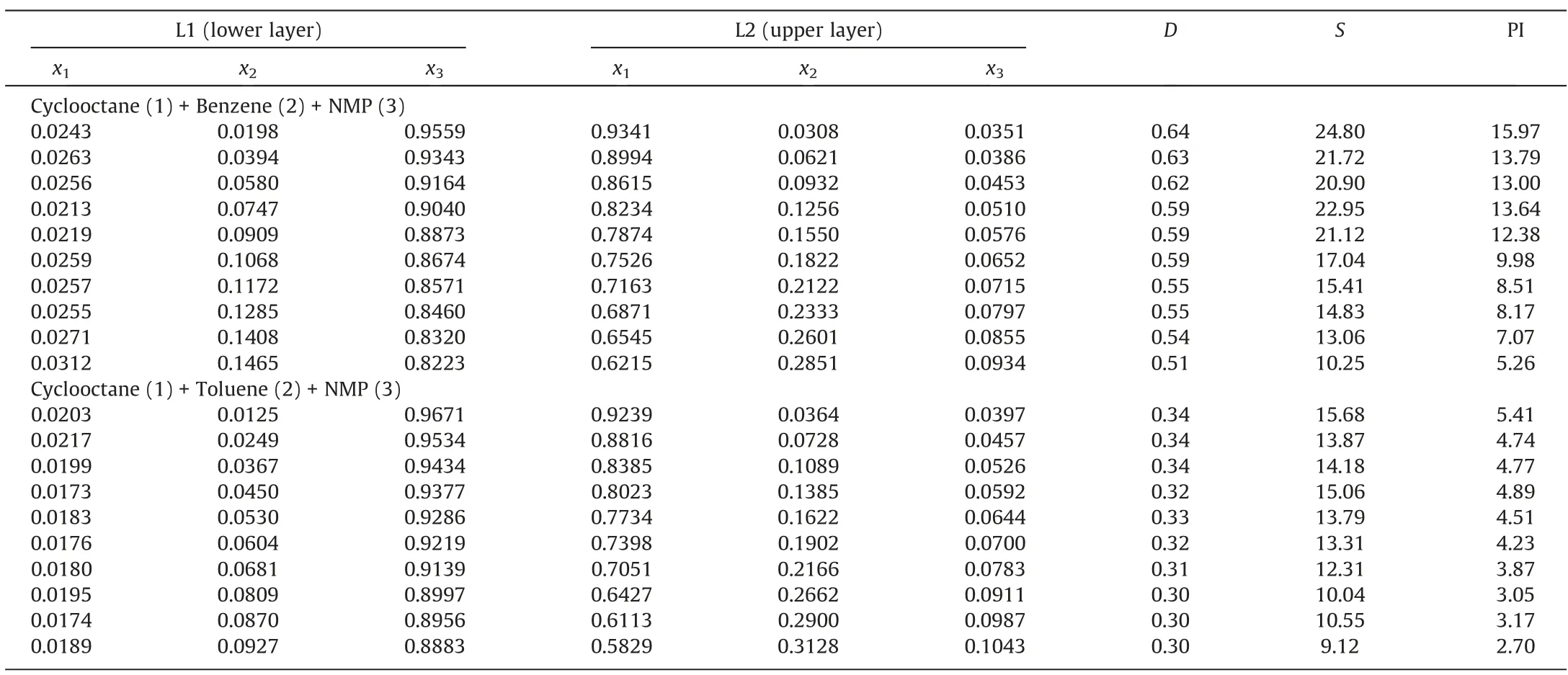
Table 2 Experimental LLE data(mole fraction),distribution coefficient(D),separation factor(S)and performance index(PI)of cyclooctane(1)+benzene/toluene(2)+NMP(3)at 313.15 K under 101.3 kPa①

Table 3 Experimental LLE data (mole fraction),distribution coefficient (D),separation factor (S) and performance index (PI) of 3-methylpentane (1)+ benzene/toluene (2) + NMP (3)at 313.15 K under 101.3 kPa①

Table 5 Experimental LLE data (mole fraction), distribution coefficient (D), separation factor (S) and performance index (PI) of 3-methylpentane (1) + benzene/toluene (2) + NMP (3) at 298.15 K under 101.3 kPa①
2.2.Equipment and procedures
The LLE data were measured using the equilibrium still method at 298.15 K or 313.15 K under a pressure of 101.3 kPa.Among them, the equilibrium still body is a constant temperature jacket structure.The equilibrium still volume is approximately 60 ml with a super constant temperature water bath (Julabo F12-EH)for temperature control.The experimental apparatus is shown in Fig.1.During the experiment,20 ml of pre-prepared NMP solution and 20 ml of alkanes solution were added to the equilibrium still,then aromatic reagents were gradually added.The cooling water condensation was placed on an equilibrium still,the magnetic stirrer was activated, and it was stirred for 30 min at a constant temperature, and then stood for 1.5 h until it was layered under the experimental conditions.The upper and lower equilibrium liquids were collected thrice by a syringe for composition analysis to determine the composition of the two phases.

Fig.1.Schematic diagram of experimental set-up.

Fig.2.Comparison between the experimental values (mole fraction) of aromatics extraction system and the calculated values of NRTL and UNIQUAC models at 298.15 K under 101.3 kPa.
The composition of the two phases were analyzed by an Agilent GC7890A gas chromatograph, and the column was DB-5 capillary(30 m × 0.2 mm).The initial column temperature was 343.15 K,heated to 503.15 K with a heating rate of 20·K min-1, and kept for 2 min.The temperature of the detector was 523.15 K, and the temperature of the vaporization chamber was 493.15 K.In this experiment,the samples were analyzed by the area normalization method.In order to make the response signal generated by the sensor truly reflect the substance content, the quantitative correction factor was introduced.
2.3.Computational process
2.3.1.Data validation
The reliability of LLE data can be examined by the Othmer-Tobias and Hand equations correlation [17,18], and the relevant equations are presented as follows:
where U and L represent the raffinate phase and extract phase,respectively,x is the mole fraction,and subscripts 1,2 and 3 represent alkanes,aromatics and NMP,respectively.Additionally,a,b,c,d are parameters of the Othmer-Tobias and Hand equations.
2.3.2.Extraction capability evaluation
The ability of an extractant can be determined using distribution coefficient(D)and separation factor(S).D denoted the equilibrium distribution of NMP between two phases,S shows the ability of NMP to separate alkanes and aromatics,and performance index(PI) can more intuitively evaluate NMP extraction performance.The calculation formula is as follows [3,19,20]:
where U and L represent the raffinate phase and extract phase,respectively, x represents the mole fraction, subscripts 1, 2 represent alkanes and aromatics.
2.3.3.Quantum chemistry calculation
The mechanism of intermolecular interaction was studied and the experimental results was verified from the aspect of quantum chemistry.The Gaussian 09 software was performed to preliminarily optimize each molecule at the level of ωB97XD/6-31G*, and the optimum position between the two molecules was determined using the semi-empirical method [21,22].The implicit model was introduced at the level of ωB97XD/6-311++G**, each molecule and the binary complex were further optimized geometrically, and the dipole moment and the induced dipole moment of the molecule could be obtained.AIM topological analysis of was carried out by Multiwfn and VMD software[23,24].
Due to the different extraction abilities of extractants for different substances, molecules with similar polarity to extract are easier to be extracted using extractant.The molecular polarity index (MPI) and dipole moment were introduced to investigate why NMP has different extraction capacities for various aromatics.MPI and dipole moment can be used to describe the intensity of molecular polarity, with higher values indicating more significant polarity, the MPI was defined as follows [25,26]:
where V is the molecular electrostatic potential,the integral is integrated over the molecular surface s, and A is the molecular surface area.
2.3.4.Thermodynamic models and their tests
To study liquid-liquid equilibrium,NRTL and UNIQUAC models are typically employed[27-30].Therefore,the NRTL and UNIQUAC models were used to regress experimental data,and binary energy interaction parameters were calculated.The correlation equation of NRTL model [31] is shown in Eqs.(7)-(9):
where aijand bijare the binary interaction parameters calculated by NRTL model, gij-gjjand gji-giirefer to the binary energy interaction parameters calculated.
The correlation equation of the UNIQUAC model [32] is shown in Eqs.(10)-(13):
where aijand bijare the binary interaction parameters calculated by the UNIQUAC model,and uij-ujjand uji-uiirefer to the binary energy interaction parameters calculated.
The root means square deviation (RMSD) were calculated to estimate the difference between the experimental data and the calculated data.The formula is as follows:
where x represents the liquid phase mole fraction,n represents the number of experimental points, a, b, and c represent the component, phase, and linkage, respectively.
3.Results and Discussion
3.1.Liquid-liquid experimental data
The LLE data of cyclooctane/3-methylpentane (1) + benzene/to luene(2)+NMP(3)ternary system at 313.15 K and 298.15 K under 101.3 kPa were measured.The results are shown in Tables 2-5.The ternary phase diagrams are shown in Figs.2 and 3.In this work,the standard uncertainties were calculated based on Eq.(15), and the standard uncertainties of compositions are shown in Table 6 [33].
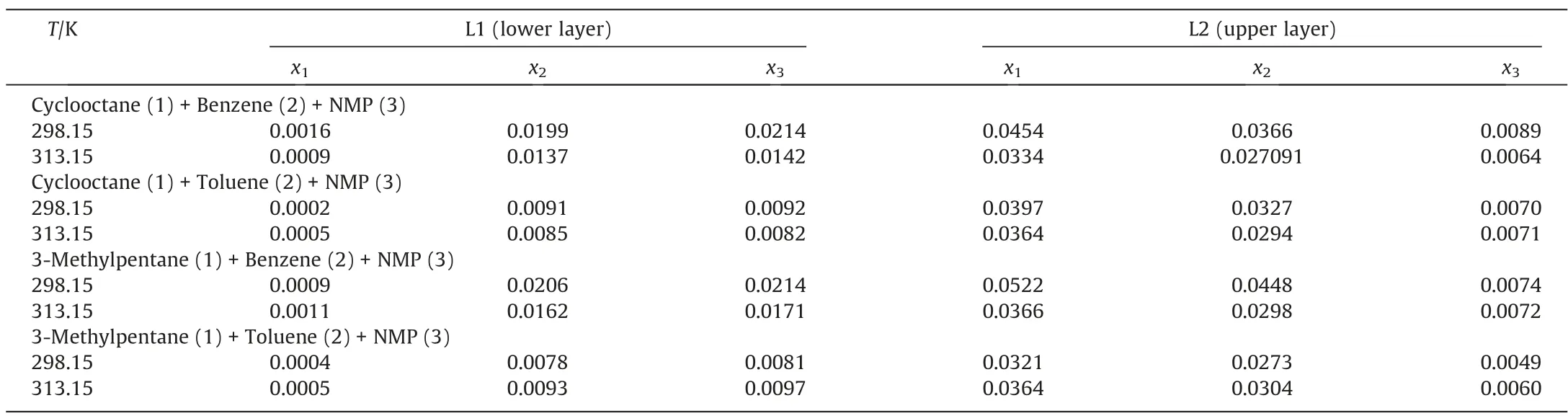
Table 6 The standard uncertainties of compositions for cyclooctane/3-methylpentane (1) + benzene/toluene (2) + NMP (3) ternary systems at 313.15 K and 298.15 K under 101.3 kPa
3.2.Consistency of experimental LLE data
Fig.4 shows the correlation fitting curves for the Othmer-Tobias and Hand equations.Table 7 shows the relevant parameters for two equations and the R2for the different LLE data.The R2values were close to 1, proving the experimental data are consistency.

Fig.4.Hand (a) and Othmer-Tobias (b) equations of ternary system.

Table 7 Constants and related factors of Othmer-Tobias and Hand equations for ternary systems
3.3.Influence of aromatics structure on extraction
According to the LLE data, the extraction capacity of NMP for benzene was superior to that of toluene.To make the extraction capacity of NMP more understandable, the D, S, and PI of NMP in various systems were calculated, and the extraction mechanism of NMP-benzene and NMP-toluene was investigated.
3.3.1.Calculation of extraction capacity of NMP
The calculation results of D, S and PI are shown in Tables 2-5,Fig.5 and Fig.S1.The S values of benzene and toluene were significantly greater than 1,indicating that aromatics and alkanes can be separated effectively using NMP.S of benzene was significantly greater than that of toluene, indicating that benzene was more readily extracted using NMP.In addition, the D of benzene ranged between 0.47 and 0.64 and that of toluene between 0.29 and 0.37,indicating that NMP has a superior extraction capacity for benzene.PI is defined as the product of D and S; the PI of benzene was significantly higher than that of toluene.Therefore, the extraction capacity of NMP for benzene was greater than that of toluene.

Fig.5.Distribution coefficient (D), Separation factor (S) and Performance index (PI) of benzene and toluene (mole fraction) in the extracted phase (L1) at 313.15 K under 101.3 kPa.
3.3.2.Extraction mechanism analysis of NMP
In order to understand effect of different aromatics structure on extraction capacity of NMP,AIM topological analysis of was carried out by Multiwfn and VMD software.AIM topology analysis diagram was obtained, as shown in Fig.6, and its topological parameters were calculated.Fig.6 shows the (3,-1) bond critical points and associated bond paths in NMP-benzene and NMP-toluene dimers.Table 8 shows the electron density (ρ(rc)), laplacian value(?2ρ(rc)), kinetic energy G(r), potential energy V(r) and total energy density H(r) at the (3, -1) bond critical points (BCP).

Fig.6.Optimized structures and graphical illustration of the interaction for dimers (a) benzene-NMP and (b) toluene-NMP.

Table 8 BCP analysis of different complexes
H-bonds are formed when the interaction meets the ρ(rc)range of 0.002 to 0.035 a.u and ?2ρ(rc) range of 0.024 to 0.139 a.u[34,35].As illustrated in Fig.6, there are four BCPs between benzene and NMP and six between toluene and NMP.The respective topological parameter values between bond critical points are listed in Table 8.Through analysis discovered that the ρ(rc)between the two complexes is between 0.002 and 0.035 a.u, but?2ρ(rc) is less than 0.024 a.u.Therefore, NMP primarily interacts with benzene/toluene through van der Waals interactions during the extraction process.
Since the extractant is extracted by the principle of ‘‘like dissolves like” [36,37], molecules with more similar polarity to the extracts are more likely to be dissolved,and NMP is a typical polar extractant.Therefore,the polarity of benzene and toluene was discussed.As shown in Table 9,the dipole moment of benzene was 0 D,and toluene was 0.62 D.However,due to the presence of NMP,the dipole moment of benzene increased from 0 D to 5.61 D,and that of toluene increased from 0.62 D to 5.06 D.This means that NMP could affect the dipole moment of benzene and toluene,benzene was the most affected by the NMP, so benzene was easier to interact with NMP.This phenomenon may be caused by the strong electron cloud in benzene and toluene due to the presence of the benzene ring,while NMP has a strong polarity, which is easy to induce induced dipoles in benzene and toluene.However,methyl has intense electronegativity, which easily affects the electron cloud on the benzene ring so that the interaction between NMP and toluene is weakened,and the induced dipole is smaller than that of benzene.
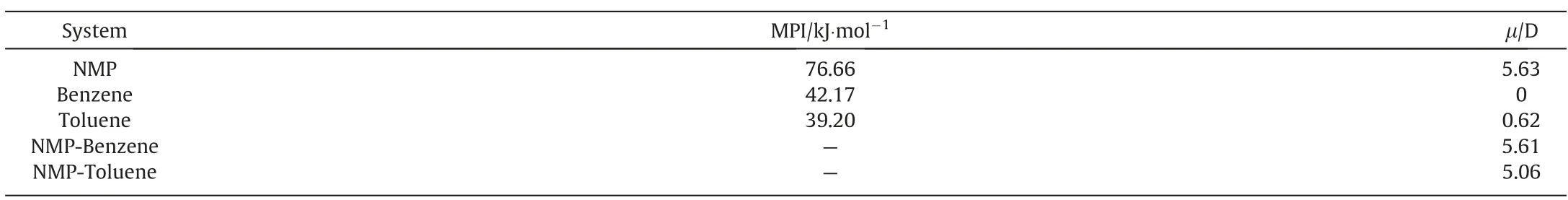
Table 9 Molecular polarity index (MPI) and dipole moment of different substance
Nevertheless, it was not enough to explain molecular polarity only by dipole moment.To further prove that benzene and NMP are more similar in polarity, the molecular polarity index (MPI)was introduced here.Unlike the dipole moment,which only represents the overall molecular polarity, MPI can quantify the local polarity caused by the uneven distribution of ESP,which can better describe the molecular polarity.As shown in Table 9, the MPI of toluene,benzene,and NMP,the order of NMP >benzene >toluene,was consistent with the dipole moment result;it indicates that the polarity of benzene and NMP were more similar.Therefore, benzene was more easily separated during NMP extraction.
It can be seen from Fig.6 that NMP combines with benzene or toluene is in the form of ‘‘plane-to-plane” manner [38], which easily produces steric hindrance and affects the extraction capacity.In the combination process of NMP with benzene and toluene,the presence of side chains in NMP will produce a particular steric hindrance.Likewise, the presence of side-chain methyl groups in toluene will increase the steric hindrance of toluene in the binding process with NMP, thereby affecting the extraction capacity of NMP.Therefore, the combination of toluene and NMP was more difficult, NMP has a greater extraction capacity for benzene.
3.4.Data calculation
The LLE data obtained by the experiments were carried out parameter regression using NRTL and UNIQUAC activity coefficient models.NRTL and UNIQUAC model’s binary energy interaction parameters were calculated.The RMSD between the calculated and experimental values of the two models was obtained.
The ternary phase diagrams of experimental and calculated LLE data are shown in Figs.2 and 3.Table 10 and Table 11 shows the corresponding energy interaction parameters and the RMSD values.It can be seen that the data points calculated by the two models correspond extremely well to the experimental data points.All RMSD of the NRTL model were less than 0.0048, and UNIQUAC model were less than 0.0063.The deviation between the calculated correlation and experimental results is slight, with reasonable consistency,which demonstrates that the two models can be used in the simulation and calculation of the extraction of aromatics extraction using NMP,and the accuracy of the NRTL model was higher.

Table 10 The NRTL and UNIQUAC binary energy interaction parameters and root mean square deviation of the cyclooctane (1) + benzene/toluene (2) + NMP (3) systems
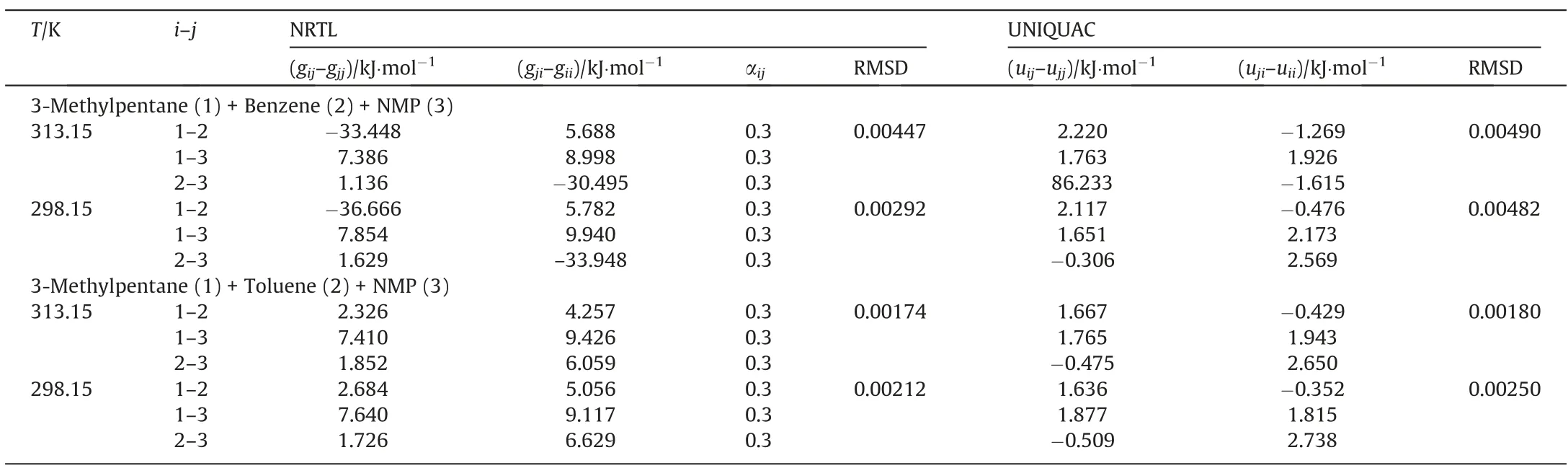
Table 11 The NRTL and UNIQUAC binary energy interaction parameters and root mean square deviation of the 3-methylpentane (1) + benzene/toluene (2) + NMP (3) systems
4.Conclusions
In this work,the LLE data of the ternary system for cyclooctane/3-methylpentane + benzene/toluene + NMP were studied, and the molecular interaction mechanism in aromatics extraction using NMP was discussed.The following were the findings:
(1) The LLE data of cyclooctane/3-methylpentane + benzene/to luene + NMP at 298.15 K and 313.15 K under a pressure of 101.3 kPa were experimentally measured.The Othmer-Tobias and Hand equations were used to test the experimental results.The result showed that the experimental data is highly consistency.
(2) The S values were all greater than unity,the D values of NMP ranged between 0.47 and 0.64 for benzene and between 0.29 and 0.37 for toluene, and the PI values of NMP to benzene was significantly higher than that of toluene in the study system.Indicating that aromatics and alkanes can be effectively separated using NMP, and benzene was more easily extracted.Quantum chemical analysis and calculation show that the interaction between benzene-NMP and toluene-NMP is mainly van der Waals, benzene is more polar than toluene,and it is easier to interact with NMP,which verifies the experimental results.
(3) Binary interaction parameters of NRTL and UNIQUAC models were obtained through regressing experimental data.All RMSD of the NRTL model were less than 0.0048, and UNIQUAC model were less than 0.0063, indicating that the regressed parameters can be reliably used to calculate the extraction of aromatics using NMP.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the support from the National Natural Science Foundation of China (22178190), and the National Youth Natural Science Foundation of China (CN) (22008129).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.05.016.
Nomenclature
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
