GA Associated Dwarf 5 encodes an ent-kaurenoic acid oxidase required for maize gibberellin biosynthesis and morphogenesis
Zulin Li, Bozhu Li, Junli Zhn, Honlin Wn, Mo Wn, Siyi Guo,b,c,Pnto Wn,b,c, Zhi Li,b,c, Dvi W.Glbrith,f, Dnn Li, Chun-Pn Son,b,*
a State Key Laboratory of Crop Stress Adaptation and Improvement, State Key Laboratory of Cotton Biology, School of Life Sciences, Henan University, Kaifeng 475004, Henan, China
b Sanya Institute of Henan University, Sanya 572025, Hainan, China
c Academy for Advanced Interdisciplinary Studies, Henan University, Kaifeng 475004, Henan, China
d Shanghai Center for Plant Stress Biology, CAS Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China
e National Engineering Research Centre for Wheat, State Key Laboratory of Wheat and Maize Crop Science, College of Agronomy, Henan Agricultural University, Zhengzhou 450046, Henan, China
f School of Plant Sciences and Bio5 Institute, The University of Arizona, Tucson, AZ 85721, USA
g China-UK-NYNU-RRes Joint Laboratory of Insect Biology, Henan Key Laboratory of Insect Biology in Funiu Mountain, Nanyang Normal University, Nanyang 473061, Henan, China
Keywords:Maize (Zea mays L.)ZmGAD5 GA biosynthesis KAO
ABSTRACT Gibberellin(GA)functions in plant growth and development.However,genes involved in the biosynthesis and regulation of GA in crop plants are poorly understood.We isolated the mutant gad5-1 (GAAssociated D warf 5), characterized by dwarfing, short internodes, and dark green and short leaves.Map-based gene cloning and allelic verification confirmed that ZmGAD5 encodes ent-kaurenoic acid oxidase(KAO),which catalyzes KA(ent-kaurenoic acid)to GA12 conversion during GA biosynthesis in maize.ZmGAD5 is localized to the endoplasmic reticulum and is present in multiple maize organs.In gad5-1,the expression of ZmGAD5 is severely reduced,and the levels of the direct substrate of KAO,KA,is increased,leading to a reduction in GA content.The abnormal phenotype of gad5-1 was restored by exogenous application of GA3.The biomass, plant height,and levels of GA12 and GA3 in transgenic Arabidopsis overexpressing ZmGAD5 were increased in comparison with the corresponding controls Col-0.These findings deepen our understanding of genes involved in GA biosynthesis, and could lead to the development of maize lines with improved architecture and higher planting-density tolerance.
1.Introduction
Maize(Zea mays L.)provides industrial raw material and animal feed worldwide [1].Preserving maize yield per plant at higher planting densities represents a promising agronomic strategy for meeting an ever-increasing demand for the crop [2,3].Improving plant morphology is a route to adapting maize to high-density cultivation and increasing biotic and abiotic stress tolerance[2,3].The Green Revolution,which began in the 1960s,was initiated with the use of dwarfing genes that permitted higher planting densities[4,5].Plant endogenous hormones,especially gibberellin,influence plant morphogenesis, yield, light energy utilization, and tolerance of high plant density [6–9].A better understanding of these roles could lead to improved maize germplasm.
GAs are diterpenoid hormones that are widely distributed across angiosperms, gymnosperms, and ferns.Of the 136 gibberellins discovered and structurally analyzed,the biological activities of only GA1, GA3, GA4, and GA7are known [10,11].Recently,Liu et al.[12] found that, in Arabidopsis thaliana, 2OG-Fe (II) oxidoreductase catalyzes the formation of a new atypical gibberellin(DHGA12) from GA12[12].DHGA12binds to the GA receptor(GID1), and shows GA-like biological activity in promoting seed germination and seedling morphogenesis, albeit with lower affinity.GA is also known[13,14]to function in regulating seed germination, stem elongation, pollen development, fruit growth, and other plant developmental processes.A study [15] of GAdeficient mutants in various plants, including maize, have shown that decreased levels of GA result in typical GA-deficient dwarf phenotypes and increased stress tolerance.Peng et al.[16]created semidwarf wheat(Triticum durum)and rice(Oryza sativa)mutants based on the regulation of GA signal transduction and biosynthesis.In maize, GA-deficient mutants (dwarf1 [d1], d3, d5, and an1) and GA-insensitive mutants (d8 and d9) displayed a filamentous phenotype in male flowers,suggesting that GA also functions in reproductive growth [17–23].
The accumulation of GA in plants depends on the catalytic activities of enzymes in its biosynthetic pathway.GA biosynthesis is thought[12,24–27]to occur in three stages;ent-kaurene synthesis in plastids, the transformation of ent-kaurenoic acid (KA) into GA12on the plastid envelope and then on the ER, and the conversion of GA12to various GAs in the cytoplasm.Terpene synthase(TPS), cytochrome P450 monooxygenase, and 2-oxyglutaratedependent dioxygenase function in GA biosynthesis and accumulation in plants [26–29].The TPS enzymes ent-copalyl diphosphate synthase (CPS) and kaurene synthase (KS) catalyze the synthesis of kaurene [11,29,30].Ent-kaurene oxidase (KO) and entkaurenoic acid oxidase (KAO), which belong to the 2-oxoglutarate-dependent dioxygenases,catalyze the transformation of KA to GA12[31–33].The production of various kinds of GAs is catalyzed by two dioxygenases of 2-oxyglutarate (GA20 oxidase[GA20ox] and GA3-oxidase [GA3ox]) from GA12[34–36].
Many enzymes in GA biosynthesis have been identified in plants.CPS and KS were both found to be encoded by genes in Arabidopsis and rice:GA1 and GA2 in Arabidopsis and OsCPS1 and OsKS1
in rice [11,29,30].In Arabidopsis, a KO-encoding gene has been cloned and named GA3, and its deletion results in severe dwarfing[32,33].In rice, Sakamoto et al.[35] identified OsKO2 and confirmed it to regulate GA synthesis and plant morphology.CYP701A26 has been confirmed [37] to function as an entkaurene oxidase in maize GA biosynthesis.Regnault and his teams[29] reported that two KAO encoding genes (KAO1 and KAO2) in Arabidopsis thaliana have functional redundancy in seed germination and seedling development, and that mutations of a single KAO gene do not result in typical GA-deficient phenotypes.In barley and pea,KAOs with catalytic activity have been identified[38].Many functional GA20 oxidases (GA20ox) and GA3-oxidases(GA3ox) have been identified in plants, such as GA 20-oxidase in pumpkin and AtGA3ox1 (GA4) in Arabidopsis [27,39,40].ZmGA3ox2 has been confirmed [41] to regulate GA synthesis and plant height.Chen et al.[42] reported that ZmGA3ox (dwarf1,D1) catalyzes the transformation of GA20into GA1and GA3, GA5into GA3, and GA9into GA4in vitro.d1 mutant plants exhibit GA deficiency and developmental abnormalities, including stunted plants and andromonoecy [43].
The identification and functional characterization of other enzymes involved in GA biosynthesis in plants,especially in crops,awaits further research.A better understanding of GA synthesis may eventually result in improvement of plant morphology, permitting higher planting densities without yield penalty.
We describe a novel maize dwarf mutant,named gad5-1,that is deficient in stem node development and plant morphogenesis.The gad5-1 mutant shows severe growth and development phenotypes,including delayed seed germination, and short plant stature and stem segment lengths.Supplementation with exogenous GA3partially restores the gad5-1 developmental deficiency phenotypes.Map-based cloning revealed that ZmGAD5 resides in the 9.03 bin of maize chromosome 9 and encodes a KAO enzyme in the GA biosynthesis pathway.Loss of expression of ZmGAD5 resulted in excessive accumulation of KA and reduced levels of GA3and GA12.The cloning and functional classification of ZmGAD5 provide a theoretical basis for the regulatory pathways of GA biosynthesis in maize and could lead to the creation of maize with improved morphology.
2.Materials and methods
2.1.Plant materials
The maize materials used were inbred lines B73, Mo17, gad5-1 and its corresponding WT (gad5-1/+, +/+), dwarf plant3-2 (d3-2), a BC5F1backcross population, an F2mapping population.As described by Wang et al.[44], the pollen of the maize inbred line B73 was treated with ethyl methanesulfonic acid (EMS), and the resulting M2seeds were screened to obtain the gad5-1 mutant.The BC5F1was constructed by crossing gad5-1 with B73 for the genetic identification of mutants, and the F2population was constructed by crossing gad5-1/+ with Mo17 for ZmGAD5 mapping.The F1population was constructed by crossing gad5-1/+ with d3-2/+ for allele verification.All materials were planted in the experimental field of Henan University in Kaifeng, Henan province in summer,and in the experimental field of Hainan in Sanya,Hainan province in winter (Table S1).Col-0, ZmGAD5-OE1 and ZmGAD5-OE6 transgenic Arabidopsis lines were grown in a culture room in the laboratory.
2.2.Phenotypic analysis of gad5-1
Seeds of the BC5F2generation were sown in small pots (7.5 ×7.5 × 7.5 cm), with two seeds per pot.After watering, they were cultured in an artificial climate chamber (28 °C, light intensity of 400 μmol m-2s-1,light–dark cycle of 14/10 h)[45].After germination and growth for three days, seedlings with consistent growth were selected, following Fujii et al.[46] and Wang et al.[47].Leaves of wild type (WT) and gad5-1 were sprayed with 0, 20,30, and 50 mmol L-1of GA3(gibberellic acid 3, G7645) once per day.Plant heights were recorded every two days.In experiments conducted in parallel, concentration gradients 0, 50, 100, and 200 mmol L-1of IAA (3-indoleacetic acid, I3750) and 0, 200, 300,and 500 mmol L-1of BR(2,4-epibrassinolide,E1641)were applied by foliar spraying to WT and gad5-1 plants, respectively, and changes in plant heights were recorded.
2.3.Map-based cloning of ZmGAD5
2.3.1.Map-based cloning
The F2population was used for map-based cloning of ZmGAD5 using the gad5-1 dwarf phenotype.As described by Wang et al.[44], Wang et al.[48] and Li et al.[49], 31 pairs of gad5-1/Mo17 polymorphic markers were screened from 384 pairs of SSR markers covering the entire maize genetic and genomic database(Tables S2,S3).The DNA of 368 F2generation dwarf plants was amplified for preliminary mapping.Based on the maize B73_RefGen_v3 genome sequence (https://www.maizesequence.org), ZmGAD5 was located in the 9.03 bin between the two polymorphic markers pumc2121 and p-ufg70 on chromosome 9.Next, new SSR markers(Table S4)were developed based on all bacterial artificial chromosome sequences in the 9.03 bin, and the sequences of nearly 6000 dwarf F2plants were amplified to calculate the recombination rate.Genes involved in gibberellin synthesis or metabolism on chromosome 9 were then amplified and sequenced (Tables S5, S6).
2.3.2.Whole-genome sequencing
Thirty gad5-1 mutants and WT plants in the BC5F2population were selected for DNA extraction.Equal amounts of DNA from individual plants were mixed to construct two pools, corresponding to dwarf plants (g-1) and plants of normal height (G-2).The pooled DNA samples were then sent to Novogene (https://www.novogene.com/) for whole-genome sequencing and bulked segregation analysis (BSA).
2.3.3.Allelic validation
Seeds representing the F1generation population generated by crossing heterozygous d3-2/+ plants with the corresponding heterozygous gad5-1/+ plants were planted, and the phenotypes recorded.Dwarf plants similar to gad5-1 and d3-2 were observed in the F1generation, with a 3:1 segregation ratio that indicated Mendelian monogenic recessive inheritance (Table S7).
2.4.Gene sequencing
Leaf genomic DNA and RNA of B73,Mo17,gad5-1 mutants,and WT were extracted,and the RNA samples were reverse-transcribed into cDNA.Primers were designed to amplify promoter sequences,genome DNA sequences, and CDS sequences (Table S6).A highfidelity enzyme (Vazyme Biotech Co., Ltd., Phanta Max Super-Fidelity DNA Polymerase,P505)was used to amplify the promoter region and gDNA region with genomic DNA as a template,and the CDS region was amplified with cDNA as a template.All amplification products were sequenced.
2.5.Subcellular localization assay
The 35S::ZmGAD5-GFP and Ubipro::ZmGAD5-YFP fusion vectors were constructed using the following primers: 35 s::ZmGAD5-GFP-F: 5′-ataccaccaatcgactctagaATGCTGGGTGTGGGGATGG-3′,35 s::ZmGAD5-GFP-R:5′-gcccttgctcaccatggtaccGCTACCGACTCTGGT GATCTTG-3′, Ubipro::ZmGAD5-YFP-F: 5′-gcagcccgggggatcaagct tATGCTGGGTGTGGGGATGG-3′, Ubipro::ZmGAD5-YFP-R: 5′-tactag tatttaaatggcgcgccGCTACCGACTCTGGTGATCTTGG-3′.
For transfection assays,protoplasts were isolated from etiolated maize B73 seedlings that had been grown for 15 days in darkness[50].A Ubipro::YFP empty vector was used as negative control.Recombinant vectors composed of Ubipro::ZmGAD5-YFP and Ubipro::YFP were separately co-transformed into protoplasts, along with theendoplasmic reticulum (ER) localization marker.As described by Yoo et al.[51] and Zhang et al.[52], 10 μg plasmid DNA was added to 100 μL protoplast suspension (106per mL),transformed, and incubated at 23 °C in darkness for 14 h.
For transient assays involving plant infiltration, the recombinant 35S::ZmGAD5-GFP and 35S::GFP constructions were transformed into Agrobacterium tumefaciens (GV3101), and the bacteria were co-injected into Nicotiana benthamiana along with an ER localization marker.As described by Liang et al.[53], the Agrobacterium cells containing the GFP constructs and the ER marker were collected and diluted with tobacco incubation buffer to OD600= 0.6–0.8, mixed in equal amounts, treated with acetosyringone, and injected into N.benthamiana leaves.The leaves were incubated in darkness for one day and then under normal light for two days.The 35S::GFP empty vector was used as the negative control.The subcellular localization of the fluorescence of YFP,GFP,and the ER localization mCherry marker were observed by confocal microscopy (LSM980).
2.6.RNA extraction and reverse transcription
Trizol (T9424) was used to extract total plant RNA from roots,stems, leaves, tassels and pistils of maize materials, transformed tobacco leaves and protoplasts.RNA was reverse-transcribed into cDNA with Promega’s reverse transcriptase (M-MLV, M1701/M1705), using 2 μg RNA per 25 μL.The PCR products were stored at –20 °C.
2.7.qRT-PCR detection and analysis
Using cDNA from different tissues as the template and ZmUbiquitin 2 (primers: UBI2-qRT-F: TGGTTGTGGCTTCGTTGGTT,UBI2-qRT-R: GCTGCAGAAGAGTTTTGGGTACA) as an internal control, the relative expression levels of ZmGAD5 (Primers:ZmGAD5-qRT-F: AGGGACTTCAGGAAGATGGA, ZmGAD5-qRT-R:GAAGGAGACGAAGGAGATGTTG) in gad5-1 and the corresponding control were detected using a Roche 480II System(Roche Diagnostics GmbH) fluorescence quantitative PCR instrument [54].Three biological replicates were used for each experiment.
2.8.Extraction and HPLC-MS analysis of GAs
Three-day-old seedlings of maize gad5-1 and WT plants, and seven-day-old seedlings of Arabidopsis Col-0, ZmGAD5-OE1 and ZmGAD5-OE6 plants were selected,with three biological replicates(1 g fresh weight).According to the methods of Chen et al.[55]and Xin et al.[56], the samples were frozen in liquid nitrogen, ground to fine powder, and extracted with 1.0 mL 80% methanol (v/v) at 4 °C for 12 h.After centrifugation (10,000×g, 4 °C, 20 min), the supernatants were collected, and the solvent evaporated under mild nitrogen stream at 35 °C, followed by re-dissolving in 100 μL H2O/acetonitrile (90/10, v/v).
GASwere quantified by ultra-high-performance liquid chromatography-tandem mass spectrometry(UPLC-MS/MS).Samples were analysis with a Xevo TQ-XS system(Waters,Milford,MA,USA)equipped with an ESI ion source.Chromatographic separation was performed with an ACQUITY UPLC HSS T3 column (2.1 × 100 mm,1.8 μm)maintained at 40°C.The mobile phase was composed of solvent A (0.1% formic acid, water) and solvent B (methanol),with a flow rate of 0.3 mL min-1.The linear gradient system was set as follows: 0–2 min, 2% B; 2–10 min, to 80% B; 10–12 min,80%B;12–13 min,to 2%B;13–15 min,2%B.The autosampler temperature was set to 4 °C and the sample injection volume was 10 μL.GA3and GA12MS data were collected in negative-ion mode.KA MS data were collected in positive-ion mode, with multiple reaction monitoring (MRM) mode.Precursor and fragment ions were: GA3(m/z 345.16–239.11), d2-GA3(m/z 347.16–239.11),GA12(m/z 331.10–269.01), d2-GA12(m/z 333.10–269.01), and KA(m/z 302.15–88.00).Data was performed with the spectrometer software (Masslynx v.4.2).
3.Results
3.1.gad5-1 mutants showed severe defects in morphogenesis,growth,and development
To identify the regulatory mechanisms of maize morphogenesis, we collected many abnormal plants from maize mutant libraries.gad5-1, a GA biosynthesis-associated mutant, was obtained by screening ~50,000 M2mutant seeds from M1selfcrossing pollinated by the maize inbred line B73 treated with EMS,which was used for further studies concerning the regulation of plant morphogenesis.
I have served thee in much, Tsarevitch Ivan, said the Gray Wolf, but I will also do thee this service. Listen. When we come near to the Palace, I myself will take the shape of the Tsar s daughter, and thou shalt lead me to Tsar Afron, and shalt take in exchange the Horse with the Golden Mane. Thou shalt mount him and ride far away. Then I will ask leave of Tsar Afron to walk on the open steppe, and when 1 am on the steppe with the Court ladies-in-waiting, thou hast only to think of me, the Gray Wolf, and I shall come once more to thee.
Compared to the WT, gad5-1 showed obvious developmental abnormalities (Figs.1, S1A,B).After germination, gad5-1 seedlings were severely dwarfed(Fig.S1A,B),and seedlings were unearthed late (Fig.S1C).Morphological comparisons of adult plants showed that gad5-1 dwarfism was accompanied by blunt,round,and short leaf edges (Fig.1A, B, D, H).gad5-1 showed no differences in root development relative to the WT(Figs.1F,G,S2).There was no significant difference in the number of stem nodes between gad5-1 and WT,but node lengths were significantly shorter in the mutant(Fig.1F, G, M).This phenotype indicated that the decrease in gad5-1 plant height was due to abnormal development of stem segments.The gad5-1 mutant also displayed defects in reproductive development, with a short male flower axis and few branches(Fig.1C, I).Male flowers did not produce pollen grains naturally(data not shown) and had short female ears (Fig.1E, K).These observations indicated that ZmGAD5 functions in the regulation of maize growth, development, and morphogenesis.
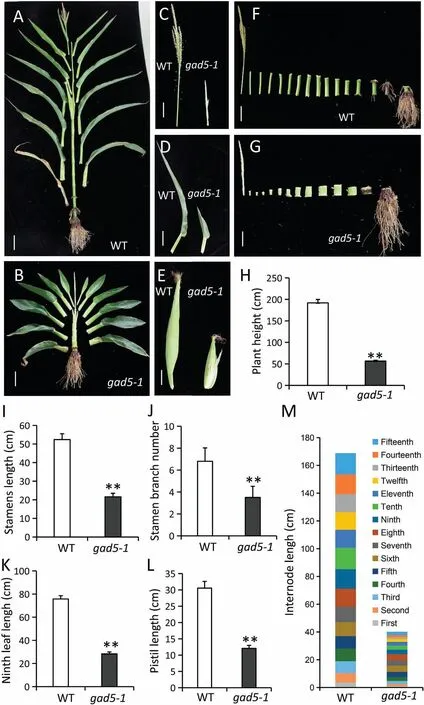
Fig.1.Anatomical phenotypes of WT and gad5-1.(A)Mature WT plants grown in the field under unstressed conditions displayed normal plant heights,normal lengths of stem nodes,and normal leaves(Scale bar,10 cm).(B)Mature gad5-1 plants grown under unstressed conditions in the field showed dwarfing,short stem nodes,and short leaves(Scale bar,10 cm).(C)The mature tassel phenotype of WT and gad5-1(Scale bar,5 cm).(D)The ninth leaf-pistil node leaf blade phenotype of WT and gad5-1(Scale bar,5 cm).(E)The mature pistil phenotype of WT and gad5-1(Scale bar,5 cm).(F)The segmented anatomical phenotype of WT(Scale bar,10 cm).(G)The segmented anatomical phenotype of gad5-1(Scale bar,5 cm).(H)Plant height quantification of WT and gad5-1 mutant mature plants.(I)Length of central axis of the mature tassel of WT and gad5-1.(J)The mature tassel branch number of WT and gad5-1.(K)The mature pistil length of WT and gad5-1.(L)The ninth leaf length(length of the pistil node leaf blade)of WT and gad5-1.(M)Stem internode lengths of WT and gad5-1.Error bars indicate standard deviations.**indicates significant differences from WT at P<0.01 by(Student’s t-test,n=30).
3.2.Map-based cloning and identification of ZmGAD5
Given that the homozygote could not complete normal growth and development, we crossed a gad5-1 heterozygote (gad5-1(+/-))to the inbred line B73, to generate F1hybrid progeny and observe the genetic characteristics of gad5-1.The F2population produced by F1selfing was screened based on the gad5-1 phenotype.In the F2population, the ratio of dwarf plants to normal plants was 1:3(Table S8)., indicating that the gad5-1 phenotype is determined by a single recessive gene.
To further characterize ZmGAD5, a mapping population was constructed by crossing the gad5-1 mutant with the inbred line Mo17.The F2population was screened based on the gad5-1 phenotype.The initial mapping placed the ZmGAD5 locus in a 2.02 Mb region between the simple sequence repeat (SSR) markers 27 M25 and 25 M47 in the 9.03 bin of chromosome 9 (Fig.2A).Investigation of the genes in this region revealed only one(Zm00001d045563) involved in GA biosynthesis.Single nucleotide polymorphism(SNP)and indel linkage diagrams of individual progeny based on whole-genome sequencing technology indicated that the mutant phenotype was tightly linked to Zm00001d045563 (Fig.S3).The phenotypic characterization of the d3-2 (dwarf plant3) mutant of Zm00001d045563 in Maize GDB(https://www.maizegdb.org/) revealed that the seedling phenotype of gad5-1 was identical to that of d3-2 (Fig.S4A, B, C).We accordingly crossed the gad5-1 heterozygote (gad5-1/+) with the d3-2 heterozygote (d3-2/+) In the F1generation, 1/4 of plants showed the dwarf phenotype (Fig.S4D, E; Table S7), confirming that the ZmGAD5 gene is Zm00001d045563.
Bioinformatics analysis indicated that ZmGAD5 encodes a typical cytochrome P450 protein family member (CYP88A1), which amino acid sequence shows high similarity to the KAO protein in Arabidopsis thaliana and rice (Fig.S5).These reports indicate that ZmGAD5 is a typical KAO protein, which likely functions in one step of GA biosynthesis.
In plants,the synthesis and accumulation of GAs are closely regulated across growth stages and within tissues,as has been shown for key enzymes of GA biosynthesis,such as CPS and KS[32,57–59].We accordingly examined the expression pattern of ZmGAD5 across multiple tissues.qRT-PCR analysis of ZmGAD5 transcripts indicated that ZmGAD5 was highly expressed in all examined tissues (leaves and roots of seedlings grown for three days after germination, stems of plantlets grown for seven days after germination, and ears and filaments of plants grown for 45 days after germination) (Fig.2B).In comparison with the WT, the expression of ZmGAD5 in gad5-1 plants was significantly lower across all of these tissues (Fig.2B, C), suggesting the cause of the mutant’s abnormal growth and development phenotype.
3.3.ZmGAD5 is located in the endoplasmic reticulum, and its abundance is decreased in gad5-1 plants
In the GA biosynthetic pathway, the conversion of ent-kaurene to GA12is catalyzed by KO and KAO in the endoplasmic reticulum[11,60–62].To further verify the subcellular location of ZmGAD5,we used two transformation systems (maize protoplast transformation and N.benthamiana transient transformation).The subcellular location of ZmGAD5-YFP and ZmGAD5-GFP was identified by confocal fluorescence microscopy.In both cases, when the coding sequences of YFP and GFP were separately fused with ZmGAD5,the resulting yellow fluorescence from YFP in maize mesophyll protoplasts, and the green fluorescence from GFP in N.benthamiana, co-localized with the red fluorescence of a marker for the ER(Figs.3A, S6).This finding implies that ZmGAD5 is located on the endoplasmic reticulum, consistent with its proposed function as a KAO enzyme.
3.4.GA biosynthesis was inhibited in gad5-1, and exogenous GA3 application rescued the phenotypic defects of gad5-1 seedlings
As a KAO protein, ZmGAD5 was predicted to catalyze the conversion of KA into GA12and to provide precursors for the production of bioactive GAs via the GA synthesis pathway in the endoplasmic reticulum (Fig.4A).To confirm this prediction, we examined the substrate and product levels of the KAO enzyme in gad5-1 plants using high-performance liquid chromatography–mass spectrometry (HPLC-MS).In gad5-1 plants, the content of KA was significantly increased, whereas that of GA12was greatly reduced (Fig.4B, C).Consequently, the content of bioactive gibberellin (GA3), was also severely reduced in gad5-1 plants(Fig.4D).These findings confirmed that ZmGAD5 catalyzes the transformation of KA to GA12in the GA biosynthesis pathway,and thus behaves as a typical KAO enzyme.
To further confirm the role of ZmGAD5 in GA biosynthesis, we sprayed gad5-1 seedlings with bioactive GA3in situ.Exogenous application of GA3rescued the growth of gad5-1 seedlings in a concentration-dependent manner.After treatment with 50 μmol L-1GA3for seven days,the growth of gad5-1 seedlings was comparable to that of WT(Fig.4E,F).However,exogenous application of IAA or BR did not rescue the abnormal developmental phenotype of gad5-1 (Fig.S7).These results suggested that the phenotypes of gad5-1 are caused by the loss of endogenous active GA.
3.5.Overexpression of ZmGAD5 in Arabidopsis promotes GA biosynthesis and plant growth
To further confirm the function of ZmGAD5 as a KAO, we produced transgenic Arabidopsis thaliana lines that overexpressed ZmGAD5.GA synthesis and plant growth were both increased in several of these lines(Figs.5,S8).Compared to Col-0 wt,the transgenic plants had larger rosette leaves (Fig.5A), higher stature(Figs.5C, S8D), and increased biomass (Fig.5B).The transgenic lines (OE1, OE6) had a lower KA content and higher GA3and GA12contents (Fig.5D–F).These results indicate that ZmGAD5 is a KAO enzyme that regulates GA biosynthesis and plant growth and development.
4.Discussion
This study has provided evidence that ZmGAD5 is a maize KAO enzyme that functions in GA biosynthesis and plant morphogenesis.The gad5-1 mutant with downregulated ZmGAD5 transcription levels showed severe defects in GA synthesis and accumulation in both vegetative and reproductive growth stages (Fig.1).The growth-defect phenotype of gad5-1 seedlings was restored by exogenous GA3application (Fig.4E, F).ZmGAD5 is a maize KAO enzyme that is localized to the endoplasmic reticulum and catalyzes the conversion of KA to GA12in the GA biosynthesis pathway.In gad5-1, KA accumulation is greatly increased, and combined GA content (GA12and GA3) is decreased (Fig.4B–D).Our findings reveal the function of ZmGAD5 as a maize KAO enzyme and reveal its roles in controlling GA biosynthesis to regulate plant growth and development.

Fig.2.Map-based identification of ZmGAD5, and molecular biological analyses of the mutant and WT phenotypes.(A) ZmGAD5 was mapped to bin 9.03 of chromosome 9 between the 25 M47 and 27 M25 SSR markers, representing a 2.02 Mb region containing 137 genes (the target gene Zm00001d045563 is labeled in red).(B, C) Relative expression of ZmGAD5 in tissues of WT and gad5-1 detected by qRT-PCR.**indicates significant differences between the WT and gad5-1 at P < 0.01 (Student’s t-test, n = 3).
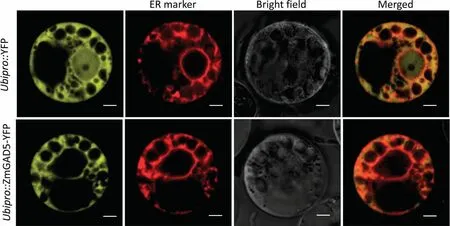
Fig.3.Subcellular localization of ZmGAD5 and ZmGAD5 expression detection.Confocal microscopy images revealing the subcellular co-localization of an ER localization marker with Ubipro::YFP (above) and Ubipro::ZmGAD5-YFP fusion protein (below) in B73 maize mesophyll protoplasts.The yellow fluorescence of Ubipro::ZmGAD5-YFP overlapped well with the red fluorescence of the ER localization marker (Scale bar, 20 μm).

Fig.4.Functional characterization of ZmGAD5 as a KAO involved in maize GA biosynthesis.(A) The role of KAO encoded by ZmGAD5 in gibberellin biosynthesis.(B–D)Kaurenoic acid(KA),GA12 and GA3 content in gad5-1 and the corresponding WT(n=3).(E)Phenotypes of WT and gad5-1 after treatment with GA3(Scale bar,5 cm).(F)Plant height quantification of WT and gad5-1 during GA3 treatment(n=10).Error bars indicate standard deviations.**indicates significant differences from WT plants at P<0.01(Student’s t-test).
Abnormal GA synthesis results in defects in plant growth and development.Fu et al.[23] showed that the loss of ent-kaurene synthase activity in maize results in a defect in GA synthesis that causes a severe dwarfing phenotype.D1, which catalyzes the biosynthesis of GA1, GA3, and GA4in maize, is expressed specifically in tassel primordia, and its deletion inhibits tassel development [42].Dwarf11 (d11), a maize GA synthesis mutant, has a dwarf phenotype that can be restored by treatment with exogenous GA3[46].The finding that severe defects in the growth and development of gad5-1 were restored by exogenous GA3but not by auxin or BR suggests that the gad5-1 phenotype is associated with GA deficiency.
Gene cloning, whole genome sequencing, and allele testing indicated that ZmGAD5 encodes a maize KAO enzyme that catalyzes the conversion of KA to GA12in the GA biosynthesis pathway.This gene was found to be homologous to AtKAO1 and AtKAO2 in Arabidopsis [18,29].The transcript level of ZmGAD5 was severely down-regulated in the gad5-1 mutant (Fig.2C),whereas the catalytic substrate of ZmGAD5, KA, accumulated and GA content decreased (Fig.4B–D).Overexpression of ZmGAD5 in Arabidopsis promoted GA synthesis and growth of transgenic plants,while KA content decreased(Fig.5).We infer that ZmGAD5 acts as a KAO that catalyzes the conversion of KA to GA12, thus affecting GA biosynthesis and plant morphogenesis.
ZmGAD5 is widely expressed in maize tissues undergoing rapid growth(Fig.2B),indicating that it functions in GA biosynthesis and plant growth and development.We also identified a second homolog (ZmKAO2) of ZmGAD5 in maize.In comparison with the WT,there were no differences in the transcription levels or the sequence of ZmKAO2 in gad5-1 (Fig.S9), and the EMS mutant of ZmKAO2 also showed no abnormalities in growth and development in comparison with the corresponding control (Fig.S10).These findings indicate that ZmGAD5 is an irreplaceable component of maize GA biosynthesis.
Many conserved cis-acting elements involved in the initiation and regulation of gene expression reside in promoter sequences and function in the binding of transcription factors to promoter regions as well as the initiation of transcription.Mutations in gene promoters thus frequently result in gene expression changes.When a fragment is inserted in front of the promoter TATA box,the gene sequence is altered,resulting in the alteration of the promotion effect of the TATA box on gene transcription initiation,which may affect the expression of this gene and its regulated phenotype [63,64].In this study, by comparing the gene sequences of ZmGAD5 in the mutant and wild-type plants, we observed an oligonucleotide motif (TATATTTTG) inserted in the promoter sequence of ZmGAD5 in gad5-1, which was 360 bp upstream of the transcription start site close to the TATA box.This insertion may change the ZmGAD5 promoter structure.Whether it influences ZmGAD5 expression and regulation awaits confirmation.Although we have not identified the cause of the severe downregulation of gene expression in gad5-1, these findings may lead to modification of maize morphology by genome editing.

Fig.5.ZmGAD5 is involved in plant development and regulation of gibberellin biosynthesis in Arabidopsis thaliana.(A)The phenotype of ZmGAD5-OE transgenic Arabidopsis plants at the seedling stage(4 weeks).Scale bar,1 cm.(B)Biomass(dry weight)statistics of mature plants after two months of growth.The aerial portions of the plants were excised,and their weights determined after drying in an oven at 80°C(**,P<0.01,Student’s t-test,n=40).(C)Plant height measurements of mature plants after two months of growth (**,P <0.01, Student’s t-test, n= 20).(D–F) Determination of KA, GA12, and GA3 contents in ZmGAD5-OE transgenic Arabidopsis lines (**, P <0.01, Student’s t-test,n = 3).Error bars indicate standard deviations.
We have cloned and characterized ZmGAD5, which encodes a KAO enzyme involved in GA biosynthesis in maize.ZmGAD5 catalyzes the conversion of KA to GA12and functions in GA biosynthesis and plant morphogenesis.Our findings lay a foundation for regulating plant morphogenesis and breeding new maize varieties with high quality and yield by manipulating ZmGAD5 expression.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Zuliang Li:Investigation,Data curation,Visualization,Methodology,Writing–original draft.Baozhu Li:Investigation,Writing–original draft.Junli Zhang:Software, Methodology.Hongliang Wang:Investigation, Methodology.Mao Wang:Investigation.Siyi Guo:Project administration.Pengtao Wang:Resources.Zhi Li:Validation.David W.Galbraith:Validation.Dandan Li:Validation.Chun-Peng Song:Conceptualization, Project administration,Supervision, Funding acquisition.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (U21A20206, Chun-Peng Song), the Project of Sanya Yazhou Bay Science and Technology City (SCKJJYRC-2022-78, Baozhu Li), the Program for Innovative Research Team(in Science and Technology)in University of Henan Province(21IRTSTHN019, Siyi Guo),and the 111 Project of China(D16014).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.04.008.
- The Crop Journal的其它文章
- OsSPL10 controls trichome development by interacting with OsWOX3B at both transcription and protein levels in rice (Oryza sativa L.)
- Ectopic expression of OsNF-YA8, an endosperm-specific nuclear factor Y transcription-factor gene, causes vegetative and reproductive development defects in rice
- Mechanisms of autophagy function and regulation in plant growth,development, and response to abiotic stress
- ZmDRR206 functions in maintaining cell wall integrity during maize seedling growth and defense response to external stresses
- The plasmodesmata-associated β-1,3-glucanase gene GhPdBG regulates fiber development in cotton
- The MabHLH11 transcription factor interacting with MaMYB4 acts additively in increasing plant scopolin biosynthesis

