ZmDRR206 functions in maintaining cell wall integrity during maize seedling growth and defense response to external stresses
To Zhong, Suining Deng, Mng Zhu, Xingming Fn, Mingling Xu, Jinrong Ye,*
a National Maize Improvement Center/Center for Crop Functional Genomics and Molecular Breeding, China Agricultural University, Beijing 100193, China
b Institute Food Crops, Yunnan Academy of Agricultural Sciences, Kunming 650205, Yunnan, China
Keywords:Cell-wall integrity Cell-wall biosynthesis Dirigent protein ZmDRR206 Defense response Seedling growth
ABSTRACT Plants adaptively change their cell wall composition and structure during their growth,development,and interactions with environmental stresses.Dirigent proteins (DIRs) contribute to environmental adaptations by dynamically reorganizing the cell wall and/or by generating defense compounds.A maize DIR,ZmDRR206,was previously reported to play a dominant role in regulation of storage nutrient accumulation in endosperm during maize kernel development.Here we show that ZmDRR206 mediates maize seedling growth and disease resistance by coordinately regulating biosynthesis of cell wall components for cell-wall integrity(CWI)maintenance.Expression of ZmDRR206 was induced in maize seedlings upon pathogen infection.ZmDRR206 overexpression in maize resulted in reduced seedling growth and photosynthetic activity but increased disease resistance and drought tolerance, revealing a tradeoff between growth and defense.Consistently, ZmDRR206 overexpression reduced the contents of primary metabolites and down-regulated genes involved in photosynthesis, while increasing the contents of major cell wall components, defense phytohormones, and defense metabolites, and up-regulated genes involved in defense and cell-wall biosynthesis in seedlings.ZmDRR206-overexpressing seedlings were resistant to cell-wall stress imposed by isoxaben, and ZmDRR206 physically interacted with ZmCesA10, which is a cellulose synthase unit.Our findings suggest a mechanism by which ZmDRR206 coordinately regulates biosynthesis of cell-wall components for CWI maintenance during maize seedling growth, and might be exploited for breeding strong disease resistance in maize.
1.Introduction
The plant cell wall influences cell shape and size, providing structural support for the plant body, protecting cells from invading pathogens, and acting as nodes for communication between the symplast and apoplast.Cell walls participate in defense against biotic and abiotic stresses, both of which reduce crop yield.Cellwall composition and structure are under active modification during various biological processes and stress responses.Maintaining cell-wall integrity (CWI) is crucial for plant growth, development,and interactions with the environment.The regulatory processes controlling active and adaptive modifications of cell-wall composition and structure also influence growth and defense[1,2].A developmental process (cell elongation), abiotic stress factors (drought,salt,or cold)and the breaching of a cell wall by a pathogen during infection can cause in vivo cell-wall damage (CWD).If the cell experiences CWD, the cell wall becomes distorted, a state that is detected by CWI monitoring components and leads to growth arrest.In response, the production of lignin, jasmonic acid (JA),and salicylic acid (SA) increases to activate general stress responses.Thus, higher levels of phytohormone (JA and SA) and lignin deposition are classic hallmark responses of CWI impairment.Plant cells must correct detrimental alterations to their cell wall to support growth upon challenges to the CWI, and they also must regain their ability to loosen their walls and incorporate new polymers to avoid wall failure for subsequent growth recovery[3,4].
The plant cell wall is a highly dynamic structure composed of polysaccharides(cellulose,semi-celluloses,and pectins),structural proteins,and phenolic compounds[5].Flavonoids,lignins,lignans,and monolignols are phenolic compounds derived from the phenylpropanoid pathway and function in plant biotic defense and abiotic stress tolerance.Lignin and lignan are phenolic polymers that consist of p-hydroxyphenyl, guaiacyl, and syringyl units formed by the oxidative coupling of p-coumaryl, coniferyl, and sinapyl alcohols, respectively [6].Bimolecular phenoxy radical coupling is needed for lignin and lignan biosynthesis during vascular plant development.Oxidases, like peroxidases (PODs) and laccases (LACs), are required for the formation of the monolignol radicals and are involved in monolignol oxidation during lignin and lignan biosynthesis [7].Coniferyl alcohol is oxidized by POD/H2O2or LAC/O2to produce the corresponding radical,and dirigent proteins (DIRs) then guide the phenoxy radical coupling reaction and mediate the stereoselective formation of either (+) or (-)-pinoresinol [8,9].DIRs are extracellular glycoproteins with a high β-strand content in all reported land plants.DIRs have no catalytic activity on their own or with a suite of potential cofactors, but direct the outcome of bimolecular phenoxy radical coupling reactions toward regio- and stereospecific products in presence of the oxidases.DIRs play a central role in plant secondary metabolism,especially the biosynthesis of lignan, flavonolignan, and alkaloids.Several genes encoding (+)- or (–)-pinoresinol-forming DIRs have been described in various plant species [10–12].
The spatial deposition of lignin is under critical regulation during plant defense responses to biotic or abiotic stress.The participation of DIRs in this process was first supported [13] by their colocalization with lignin initiation sites, as determined by immunohistochemistry with anti-DIR polyclonal antibodies.The Casparian strip(CS),composed mainly of lignin,constitutes a physical and chemical barrier that tightly controls water and nutrient transport and provides protection against soilborne pathogens[14].The location of CS-domain proteins (CASPs) specifies the site of CS formation in cell membrane domains by recruiting proteins required for CS formation and lignification.In Arabidopsis, the DIR protein AtDIR10/ESB1 is targeted to the lignification initiation sites in a CASP-dependent manner during CS formation and is required for both early deposition of lignin patches and their fusion in generating the mature CS [15,16].The soybean (Glycine max) DIR-like protein PDH1 participates in regulation of pod dehiscence by controlling the spatial deposition of lignin[17].Arabidopsis DIR7 functions in CWI maintenance.Loss of function of DIR7 causes alterations in cell-wall structure, impaired SA accumulation induced by isoxaben (ISX, a cellulose biosynthesis inhibitor), and increased susceptibility to a necrotrophic fungus [18].Thus, DIRs participate in regulating cell-wall modification or reinforcement during CWI maintenance.
Lignin biosynthesis is central to plant defense responses to biotic and abiotic stresses.Lignin confers stability and hydrophobicity to the plant vascular system.It also forms a barrier against microbial pathogens to limit the spread of pathogen-derived toxins and enzymes into the host by altering the compressibility and porosity of the cell wall [19,20].The participation of DIRs in response to pathogens that cause physical damage to the cell wall during infection has been reported in various species, where DIRs regulate the dynamic reorganization of the cell wall and/or generate defense compounds.The dirigent-like protein GhD2 modulates disease resistance by interacting with GhJAZ2, a member of the jasmonate-ZIM-domain family, in cotton [21].Various lignan stereoisomers display antibacterial activities.(+)-Gossypol is a phenolic aldehyde that functions in pathogen responses, and DIRs participate in the formation of the phenolic terpenoid(+)-gossypol in cotton (Gossypium sp.) [22].AtDIR12/DP1 is expressed specifically in outer integument cells of developing seeds and is coexpressed with the laccase gene LAC5 to regulate neolignan biosynthesis in Arabidopsis[23].In plants exposed to abiotic stress,lignification levels are frequently modulated by DIRs and peroxidases.Increased PEROXIDASE5 and DIR2-like protein abundance in soybean coincided with elevated accumulation of H2O2in roots induced by manganese toxicity [24].
Maize is a major crop used for food,feed,and fuel.Because ever more frequently occurring extreme weather conditions and maize diseases cause severe yield loss, new maize varieties with strong disease resistance,high yield,and good quality are a constant goal of maize breeding.Several mechanisms monitor and maintain the functional integrity of plant cell walls,but knowledge of the underlying genes,molecular processes,and their function in growth and defense is limited.A maize DIR, DISEASE RESISTANCE RESPONSE206 (ZmDRR206), was previously reported [25] to play a dominant role in regulation of storage nutrient accumulation in developing maize endosperm, ZmDRR206 overexpression resulted in small and shrunken kernels with reduced starch content.Here,the mechanism by which ZmDRR206 mediating maize seedling growth and disease resistance against Fusarium graminearum infection was investigated.The induced expression of photosynthesisand defense-associated genes, especially genes encoding enzymes mediating cell-wall biosynthesis, and changes of main cell wall components and defense phytohormones, were evaluated in ZmDRR206-overexpresing maize seedlings.And the interaction of ZmDRR206 and its partners was studied.The aim of this work was to improve our knowledge of DIRs in maintaining cell wall integrity by regulating cell-wall biosynthesis during maize seedling growth and defense responses.
2.Materials and methods
2.1.Fusarium graminearum inoculation, disease severity scoring, and seedling phenotypic analysis
ZmDRR206-overexpressing maize plants, named DRR-OE, were generated as described [25].The fungal pathogen F.graminearum preparation and inoculation in the field were performed as previously described [26] with maize plants grown and inoculated in Shangzhuang experimental station, Beijing, China in 2017 and 2018.Young seedling inoculation of primary roots of 5-day-aftergermination (DAG) seedlings and disease severity scoring were performed as previously described[27].Primary roots with typical symptoms were scored at 48 h after inoculation (hai).Wild-type(WT, LH244 inbred line) and DRR-OE seedlings were cultured in a controlled growth room under conditions of 28/22 °C (day/night)at a light intensity of 500 μmol m-2s-1(16 h light/8 h night)and 50%relative humidity.Three replicates were set for each genotype with about 15 seedlings per replicate.For measurement of ZmDRR206 expression, WT seedlings were inoculated with F.graminearum at 5 DAG,then cultured in a controlled growth chamber under light and sampled at 0, 6, and 18 hai.For comparing seedling root growth rate, the lengths of 7-DAG seedling primary roots cultured with paper-rolling were measured and shoot growth rate was measured on soil-grown young seedlings at 12 or 15 DAG.The experiments were repeated at least three times.
2.2.Drought-stress treatment
Seedlings(DRR-OE and WT)were grown in a controlled growth chamber as described above under well-watered conditions until drought treatment.Drought stress was imposed on the two-leaf seedlings by withholding water and keeping the seedlings under observation for the following 15 days.At this time, indications of severe wilting(leaves and stems becoming soft,dry,and drooping)were visible in all of the WT seedlings and the seedlings were rewatered.Measurements were made 6 days after re-watering.The number of surviving seedlings and the total seedling number were used to obtain the survival rate.For Arabidopsis dehydration assay,7-day-old seedlings grown on ? MS medium were transferred to soil, grown for 14 days under 8 h light/16 h dark conditions, at 22 °C, and subjected to drought stress for an additional 10 days.Then the plants were re-watered, and the number of plants that survived the stress and recovered their developmental phenotype was recorded 5 days after re-watering.These experiments were repeated three times.
2.3.Subcellular localization
The coding sequence of ZmDRR206 was cloned into a pCaMV35S:GFP vector to fuse in frame and upstream of the green fluorescent protein (GFP) coding sequence and under the cauliflower mosaic virus (CaMV) 35S promoter for constructing pCaMV35S:ZmDRR206-GFP vector.A partial cDNA of ZmCesA10 encoding a peptide containing the first four transmembrane domains (shared by all four proteins encoded by the splicing variants of ZmCesA10,corresponding to amino acids (aa) 752 to 997 of a protein with 1087 aa) was obtained and fused in frame with the mCherry (or Myc)-coding sequence and under the Super promoter for constructing pSuper:ZmCesA10p-mCherry (-Myc) vector (primers are listed in Table S2).Agrobacterium strain EHA105 containing the vector was cultured at 28 °C overnight.Agrobacterium cells were harvested by centrifugation and resuspended in buffer (10 mmol L-1MES, pH 5.7, 10 mmol L-1MgCl2, and 200 mmol L-1acetosyringone)at OD600=0.8.Leaves of 5-week-old soil-grown Nicotiana benthamiana were infiltrated with Agrobacterium cultures,carrying the vectors pCaMV35S: GFP, pCaMV35S: ZmDRR206-GFP, or pSuper:
ZmCesA10p-mCherry for expressing GFP, ZmDRR206-GFP, or ZmCesA10p-mCherry, respectively.The plants were incubated in growth chambers under 16 h light/8 h dark at 25 °C, with light intensity of 500 μmol m-2s-1and ~50% relative humidity.The excitation wavelengths of GFP and mCherry were 488 nm and 561 nm, respectively.The emitted signals of GFP and mCherry were collected between 500 and 535 nm and between 580 and 620 nm, respectively.Fluorescence images were examined and acquired with confocal laser microscope systems (Laser Scanning Microscopy 880, LSM 880) and images were processed using LSM microscope imaging software.The Agrobacterium tumefaciens strain containing pCaMV35S: ZmDRR206-GFP construct was transformed into Col plants using the floral dip method, to generate ZmDRR206-overexpressing transgenic Arabidopsis plants.
2.4.Measurement of leaf photosynthetic parameters
WT and DRR-OE seedlings were grown in the field under natural conditions in May 2019 or in a glass greenhouse with a controlled temperature of 25 °C, 50% relative humidity, with light intensity reaching 1100 μmol m-2s-1from 09:00 to 14:00 on sunny days.Seedlings at the three-leaf stage grown under the same conditions were used for photosynthetic parameter measurements on a sunny day as previously described [28].The middle part of the lastexpanded leaf of every seedling, namely the second leaf of 12-DAG seedlings and the third leaf of 15-DAG seedlings, was used for measuring soil plant analysis development (SPAD) value (leaf chlorophyll content), with a SPAD meter (SPAD-502 Plus, Konica Minolta, Inc.Tokyo, Japan).Net photosynthetic rate (Pn) and transpiration rate(Tr)were measured with a Li-6400XT Portable Photosynthesis System(LI-COR,LiCor Inc.,Lincoln,NE,USA),from 09:00 to 12:00, following the manufacturer’s instructions.Replicates were measured for each genotype.
2.5.Transcriptome sequencing (RNA-seq) and quantitative real-time reverse transcription PCR (RT-qPCR)
For RNA-seq, seedlings were cultured as described.Five-DAG seedlings with a primary root length of 6–8 cm were selected and inoculated at 10:00, cultivated under light for the subsequent 10 h and then in the dark for 8 h and sampled on the following day at 18 hai.Uninoculated seedlings cultured under the same conditions were collected at the same time.Seedlings were frozen in liquid nitrogen and used for RNA extraction and deep sequencing in 2016.RNA-seq libraries were constructed according to the protocol of the VAHTS mRNA-seq Library Prep Kit (Vazyme, Nanjing,Jiangsu, China) and sequenced on an Illumina HiSeq 2500 instrument to generate 150-nt paired-end reads.Differentially expressed genes (DEGs) were identified with criteria P < 0.05 and log2(fold change)>1 by paired Student’s t-test.Gene Ontology(GO)enrichment analysis of the DEGs was performed with the agriGO enrichment analysis tool (https://bioinfo.cau.edu.cn/agriGO).
For RT-qPCR, WT seedlings were grown in the dark under the described growth-chamber conditions and transferred to light for 1 and 2 h to measure light-responsive expression of ZmDRR206.The seedlings were cultivated under the same conditions for six days and used for expression measurement of selected genes.Total RNAs were extracted from young seedling tissues with RNAiso Plus (Takara, Dalian, Liaoning, China), One microgram of total RNA was incubated with DNase I at 37°C to remove genomic DNA, and used for first-strand cDNA synthesis using PrimeScript RT Master Mix (Takara).qPCR assays were performed with the TB Green Premix Ex Taq kit (Takara) following the manufacturer’s protocols on a 7500 Real-Time PCR system (Applied Biosystems,USA) to detect selected genes.The primers used are listed in Table S2.Relative expression of genes was calculated using the relative quantification method with maize GAPDH(NCBI accession no.X07156) and ZmTubulin1 as endogenous control.The variation in expression was estimated using three independent biological replicates.
2.6.Measurement of the contents of cell-wall components
The 7-DAG seedling roots and 12-DAG maize seedlings, cultivated as described above,were harvested,dried,and homogenized to fine powder using a mixer mill at 25 Hz for 2 min.The powdered root or seedling tissues were prepared to exclude protein and other UV-absorbing materials following Moreira-Vilar et al.[29] and used for measuring the contents of major cell-wall components.Acid-soluble lignin was measured on a UV–visible spectrophotometer (TU-1901), against a background of 4% sulfuric acid.The insoluble fractions were used to assay acid-insoluble lignin.Lignin content was determined as the sum of insoluble and soluble lignins.For measurement of cellulose and semi-cellulose content,the de-starched alcohol-insoluble residues were hydrolyzed with trifluoroacetic acid.The supernatants were separated and the remains were treated in Updegraff reagent and hydrolyzed with 72% sulfuric acid.The sugars in the supernatant were separated using an SP0810 column (Shodex) on a UHPLC system (Agilent-1260).The content of the detected sugars was calculated based on standard curves of glucose,xylose,mannose,galactose,and arabinose and was used for determination of cellulose and semicellulose content.
2.7.Measurement of mineral element content
Seedlings at 7 DAG were sampled and dried at 60°C to constant weight for 24 h.Tissue (0.3 g dry weight) of the seedlings was digested with HNO3at room temperature overnight.The working parameters of ICP-AES (Model ICAP-6300, Thermo Electron, Beverly, MA, USA) were as follows: RF power: 1150 W; peristaltic pump speed:50 r min-1; auxiliary gas flow rate:0.5 L min-1; atomizer gas flow rate:0.7 L min-1.The mineral-element contents per unit dry weight of tissue were calculated from the assayed concentrations of the mineral elements in the diluted solution.Replicates were measured for each genotype.
2.8.Measurement of phytohormones
Endogenous SA, JA, and ACC measurement was performed by Wuhan Greensword Creation Technology Co.Ltd., (Wuhan, Hubei,China) (https://www.greenswordcreation.com/index.html) based on UHPLC-MS/MS separation (Thermo Scientific Ultimate 3000 UHPLC coupled with TSQ Quantiva).To measure the endogenous concentrations of SA and JA,500-mg samples of 6-DAG maize seedlings were harvested and extracted with acetonitrile.Briefly, samples were frozen, ground in liquid nitrogen, and extracted with 0.5 mL 80% acetonitrile at –20 °C for 12 h.After centrifugation,the supernatants were collected and evaporated under a gentle nitrogen stream at 35 °C, followed by redissolution in 100 μL acetonitrile for UHPLC-MS/MS separation.For endogenous ACC measurement, 500-mg samples were frozen in liquid nitrogen,ground to fine powder, and extracted with 0.5 mL MeOH at–20 °C for 12 h.After centrifugation (10,000×g, 4 °C, 20 min), the supernatants were collected and evaporated under a gentle nitrogen stream at 35°C,followed by redissolution in 100 μL 50%MeOH for UHPLC-MS/MS separation.[2H2]-JA, [2H4]-SA, and [2H4]-ACC(Olomouc, Czech Republic) were added as internal standards for quantification.Data acquisition and analysis were performed with a Thermo Scientific Xcalibur 2.1 data system.Three replicates were collected for each sample.
2.9.Lignin measurement
Seedlings were grown for 5 days and cultured under the growth condition described.Seedlings with similar primary root length were chosen for isoxaben (ISX, a cellulose biosynthesis inhibitor)treatment.ISX was dissolved in Dimethyl sulfoxide (DMSO).Root tips(~3 cm)of 5-DAG seedlings were immersed in water containing 1 mmol L-1ISX and cultivated under the same growth conditions for 10 h, treatment ISX (+).Root tips that were immersed into water to which DMSO had been added with the same amount as the added ISX,were used as control,ISX(-).Lignification in seedling roots(n>10)was measured 10 h after start of treatment.Lignified regions were detected with phloroglucinol-HCl as described[30].
2.10.Luciferase complementation image (LCI) assay
For LCI assay, the coding sequence of ZmDRR206 was cloned into JW772 (C-terminal half of luciferase, cLUC) to produce cLUCDRR206.The partial cDNA of ZmCesA10 described above and cDNA of ZmDIN1, ZmRin1 were cloned into JW771 (N-terminal half of luciferase, nLUC) to produce CesA10p-nLUC, DIN1-nLUC, and RIN1-nLUC,using ClonExpress II One Step Cloning Kit(Vazyme Biotech)(primers are listed in Table S2).These constructs were transformed into A.tumefaciens (strain GV3101), after which the Agrobacterium cells were cultured to OD600 = 0.8, pelleted, and suspended in a buffer (10 mmol L-1methylester sulfonate,10 mmol L-1MgCl2, and 150 mmol L-1acetosyringone, pH 5.7).Equal amounts of OD600-normalized Agrobacterium cultures for cLUC and nLUC constructs were mixed to a final concentration of OD600 = 1.0.Transient expression in N.benthamiana leaf tissues was achieved by Agrobacterium infiltration following Zhang et al.[28],and fluorescence signal was imaged with a Tanon-5200 imaging system.These experiments were independently repeated three times and each combination was infiltrated with multiple leaves each time.
2.11.Yeast two-hybrid assay
The DUALmembrane(www.dualsystems.com)pairwise interaction assay in yeast was used to investigate the interaction between ZmDRR206 and ZmCesA10,ZmDIN1,and ZmRIN1 according to the DUALmembrane user manual.ZmDRR206 coding sequence without the N-terminal signal peptide was subcloned into a pBT3-SUC DUALmembrane bait vector using the ClonExpress II One Step Cloning Kit, to generate a pBT3-SUC-DRR construct in which ZmDRR206 was fused to the C-terminal half of ubiquitin (Cub)and the artificial transcription factor LexA-VP16.The coding sequence of ZmDIN1, ZmRIN1, and partial cDNA of ZmCesA10 was subcloned into sfiI-digested pPR3-N (the pPR3-N DUALmembrane prey vector)) to generate pPR3-N-RIN1, pPR3-N-DIN1, and pPR3-N-CesA10p constructs, thus, these preys were fused to the mutated N-terminal half of ubiquitin (NubG) with primers listed in Table S2.pBT3-SUC-DRR plasmids were co-transformed into NMY51 yeast cells with pPR3-N-CesA10p, or pPR3-N-DIN1 or pPR3-N-RIN1, respectively.The combination of these prey constructs with pBT3-SUC empty,as well as the combination between pBT3-SUC-DRR and pPR3-N empty, was used as negative controls.Subsequently, the interaction was determined by spotting the resulting transformants onto synthetically defined (SD) /-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp selection plates following the manufacturer’s protocols.
2.12.ZmDRR206-antibody production, immunoblotting, and co-IP assay
To develop a ZmDRR206-specific antibody, the PSAVLAADDDGTTC peptide was chemically synthesized and used as an antigen to immunize New Zealand white rabbits in Hangzhou Huaan Biotechnology (https://huabio.bioon.com.cn/).For Western blotting, total proteins were extracted from 6-DAG seedlings with protein extraction buffer(50 mmol L-1Tris-HCl,pH 7.5,150 mmol L-1NaCl, 10 mmol L-1MgCl2, 0.2% NP-40, 5 mmol L-1DTT, and 1× protease inhibitor cocktail).The membrane proteins were extracted from 6-DAG seedlings with a Membrane Protein Extraction Kit (Sangon Biotech, C500049).Proteins were resolved by SDS–PAGE and then transferred to a PVDF membrane.The membrane was then incubated with primary and secondary antibodies and visualized with ECL Plus reagent.To measure the ZmCES10-ZmDRR206 interaction in vivo, Co-IP assays were performed.Briefly, N.benthamiana leaves co-expressing ZmDRR-GFP/GFP(control) and ZmCES10p-Myc were homogenized in immunoprecipitation buffer and centrifuged twice at 20,000×g for 20 min at 4 °C.GFP or GFP-tagged proteins were immunoprecipitated with GFP-Trap Magnetic Agarose beads (MBL, #D153-11).For protein blots, GFP and Myc fusion proteins were probed with anti-GFP(ABclonal, #AE012) and anti-Myc (ABclonal, #AE010) antibodies,respectively.
2.13.Statistical analysis
The means of the WT and DRR-OE seedlings were compared by Student’s t-test.All gene expression experiments were performed at least three times with independent samples.
3.Results
3.1.Expression of ZmDRR206 in maize seedlings responded rapidly to Fusarium graminearum infection and light illumination
In previous studies,we discovered that expression of ZmDRR206 was induced in infected seedling roots of near-isogenic lines(NILs)carrying the resistant qRfg1 (Resistance to F.graminearum) allele(R-NIL),but not in infected roots of the NIL carrying the susceptible qRfg1 allele (S-NIL).Similarly, another maize resistance allele developed from a second resistance locus(qRfg2)also showed elevated ZmDRR206 expression in response to F.graminearum infection [27,31] (Fig.S1A, B).Expression of ZmDRR206 rose rapidly 6 and 18 h after inoculation with F.graminearum and was also induced rapidly by light illumination in seedlings of the inbred line LH244 (Fig.1A, B).ZmDRR206 was annotated as an inducible pathogenesis-related (PR) gene involved in defense response to biotic stimulus and encoding a DIR family protein with a predicted molecular function of isomerase activity.Based on published highspatial-resolution deep transcriptome-sequencing datasets, we observed that ZmDRR206 was expressed in several young tissues:developing endosperm and young seedling shoots, roots, young leaves,and tassels[32], as well as in the basal endosperm transfer layer(BETL),the embryo-surrounding region,the aleurone,and the conducting zone in developing maize kernels [33] (Fig.S1C, E).

Fig.1.Pathogen-and light-inducible expression of ZmDRR206 and the plasma-membrane location of ZmDRR206.(A)Fungus-inducible expression of ZmDRR206.0 h,6 h and 18 h are wild-type (WT, inbred line LH244) seedlings at 0, 6,and 18 h after inoculation with F.graminearum.(B) Light-inducible expression of ZmDRR206.D represents WT seedlings grown under continuous dark for 5 days after germination(DAG),L1 and L2 are dark-grown 5-DAG WT seedlings transferred to light for respectively 1 and 2 h.The statistics in(A)were derived from paired Student’s t-test comparing WT(0)and the inoculated WT at indicated timepoints 6 or 18 h after inoculation(hai)in three replicates;and comparing WT grown under dark with dark-grown WT at 1 h(L1)and 2 h(L2)after light-illumination.Asterisks(*)correspond to absolute fold change>1.5,P<0.05 and(**) to absolute fold change > 2 under P < 0.01.(C) The ZmDRR206-GFP fluorescence signal is associated predominantly with the periphery of the leaf cells detected by confocal microscopy.The arrows indicate the punctate ZmDRR206-GFP signal distribution in the cell periphery.The rightmost image in the top row is the overlapping image of another cell with punctate fluorescence signal in the cell periphery.Scale bar, 20 μm.(D) ZmDRR206 protein is detected in the membrane pellet (MP) with ZmDRR206-specific antibody by Western-blot analysis.Total protein (WT), soluble protein (SP), and MP were extracted from 6-DAG WT seedlings.
To investigate the subcellular localization of ZmDRR206, we transiently infiltrated N.benthamiana leaves with Agrobacterium carrying a construct expressing a ZmDRR206-GFP fusion.ZmDRR206-GFP fluorescence was associated with the cell periphery, in contrast to the even distribution of free GFP in the cytoplasm and the nucleus.A punctate pattern for ZmDRR206-GFP fluorescence was observed at the cell periphery (Fig.1C).With a ZmDRR206 specific polyclonal antibody, tissue fractionation and immunoblotting analysis of 6-day-old WT seedlings revealed a strong ZmDRR206 signal only in the membrane pellet, and not among soluble proteins(Fig.1D)indicating ZmDRR206 is localized primarily to the plasma membrane (PM).
3.2.ZmDRR206 functioned in maize seedling growth and kernel development
To investigate the biological function of ZmDRR206, the homozygous progeny of transgenic maize plant, designated as DRR-OE [25], were used.The mean hundred-kernel weight(HKW), and length and width of DRR-OE kernels were all significantly smaller than those of the WT.DRR-OE kernels exhibited a shrunken and reduced endosperm,but their embryos were similar to those of WT,resulting in a higher embryo:endosperm ratio than that of the WT.The germination rate of DRR-OE lines was comparable to that of the WT (Fig.S2A–I), indicating the unaffected embryo development.
Relative ZmDRR206 transcript levels were significantly higher in the primary roots of DRR-OE lines,as determined by RT-qPCR,with a fold increase of 176 in DRR-OE3 and of 39 in DRR-OE6 relative to WT roots 6 DAG.ZmDRR206 protein also accumulated to much higher levels in DRR-OE than in WT seedlings, based on immunoblot analysis with the ZmDRR206-specific antibody (Fig.2A, B).Both primary root length of 7-DAG seedlings and the height of 12-DAG seedlings were shorter in the DRR-OE3 and DRR-OE4 than in WT seedlings,but not DRR-OE6 roots (Fig.2C–F).DRR-OE plants grew more slowly than the WT during the early growth stage (~8 weeks)in the field.However,DRR-OE plants later reached WT stature and did not differ from the WT in mature plant height(Fig.S2J).These growth dynamics (delayed early growth) suggest that ZmDRR206 may not affect overall plant growth and development.ZmDRR206 overexpression also retarded growth of the transgenic Arabidopsis seedling, which had shorter primary roots and developed smaller seeds than that of Columbia wild-type seedling(Fig.S2K–N).These results indicate that ZmDRR206 functions in maize kernel development and seedling growth.
3.3.Photosynthetic activity was reduced in DRR-OE maize seedlings
As the expression of ZmDRR206 was light-inducible and the growth of DRR-OE seedlings was retarded, we measured chlorophyll contents and Pn of DRR-OE and WT seedlings.Because DRROE3 seedlings were much smaller than WT seedlings, we focused on DRR-OE4 and DRR-OE6 seedlings.The mean SPAD value of 12-DAG WT leaves was 34.3, while that of 12-DAG DRR-OE leaves was lower a 23% (DRR-OE4) and 19% (DRR-OE6).The mean SPAD values of the 15-DAG DRR-OE leaves were also lower than that of WT.The Pnvalues ranged from 11.3 to 12.7 μmol CO?m-2s-1in the WT and from 6.7 to 7.5 μmol CO?m-2s-1in DRR-OE seedlings,corresponding to decreases of 45% (DRR-OE4) and 38% (DRR-OE6),relative to WT seedlings(Fig.2G,H).Thus,chlorophyll biosynthesis and photosynthetic activity were reduced in DRR-OE seedlings,possibly contributing to their delayed growth.
3.4.DRR-OE seedlings showed higher disease resistance and drought tolerance
DRR-OE seedlings showed higher resistance to F.graminearum infection, as evidenced by their lower disease severity index(DSI) than that of WT seedlings.DRR-OE seedlings also displayed better growth of both shoots and roots after inoculation with F.graminearum than did WT seedlings.This increased resistance was confirmed in field trials of mature maize plants (Fig.3).Thus,ZmDRR206 positively regulates maize disease resistance to F.graminearum-induced stalk rot.
DRR-OE seedlings were much more tolerant to drought stress than WT seedlings.Water withholding after the two-leaf stage resulted in severe wilting of all WT seedlings at ~25 DAG, in contrast to the green leaves and upright stems of DRR-OE plants.We quantified this apparent tolerance to drought by scoring survival rate (SR) of DRR-OE and WT seedlings.The SR of both DRR-OE4 and DRR-OE6 seedlings was over 95% upon drought stress treatment, whereas WT seedlings reached only about 50% survival(Fig.4A, B).We also estimated the transpiration rate (TR) at the center of the widest part of the newest expanded leaves of 15-DAG seedlings.In agreement with their SRs, TRs ranged from 0.88 to 0.96 μmol H2O m-2s-1for WT seedlings, but were much lower in DRR-OE seedlings, ranging from 0.50 to 0.55 μmol H2O m-2s-1in DRR-OE seedlings, for decreases of 46.15% (DRR-OE4)and 40.4%(DRR-OE6)(Fig.4C).Similarly,ZmDRR206 overexpression in Arabidopsis increased the drought tolerance of transgenic seedlings, which showed significantly increased SR and more growth under severe water deficiency (Fig.4D, E).
3.5.Down-regulated photosynthesis-associated genes in DRR-OE maize seedlings
We conducted deep transcriptome sequencing to assess the effects of ZmDRR206 overexpression on gene expression in maize control seedlings (WT and DRR) and seedlings inoculated with F.graminearum (WTi).Comparing gene expression between DRR-OE and WT seedlings identified 2101 DEGs,of which 775 were upregulated and 1326 downregulated.Statistical GO term enrichment analysis revealed that the cellular components of the proteins encoded by many downregulated DEGs were:ribosome,ribonucleoprotein complex, photosystem, plastoglobules, photosynthetic membrane, and ribosomal subunits.Almost all photosynthesis antenna protein and photosynthesis light harvesting-associated genes were simultaneously down-regulated by ZmDRR206 overexpression and by pathogen infection (Fig.S3A).The downregulated DEGs also showed enrichment in translation-and photosynthesisassociated functional categories: ribosome, ribosome biogenesis,ribonucleoprotein complex biogenesis, photosynthesis antenna proteins, photosynthesis light harvesting, and photosynthesis(Fig.5A, B), suggesting reduced participation of DRR-OE seedlings in these processes.Among the dramatically down-regulated genes in DRR-OE seedlings, the trihelix transcription factor (TF) gene ZmGT-3b exhibited an expression profile similar to those of photosynthesis-associated genes.The transcripts of ZmGT-3b,PHOTOSYSTEM II3 (ZmPSII3), LIGHT HARVESTING COMPLEX II(ZmLHCII) and mesophyII7 (ZmLHCII7), were reduced to less than a fold-change of 0.4 in DRR-OE4 and DRR-OE6 seedlings relative to WT, as seen in our RNA-seq data (Fig.S3B, C) and by RT-qPCR(Fig.5C).These results were consistent with the lower photosynthetic rates of DRR-OE relative to WT seedlings(Fig.3H),suggesting that the retarded growth of DRR-OE seedlings may be associated with the repression of growth-promoting (translation- and photosynthesis-associated) genes.

Fig.2.Characterization of the seedling growth phenotype of ZmDRR206-overexpressing transgenic maize lines.(A,B)Transcript(A)and protein(B)levels of ZmDRR206 in the WT and DRR-OE seedlings.ZmDRR206-specific antibody was used for Western-blot analysis with total proteins extracted from maize seedlings at 6 DAG.(C,D)Primary root length (C) and phenotype (D) of maize seedlings at 7 DAG.Values are mean ± SD (n > 10) with three replicates per genotype.(E, F) Height (E) and phenotype (F) of maize seedlings at 12 DAG.Values are mean±SD(n>10)with three replicates per genotype.(G)SPAD value of maize seedlings at 12 or 15DAG.Values are mean±SD(n>10)with three replicates per genotype.(H)Net photosynthetic rate of maize seedlings at 15 DAG.Values are mean±SD(n=5).The values of SPAD and Pn were measured on the middle part of the newly expanded leaf:the second leaf(WT-2,DRR4-2 and DRR6-2)of the 12-DAG seedlings,and the third leaf(WT-3,DRR4-3 and DRR6-3)of the 15-DAG seedlings were used.WT is the wild-type line LH244;DRR3,DRR4 and DRR6 are ZmDRR206 over-expressing transgenic everts DRR-OE3,DRR-OE4 and DRR-OE6.*,P<0.05;**,P<0.01;***, P < 0.001 by paired Student’s t-test.
We independently validated the repression of photosynthesis by quantitative proteomics of whole protein extracts from maize leaves (2nd leaves of 12-DAG seedlings).We identified 376 proteins with differential abundance (DAPs) by comparing protein levels in DRR-OE and WT leaf extracts, finding a two-foldincrease of ZmDRR206 in DRR-OE relative to WT seedlings.Of the 376 DAPs, 168 were predicted to localize to the chloroplast and 108 showed lower abundance in DRR-OE samples.All DAPs enriched in the Kyoto Encyclopedia for Genes and Genomes(KEGG)pathways zma00195 (photosynthesis), zma00910 (nitrogen metabolism),and zma00196(photosynthesis-antenna proteins)showed lower abundances in DRR-OE than in WT seedlings.More-abundant DAPs were enriched mainly in the KEGG pathways zma00940(phenylpropanoid biosynthesis),zma04626(plant–pathogen interaction),zma00480(glutathione metabolism),and zma03410(base excision repair) (Fig.S3D–G).Metabolite profiling was used to identify differentially abundant metabolites in the 2nd leaves of DRR-OE relative to WT seedlings at 12 DAG.The contents of all measured carbohydrates,organic acids,and four amino acids were decreased(<0.65-fold,P-value<0.05),while the contents of asparagine, valine, lysine, protectants (allantoin, choline, and melanin),and some secondary metabolites were increased (> 1.5-fold, Pvalue < 0.05) in DRR-OE relative to WT seedlings.However, flavonoid contents varied, the contents of some flavones rose, and the contents of flavonols decreased in DRR-OE relative to WT seedlings.The contents of cinnamic acid(CA)showed the largest fold-change increase(77.6)(Fig.S4).Thus,ZmDRR206 affects both primary and secondary metabolism in maize seedlings.
3.6.Defense-related transcriptional reprogramming was induced by ZmDRR206 overexpression
Functional annotations of the DEGs upregulated by ZmDRR206 overexpression revealed their association with oxidoreductase activity, monooxygenase activity, peroxidase activity, and ion binding(Fig.5B),suggesting their association with redox,synthesis and metabolism processes.KEGG pathway enrichment analysis emphasized gene functions in the biosynthesis of secondary metabolites, phenylalanine metabolism, phenylpropanoid biosynthesis, plant–pathogen interaction, and plant hormone signal transduction.Among the pathways for biosynthesis of secondary metabolites,biosynthesis of flavones,flavonols,stilbenoids,diarylheptanoids,gingerol,benzoxazinoids,diterpenoids,flavonoids,and isoflavonoids was enriched (Fig.5A).This enrichment was consistent with the metabolome analysis,which showed various changes in benzoxazinoids and flavonoids(Fig.S4).Almost all of these functional categories can be summarized as support of basal defense responses to various stresses, suggesting that ZmDRR206 is positively associated with plant defense responses.
Plants undergo transcriptional reprogramming to prioritize defense-over growth-associated cellular functions upon pathogen infection.Inoculation with F.graminearum induced 1026 DEGs in WT seedlings, including the 304 DEGs that were also induced by ZmDRR206 overexpression, pointing to commonalities in the transcriptional reprogramming induced by ZmDRR206 overexpression(DRR/WT)and inoculation(WTi/WT).DEGs induced by inoculation(WTi/WT) were also enriched for GO and KEGG terms associated with biosynthesis of secondary metabolites, in particular of phenylpropanoids,stilbenoids,diarylheptanoids,gingerol,benzoxazinoids, flavonoids, diterpenoids, flavones, flavonols, and carotenoids.The transcriptional reprogramming induced by ZmDRR206 overexpression (DRR/WT) was stronger for these defense response–associated functional categories than that induced by inoculation of WT seedlings (WTi/WT), as indicated by their greater number of DEGs in DRR/WT relative to the WTi/WT comparison (Fig.S5).Thus, ZmDRR206 overexpression induced defense-associated transcriptional reprogramming in DRR-OE seedlings.

Fig.3.Disease resistance of ZmDRR206-overexpressing transgenic maize lines.(A)Disease severity index(DSI)of the WT and DRR-OE seedlings at 48 h after F.graminearum inoculation.Values are mean±SD(n>15)with three replicates per genotype.(B)Disease phenotype of the inoculated maize seedlings(upper)and stem of the mature fieldgrown plants (lower).(C) Primary root length of inoculated WT and DRR-OE seedlings with disease symptoms.Values are mean ± SD (n > 15) with three replicates per genotype.(D)The images of WT and DRR-OE in the field stalk rot tests.Scale bar,10 cm.(E,F)DSI of the WT and DRR-OE mature plants inoculated with F.graminearum in the field in 2017 (E) and in 2018 (F).Values are mean ± SD (n > 30) with three replicates per genotype.WT is the wild-type line LH244, DRR4 and DRR6 are ZmDRR206-overexpressing transgenic events DRR-OE4 and DRR-OE6.*, P < 0.05; **, P < 0.01; ***, P < 0.001, by paired Student’s t-test.
3.7.ZmDRR206 functions in CWI maintenance by regulating cell-wall biosynthesis
Defense-induced biosynthesis of lignin supports basal immunity.We investigated the cell-wall composition of roots from 7-DAG seedlings and from 12-DAG whole seedlings.We detected significant increases in contents of acid-soluble lignin (ASL, 13.0%),acid-insoluble lignin (AIL, 11.1%), and lignin (11.5%) in roots of 7-DAG DRR-OE seedlings, while the contents for cellulose and semicellulose were similar, relative to WT seedlings (Fig.6A).By 12-DAG, DRR-OE seedlings showed greater contents for cellulose(8.0%) and semicellulose (9.7%), together with increased contents of AIL(6.7%)and lignin(6.1%),although ASL contents were not different from those of WT seedlings.Arabinose levels are positively correlated with lignin levels, cellobiose activates plant immune responses, and shifts in the xylose content of the cell-wall influence CWI [19,34].We measured higher levels of cellobiose(14.1%), glucose (4.5%), arabinose (6.5%), and xylose (4.6%) in DRR-OE relative to WT seedlings at 12 DAG (Fig.S6A, B).The leaf blade and midrib were thinner in 12-DAG DRR-OE relative to WT seedlings (Fig.S6C).The observed increases in contents for the main cell-wall components in DRR-OE seedlings may increase the structural integrity and strength of the cell wall and suggest that ZmDRR206 coordinately regulates the biosynthesis of cell-wall components.
GO analysis revealed that many DEGs were enriched in cell-wall organization- or biogenesis–associated functional categories,including xyloglucan metabolism, semi-cellulose metabolism,cell-wall polysaccharide metabolism, cell-wall modification, and glucan metabolism (Fig.S7A), indicating that these genes contribute to biosynthesis or modification of cell-wall components.The influence of ZmDRR206 overexpression on cell-wall composition prompted us to assess the expression profiles of the associated genes in DRR-OE seedlings.Consistent with the higher contents of the major cell-wall components,transcript levels of 4 cellulose synthase (Ces) genes were increased in DRR-OE relative to WT seedlings, and the expression of all Ces but CesLG3 were increased specifically in response to ZmDRR206-overexpression (DRR/WT),but not to inoculation (WTi/WT) (Fig.S8A).Similarly, many genes encoding enzymes in the lignin biosynthesis pathway were upregulated in DRR-OE seedlings, including genes encoding phenylalanine ammonia-lyase (PAL), cinnamate 4-monooxygenase(C4M), 4-coumarate CoA ligases (4CLs), caffeoyl-CoA Omethyltransferases (AOMTs), cinnamyl alcohol dehydrogenases(CADs), laccases (LACs), dirigent proteins (DPs) and CASP(Fig.S8B–D; Table S1).Of the genes involved in xyloglucan metabolism and cell-wall remodeling, including nine DEGs encoding xyloglucan endotransglucosylase/hydrolases (XTHs) and 21 DEGs encoding expansins (EXPs) were down-regulated; while many POD, UDP-glucosyltransferase (UGT), chitinase and ABC transporter encoding genes were up-regulated in DRR-OE relative to WT seedlings (Table S1).
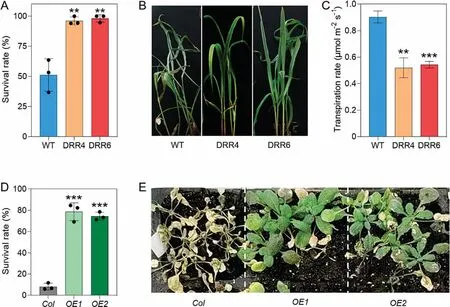
Fig.4.Drought tolerance of transgenic plants overexpressing ZmDRR206.(A, B) Survival rates (A) and growth phenotypes (B) of WT and DRR-OE seedlings after drought treatment.Values are mean±SD(n>15)with three replicates per genotype.(C)Transpiration rates of the last expanded leavesof 15-DAG WT and DRR-OE seedlings.Values are mean±SD(n=5).WT is the wild-type line LH244,DRR4 and DRR6 are ZmDRR206 overexpressing transgenic events DRR-OE4 and DRR-OE6.(D,E)Survival rates(D)and growth phenotypes (E) of Arabidopsis seedlings after drought treatment.Col is the wild type and OE1, OE2 are two independent ZmDRR206-overexpressing transgenic Arabidopsis lines.Values are mean ± SD (n > 25) with three replicates per genotype.***, P < 0.001 by paired Student’s t-test.
Consistent with the increased contents of cell-wall components,the transcript levels of ZmCesA10,ZmCesA11,and ZmCesA12,which encode cellulose synthases required for cellulose biosynthesis in secondary cell walls [20]; as well as those of genes encoding enzymes in the lignin biosynthesis pathway (C4M, AOMT, CADbm1 [also named brown midrib], and CAD6), were all significantly up-regulated in DRR-OE relative to WT seedlings, as determined by RT-qPCR (Fig.5D).ISX induced ectopic production of lignin in the upper parts of WT primary root tips,but not in DRR-OE seedling roots,as revealed by staining with phloroglucinol-HCl(an indicator of secondary wall thickening)(Fig.6B).Thus,ZmDRR206 may support CWI maintenance by regulating cell-wall production and remodeling during maize seedling growth.
The CWI maintenance system involves ion channels that constantly monitor the state of the cell wall and initiate adaptive changes in both cellular and cell-wall metabolisms [1,3].Multiple ion transporters were up-regulated in DRR-OE relative to WT seedlings,including genes encoding phosphate,potassium,copper,vacuolar iron, and zinc transporters (Table S1).Relative to WT seedlings,7-DAG DRR-OE seedlings accumulated more magnesium(Mg),potassium(K),sodium(Na),and phosphorus(P),but less aluminum(Al)and iron(Fe).The contents of copper(Cu)and zinc(Zn)were comparable across seedlings (Fig.6C, D), indicating that cellular osmotic conditions(ion contents)are altered in DRR-OE seedlings.These results further suggest that ZmDRR206 functions in CWI maintenance.
3.8.Altered biosynthesis of defense-associated phytohormones and constitutive expression of defense-associated genes in DRR-OE seedlings
The altered cell-wall composition observed in DRR-OE seedlings prompted us to measure the transcript levels of genes involved in JA/SA and ethylene biosynthesis in DRR-OE seedlings, as the impairment of CWI triggers responses that include the activation of defense gene transcription; JA, SA, and/or ethylene production;and lignin accumulation[30,35].It is well known that most lipoxygenase (LOX) genes can be induced by JA, eight lipoxygenase (LOX)genes were found to be specifically up-regulated in DRR-OE relative to WT seedlings, some of them potentially synthesizing JA,whereas up-regulation of the genes encoding allene oxide synthase(AOS) and allene oxide cyclase (AOC) did not reach statistical significance.Four PAL genes were up-regulated in DRR-OE seedlings,together with the significantly elevated CA content in DRR-OE(Fig.S7B; Table S1), indicating that both JA and SA biosynthesis are induced.Consistently, the contents of JA, JA-Ile (the bioactive form of JA), 12-oxo-phytodienoic acid (OPDA, the precursor for JA biosynthesis), and SA were all significantly increased, while ACC(precursor in ethylene biosynthesis) content was significantly decreased in DRR-OE relative to WT seedlings (Fig.7A).JA/SAregulated and/or defense-associated genes were constitutively up-regulated in DRR-OE seedlings, including jasmonate-regulated genes (JRGs), jasmonate-induced protein genes (JIPs), vegetative storage protein genes (VSPs), pathogenesis-related genes (PRs),disease-resistance genes and chitinase genes (Table S1).We confirmed the up-regulated expression of several defense-associated genes in DRR-OE relative to WT seedlings by RT-qPCR: two WRKY TF genes(WRKY11 and WRKY69),four PR genes(PR1,PR10a,PR10b,and PR3),three chitinase genes(CHN5[chitinase chem5],AEC[Acidic endochitinase], and BEC [Basic endochitinase A]), and five JAregulated genes (two JIPs, VSPpni288, VSP2, and LOX2) (Fig.7B).ZmDRR206 overexpression significantly upregulated genes belonging to multiple TF families:MYB(eight genes),WRKY(nine genes),basic helix-loop-helix (bHLH, eight genes), ERF (13 genes), basic leucine zipper (bZIP, five genes), and NAC (seven genes).Other genes encoding growth-promoting TFs were downregulated in DRR-OE relative to WT seedlings,including GATA genes,TCP genes,PRE3 genes (Table S1).These altered patterns of gene expression further suggest that ZmDRR206 acts in CWI maintenance and contributes to the altered growth and stress response of DRR-OE seedlings.

Fig.5.Changes in the transcriptome induced by ZmDRR206 overexpression in maize seedling.(A) The most-enriched KEGG pathways of the DEGs from DRR/WT transcriptome comparison.Circle sizes indicate DEG numbers and color gradients indicate enrichment significance.RichFactor is the ratio of the DEG number enriched in the term to the total number of the gene interpreted in the term.(B)Enrichment of ZmDRR206 down-regulated genes in translation- and photosynthesis-associated functional categories and enzyme activity of up-regulated genes.DRR and WT represent DRR-OE and WT seedlings grown under normal conditions.(C) Expression of photosynthesisassociated genes was constitutively down-regulated in DRR-OE relative to WT seedlings by RT-qPCR.ZmGT-3b (Zm00001d017752), ZmPSII3 (Zm00001d049387), ZmLHCII(Zm00001d021784), ZmLHC117 (Zm00001d039040).(D) Expression of cell-wall biosynthesis associated genes was constitutively up-regulated in DRR-OE relative to WT seedlings by RT-qPCR.tC4M (Zm00001d009858), trans-cinnamate 4-monooxygenase; AOMT (Zm00001d005998), Caffeoyl-CoA O-methyltransferase1; CAD-bm1(Zm00001d015618)/CAD6 (Zm00001d020401), cinnamyl alcohol dehydrogenases.WT represents wild-type LH244 seedlings, DRR4 and DRR6 are ZmDRR206-overexpressing transgenic events DRR-OE4 and DRR-OE6.Values are mean ± SD of three replicates per genotype.
3.9.ZmDRR206 interacts with a cellulose synthase subunit ZmCesA10
We searched for its interaction partners and culminating in the selection of three candidates: ZmCesA10, ZmDIN1 (DARKINDUCED1, a thiosulfate sulfur transferase), and ZmRin1 (ribonuclease 1).We assessed their potential interaction with ZmDRR206 in a split-ubiquitin membrane-based yeast two-hybrid system(DUALmembrane system) and by luciferase complementation imaging (LCI) assay.As the negative controls, the cotransformation of DRR206-Cub with the NubG empty vector prevented the yeast cells from growing on the selective medium.In contrast, yeast cells co-transformed with DRR206-Cub and NubGCesA10p; DRR206-Cub and NubG-DIN1; or DRR206-Cub and NubGRIN1 constructs grew on the selective medium, indicating that DRR206 and CesA10p, DRR206 and DIN1, DRR206 and RIN1 interacted in yeast to activate the expression of the reporter gene for growth selection (Fig.8A).In the LCI assay, we cloned the coding sequences of the potential interactors in-frame and upstream of the sequence of the N-terminal half of firefly luciferase (nLUC, in the JW771 vector) to produce CesA10p-nLUC, DIN1-nLUC, and RIN1-nLUC.We also cloned the coding sequence of ZmDRR206 into JW772 (harboring the sequence for the C-terminal half of luciferase, cLUC) to produce cLUC-DRR206.We detected strong luminescence signals when the pairs of constructs cLUC-DRR206 and CesA10p-nLUC; cLUC-DRR206 and DIN1-nLUC; or cLUC-DRR206 and RIN1-nLUC were co-infiltrated into N.benthamiana leaves, but not their negative control construct combination (Fig.8B), indicating that interaction occurred between DRR206 and CesA10p, DRR206 and DIN1, and DRR206 and RIN1 in planta.The interaction of ZmDRR206 and ZmCES10p was further confirmed by Co-IP assay in vivo (Fig.8C).In contrast to the random distribution of the free GFP or mCherry signal in the cytoplasm and nucleus,both DRR206-GFP and CesA10p-mCherry signal were associated with the cell periphery, CesA10p-mCherry alone accumulated into large spots in the cell periphery(suggesting its association with a specific subdomain of the PM), whereas DRR206-GFP signal colocalized with CesA10p-mCherry signal in the cell periphery of the epidermal cells and DRR206-GFP disrupted the large spot induced by CesA10p-mCherry alone (Fig.8D).
We observed marked co-expression of ZmDRR206 and ZmCesA10 during maize growth and development.They were abundantly expressed in young shoots, roots, and leaves [32],and expression of both genes was induced by F.graminearum inoculation after 6 h in 5-DAG roots of the resistant but not the susceptible NIL [27].They were specifically expressed to comparable levels in crown root nodes, non-pollinated internodes at 24 DAP,V9 eleventh leaves, V9 immature leaves, V9 thirteenth leaves, 5-day-old primary roots, 5-day-old root cortex, and 7- to 8-day-old secondary roots, based on a survey of RNA-seq data using qTeller(Fig.S9).These results further suggest an in vivo interaction between ZmDRR206 and ZmCesA10.
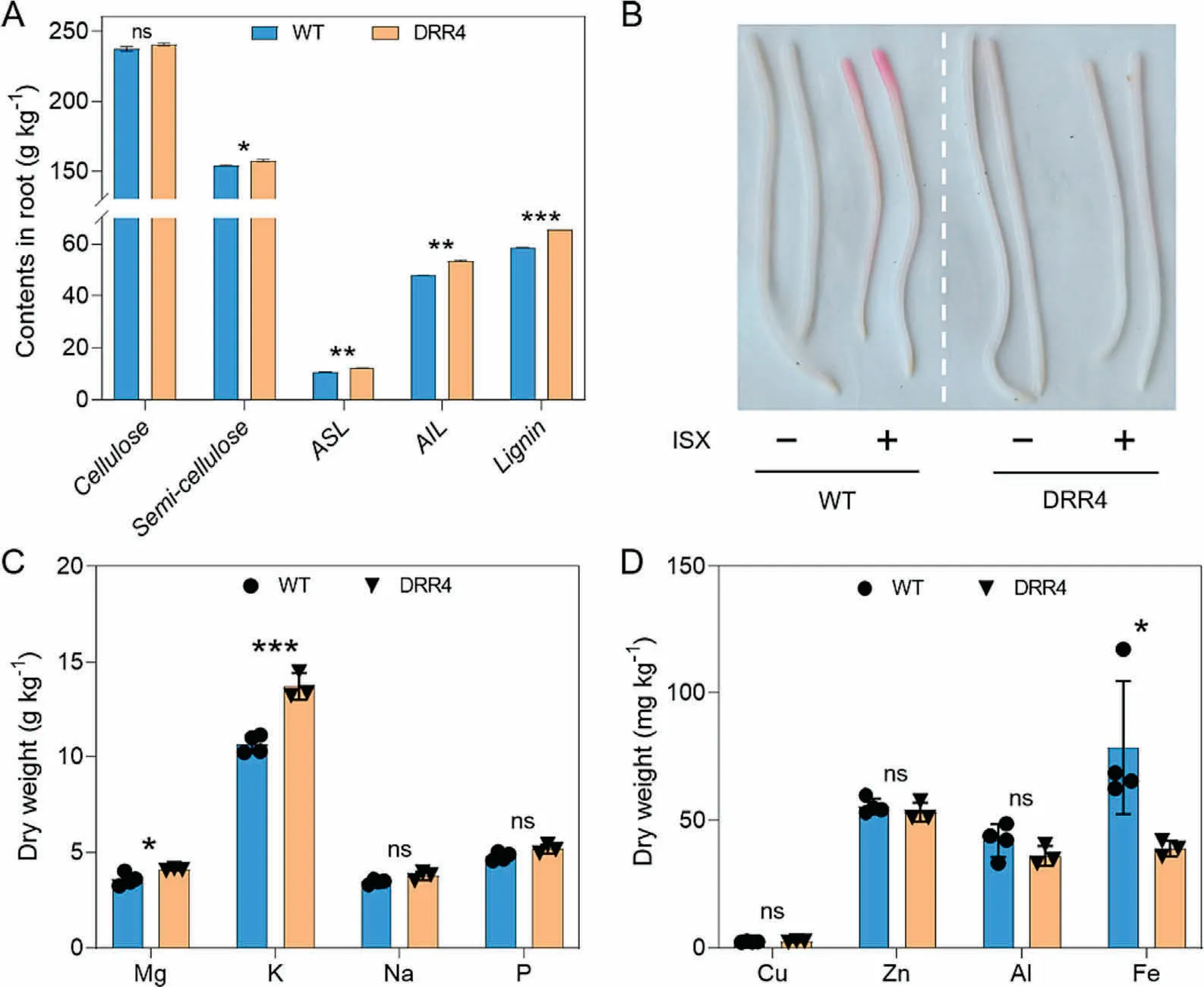
Fig.6.Role of ZmDRR206 in cell-wall integrity maintenance during maize seedling growth.(A)Contents of major cell-wall components in 7-DAG maize seedling roots.ASL,acid-soluble lignin, AIL, acid-insoluble lignin; lignin is the sum of AIL and ASL.Values are mean ± SD of three replicates per genotype.(B) DRR-OE seedlings are resistant to isoxaben(ISX,a cellulose biosynthesis inhibitor)treatment.The apical part of ISX-treated WT primary root tips was stained red,while the ISX-treated DRR-OE seedling root was not, by lignin-specific phloroglucinol-HCl staining.(C, D) Contents of the macro-elements (C) and micro-elements (D) in WT and DRR-OE (DRR4) maize seedlings at 7-DAG.Values are mean ± SD of three replicates per genotype.*, P < 0.05; **, P < 0.01; ***, P < 0.001 by paired Student’s t-test.
4.Discussion
Plant cells monitor the status of their cell walls with various types of sensors and receptors at the PM,some of which may interact with cell-wall components to coordinate mechanical changes in cell-wall structure and cellular responses [4].Disruption of CWI results in a variety of compensatory reactions, including ROS production [30], ectopic deposition of lignin [35], alteration of other cell-wall components [36], and increased JA and ethylene production [37].The reallocation of energy to defense generally comes at the expense of plant growth, owing to competition for limited resources between growth and defense responses [38].The observed stunted growth of many cell-wall mutants associated with attenuated cell-wall biosynthesis may partially reflect responses mediated by constitutive activation of defense pathways, and the ensuing tradeoff between growth and defense,rather than being directly caused by a physically weakened cell wall [19,39,40].Cell-wall strengthening can be activated by loss of CWI via the mechanisms that monitor cell-wall status.DIRs have been suggested[13,30]to represent one set of potent effectors acting downstream of the CWI signaling cascade by mediating the spatial control of lignin deposition during growth and stress responses.
The induction of ZmDRR206 expression upon pathogen infection in multiple maize lines with diverse backgrounds suggests that ZmDRR206 functions like its soybean ortholog [12] in increasing disease resistance.There were three major findings in this study.First, we identified ZmDRR206 as a regulator of maize seedling growth and basal immunity: The abundance of this protein was closely correlated with maize disease resistance, ZmDRR206 positively regulated cell-wall biosynthesis and defense responses, but negatively associated with photosynthesis, thus shedding light on how maize integrates growth, overall cell-wall biosynthesis, and adaptation to biotic stress.Second, we showed that ZmDRR206 functions in CWI maintenance by coordinately regulating the biosynthesis of cell-wall components.ZmDRR206 overexpression increased the contents of major cell-wall components and conferred resistance to ISX treatment.Interaction between ZmDRR206 and ZmCesA10 was also established.Third, we identified the negative effect of ZmDRR206 overexpression on the physiological processes of photosynthesis and translation during maize seedling growth, possibly associated with the interaction between ZmDRR206 and various other proteins.
4.1.ZmDRR206 functions in CWI maintenance during maize seedling growth and defense responses

Fig.7.Altered biosynthesis of phytohormones and constitutive expression of defense-associated genes in DRR-OE seedlings.(A)Contents of phytohormones in WT and DRROE seedlings at 6 DAG.JA-Ile(Jasmonoyl-isoleucine)is the active form of JA;OPDA(12-oxo-phytodienoic acid)is the precursor in JA biosynthesis;ACC(1-aminocyclopropane-1-carboxylate)is the precursor in ethylene biosynthesis.Values are mean±SD of three replicates per genotype.*,P<0.05;**,P<0.01;***,P<0.001 by paired Student’s t-test.(B)Constitutive up-regulation of defense-associated genes in DRR-OE seedlings by RT-qPCR.WRKY11 and WRKY69 encoding WRKY TFs;PR1,PR10a,PR10b and PR3 encoding PR proteins; CHN5, AEC and BEC encoding chitinases; JIP, VSP, and LOX2 are JA regulated genes.WT is LH244 seedlings, DRR4 and DRR6 are ZmDRR206-over-xpressing transgenic events DRR-OE4 and DRR-OE6.Values are mean ± SD of three replicates per genotype.
Plant cell-wall modifications via mutation or overexpression of cell wall-associated genes substantially influence disease resistance and/or tolerance to abiotic stresses[41,42].The observed disease resistance phenotypes in mutants or transgenic plants with wall alterations are thought [20,43]to be caused by the activation of defense signaling pathways,rather than by an inability of pathogens to overcome these modified wall composition or structures.Cellulose affects many aspects of plant life and fitness through its central role in determining the mechanical properties of plant cell walls.A functional cellulose synthase complex (CSC) requires the cooperation of at least three distinct CesA proteins for the biosynthesis of both primary and secondary cell walls[44–46].Arabidopsis CesA8, CesA7, and CesA4 are secondary cell wall–specific cellulose synthase catalytic subunits; their respective mutants, irregular xylem1(irx1),irx3,and irx5,display collapsed xylem,altered CWI, and increased disease resistance against multiple pathogens,associated with altered expression of genes involved in CWI[47,48].The constitutive activation of immune responses in irx1/3/5 plants probably explains their tradeoff phenotypes (dwarf plants,reduced seed yield, and increased disease resistance).Likewise, all mutants identified in OsCesA4, OsCesA7, and OsCesA9,which encode functional orthologs of the Arabidopsis enzymes[49], share a brittle culm (bc) phenotype and lower cellulose contents in their secondary wall,accumulate JA and ethylene,and display abnormal plant growth characterized by dwarf stature with small leaves and withered leaf tips[50–52].DRR-OE seedlings also displayed diminished growth; increased disease resistance; concomitant rise of cellulose, semi-cellulose, and lignin contents,and increased JA and SA levels, relative to WT seedlings (Figs.3–7).Consistently, CesA genes and a subset of secondary metabolite biosynthesis-associated genes, in particular genes encoding enzymes of lignin biosynthesis, including ZmCesA10, ZmCesA11,and ZmCesA12, were constitutively upregulated in DRR-OE relative to WT seedlings (Fig.5D).ZmCesA10 is an ortholog of OsCesA7.ZmDRR206 physically interacted with ZmCesA10 and their encoding genes were co-expressed in various maize tissues(Figs.8,S9A–C).ISX is frequently used to impose cell-wall stress in plant CWI maintenance research,as ISX impairs the function of CesA enzymes to induce the reorganization of primary and secondary cell walls and altered wall compositions with lower cellulose biosynthesis,which causes CWD and subsequent CWI alterations that activate immune responses, like the ectopic biosynthesis and deposition of lignin, and increased production of JA and ethylene [18,19].DRR-OE seedlings had higher contents of cell-wall components and were resistant to ISX treatment (Fig.6A, B).The resistance to ISX-induced cell-wall stress of DRR-OE seedlings may have been associated with reinforcement of their cell walls by higher contents of cell-wall components.
CWI maintenance is closely associated with mechanosensing and turgor monitoring for key processes underlying plant development and stress response.Ion channels and ion transporters are involved in monitoring turgor pressure levels [53].Compared to WT seedlings, DRR-OE seedlings accumulated more Mg, K, Na,and P (Fig.6C, D).Potassium (K+) is critical for the adaptive responses of plants to various abiotic or biotic stresses, as increased K+uptake confers higher levels of drought tolerance[54].Lignin is the first barrier for metal ions, its biosynthesis is associated with heavy metal absorption, transport, and tolerance in plants.Lignin binds to multiple heavy metal ions and reduces their entry into the cytoplasm[55].The reduced Al and Fe contents in DRR-OE versus WT seedlings might be associated with increased lignin biosynthesis.In view of the altered cell-wall composition and the cellular osmotic conditions, the upregulation of cell-wall biosynthesis-associated genes,the constitutively activated defense response, the tradeoff phenotype of DRR-OE seedlings, and the interaction between ZmDRR206 and ZmCesA10, we propose that ZmDRR206 coordinately regulates the biosynthesis of cell-wall components and functions in CWI maintenance during maize seedling growth and defense responses.

Fig.8.Physical interaction between ZmDRR206 and its protein partners.(A)Y2H assays between ZmDRR206 and ZmCesA10p,ZmDRR206 and ZmDIN1,and ZmDRR206 and ZmRIN1.NMY51 yeast cells co-transformed with DRR206-Cub and NubG-CesA10p or DRR206-Cub and NubG-DIN1, or DRR206-Cub and NubG-RIN1 constructs, grew well on synthetic dropout (SD) medium without Ade, Leu, Trp, His (right panel, SD/-Ade-Leu-Trp-His) selective medium, but not the control combination DRR206-Cub and NubG vector.(B) Interaction between ZmDRR206 and ZmCesA10p, ZmDRR206 and ZmDIN1, and ZmDRR206 and ZmRIN1 by LCI assay in N.benthamiana.Overlapping signal of luciferase and light is shown.cLUC, C-terminal LUC; nLUC, N-terminal LUC.Fluorescent signal intensity represents interaction activities.The combinations used for interaction between ZmDRR206 and the protein of interest are labeled, the combination between cLUC and the protein of interest are the negative controls.These experiments were performed three times with similar results.Scale bar,1 cm.(C)Co-IP assay to confirm the interaction of ZmCES10p-Myc and ZmDRR-GFP in vivo.Tobacco leaves co-transformed with p35S: ZmDRR-GFP (p35S: GFP as control) and pSuper: ZmCES10-Myc were used in immunoprecipitation assays with anti-GFP antibody, and the immunoblot was probed with anti-Myc antibody.(D) ZmDRR206-GFP co-localized with ZmCesA10p-mCherry in the periphery of leaf cells.Both ZmDRR206-GFP and ZmCesA10p-mCherry fluorescence signal were localized predominantly to the cell periphery of the intact leaf tissue,and ZmCesA10p-mCherry accumulated into large spots in the cell periphery.The N.benthamiana leaves were infiltrated with Agrobacterium that contained pSuper:mCherry,or pCaMV35S:GFP or pCaMV35S:ZmDRR206-GFP and/or pSuper: ZmCesA10p-mCherry.Scale bars, 20 μm.
4.2.ZmDRR206 overexpression activates constitutive defense responses in maize seedlings
The biosynthesis of cell-wall components is coordinately regulated.Deposition of cellulose and lignin in cell walls is vital for growth and defense responses[56,57].Lignin contributes to pathogen resistance,while pathogens can target the host proteasome to modify the cell-wall secondary metabolism to facilitate their invasion.Cinnamyl alcohol dehydrogenase(CAD)is the final enzyme in the monolignol biosynthetic pathway and thus directly affects lignin accumulation.Rhizoctonia solani targets ZmCAD for ubiquitination and degradation by interacting with the F-box protein ZmFBL,resulting in reduced lignin accumulation and susceptibility to banded leaf and sheath blight [58].Chitinases are PR proteins involved in non-host-specific defense and are thought [59] to be involved in cell-wall modification and activation of ethylene production.ZmDRR206 overexpressing plants displayed increased cell-wall biosynthesis, increased JA, JA-Ile and SA levels, and constitutive expression of many defense-associated genes, including multiple PAL-, PR-, chitinase- and CAD-encoding genes, and JAregulated genes (Fig.7b; Table S1).However, it was unexpected to find the lower ACC level in DRR-OE seedlings.It is possible that ACO activity is higher than ACC activity, leading to more ethylene production and stronger signaling (shown by the up-regulated 13 ERFs)in DRR-OE relative to WT seedlings(Fig.7A;Table S1).Melatonin functions in ameliorating various common abiotic and biotic stresses, including cold, drought, heavy metals, salt, and pathogen attack[60].The contents of the three protectants allantoin,choline,and melanin were all increased>1.5-fold in DRR-OE relative to WT seedlings (Fig.S4).Collectively, ZmDRR206 overexpression activated a constitutive defense response, visible as the slow growth phenotype of DRR-OE seedlings from the growth–defense tradeoff.
ZmDRR206 may affect different physiological pathways by physically interacting with various proteins.Indeed, ZmDRR206 interacted with ZmDIN1 (Fig.8A, B), which is a senescenceassociated dark-inducible protein.DIN genes may mediate the basal immune responses used by cells to cope with stresses imposed by senescence, pathogen infections, and SA, JA, darkness,or photosynthesis inhibitors[61].A common theme to these stress conditions is cellular damage caused by oxidative stress, suggesting that there may be overlapping factors that participate in multiple stress-response pathways.Arabidopsis DIN6 and DIN11 are regulators during systemic virus infection[62].Tobacco(Nicotiana tabacum)Ntdin functions in sulfur and nitrogen metabolism via its relationship with nitrate reductase activity and molybdenum cofactor (Moco) biosynthesis.Ntdin expression is also upregulated by both ABA and IAA,likely because the biosynthesis of these phytohormones requires Moco [63].The physical interaction between ZmDRR206 and ZmDIN1 may thus modulate various aspects of the constitutive defense response in DRR-OE seedlings.
4.3.The possible role of ZmDRR206 in photosynthesis and translation
Photosynthesis affects plant cellular ability to initiate a defense response by generating ROS and immune response-associated signals such as SA.Pathogen infection represses photosynthesis,affects primary metabolism,slows plant growth,and modifies secondary metabolism toward defense responses.The products of photosynthesis (sugars) can activate defense-related responses and modulate key signaling elements of defense phytohormone pathways in an attempt to strike a balance between growth and defense responses [64].Cell-wall dynamics affect photosynthetic properties.Indeed, diffusion of CO2from the leaf intercellular air space to the site of carboxylation is a potential target trait for increasing photosynthetic efficiency and crop productivity.The cell wall characteristics of thickness and porosity have been reported[65–68] to influence mesophyll conductance (gm) and photosynthesis,as dynamic changes in cell-wall composition and rearrangements of cell-wall components affect gmunder nonstress conditions.Cell-wall modifications modulated photosynthesis in rice mutants.Knockout mutants in Cellulose synthase-like family 6(OsCSLF6) were disrupted in cell-wall mixed-linkage glucan(MLG) production and showed lower gmthan the WT, suggesting that a change in cell-wall properties influences the diffusivity and availability of CO2[69].The rice fragile culm 24(Osfc24)mutant is dwarf and brittle, with cell-wall composition altered by lower contents of cellulose and pectin but higher levels of semicellulose and lignin.The Osfc24 mutant has pale leaves with lower chlorophyll contents,as well as diminished photosynthetic activity[70].Changes in pectin physicochemical properties induce modifications in wall architecture by rearranging cell-wall compounds.The Arabidopsis mutants atpme17.2 (pectin methylesterase17.2)and atpae11.1 (pectin acetylesterase11.1) are characterized by a lower proportion of cell-wall pectin, and decreased gmand photosynthesis [71].Collectively, these observations suggest that cellwall modifications strongly influence photosynthetic output.ZmDRR206 overexpression repressed the expression of photosynthetic genes and activated the expression of genes involved in cell-wall formation, resulting in increased contents of cell-wall components and reduced chlorophyll contents and photosynthesis activity in DRR-OE relative to WT seedlings (Figs.2, 5), suggesting that ZmDRR206 may affect chlorophyll biosynthesis and photosynthesis by regulating cell-wall biosynthesis.
ZmRIN1 is annotated as a ribonuclease with endoribonuclease,which is involved in RNA catabolism, and functions in RNA maturation and decay, including ribosome RNA processing [72].The physical association between ZmDRR206 and ZmRIN1 (Fig.8A, B)may explain the downregulation of DEGs enriched in ribosomeand translation-associated functional categories in DRR-OE relative to WT seedlings, reflecting a negative association between ZmDRR206 and translation (Fig.6A, B).Ribonucleases have been proposed to provide cells with the means to recognize and destroy unwanted genetic material [73].Small RNA–mediated sequencespecific cleavage is used in both prokaryotes and eukaryotes to combat virus infection via the double-stranded RNA binding domain of ribonucleases.Ribonucleases are also necessary for the generation of microRNAs (miRNAs), which are small noncoding RNAs that fine-tune protein abundance via translational repression in both the plant and animal kingdoms.miRNAs also act as sequence-specific guides for the RNA-induced silencing complex by binding to their cognate mRNA targets and inducing their cleavage [72].ZmDRR206 may thus be involved in the suppression of ribosome and translation activity via its interaction with ZmRIN1.
We propose that ZmDRR206 acts as a conserved component in maize seedling growth and defense responses to external biotic or abiotic stresses via its physical interactions with other proteins.In a working model for the role of ZmDRR206 in maize seedling growth and defense response: ZmDRR206 coordinately regulates cell-wall biosynthesis for CWI maintenance and defense response by physically interacting with ZmCesA10 and ZmDIN1, thus optimizing the temporal and spatial expression of growth promotion(photosynthesis and translation), cell-wall biosynthesis, and defense-associated genes to coordinate metabolism during seedling growth and defense response to external environmental stress(Fig.S10).
CRediT authorship contribution statement
Tao Zhong:Visualization, Data curation.Suining Deng:Data curation.Mang Zhu:Methodology, Investigation.Xingming Fan:Investigation.Mingliang Xu:Supervision.Jianrong Ye:Conceptualization, Writing – review & editing, Funding acquisition, Data curation, Supervision, Project administration, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2018ZX0800917B)and grant from Yunnan Provincial Science and Technology Department (202005AF150026).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.09.007.
- The Crop Journal的其它文章
- OsSPL10 controls trichome development by interacting with OsWOX3B at both transcription and protein levels in rice (Oryza sativa L.)
- Ectopic expression of OsNF-YA8, an endosperm-specific nuclear factor Y transcription-factor gene, causes vegetative and reproductive development defects in rice
- Mechanisms of autophagy function and regulation in plant growth,development, and response to abiotic stress
- The plasmodesmata-associated β-1,3-glucanase gene GhPdBG regulates fiber development in cotton
- The MabHLH11 transcription factor interacting with MaMYB4 acts additively in increasing plant scopolin biosynthesis
- Evolutionary genetics of wheat mitochondrial genomes

