Post-operative morbidity after neoadjuvant chemotherapy and resection for gallbladder cancer: A national surgical quality improvement program analysis
Minha Kim,Stephanie Stroever,Krist Aploks,Alexander Ostapenko,Xiang Da Dong,Ramanathan Seshadri
Abstract BACKGROUND Gallbladder cancer is the most common malignancy of the biliary tract.Neoadjuvant chemotherapy (NACT) has improved overall survival by enabling R0 resection.Currently,there is no consensus of guidelines for neoadjuvant therapy in gallbladder cancer.As investigations continue to analyze the regimen and benefit of NACT for ongoing care of gallbladder cancer patients,we examined American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database to determine if there was higher morbidity among the neoadjuvant group within the 30-day post-operative period.We hypothesized patients who underwent NACT were more likely to have higher post-operative morbidity.AIM To investigate the 30-day post-operative morbidity outcomes between patients who received NACT and underwent surgery and patients who only had surgery.METHODS A retrospective analysis of the targeted hepatectomy NSQIP data between 2015 and 2019 was performed to determine if NACT in gallbladder cancer increased the risk for post-operative morbidity (bile leak,infection rate,rate of converting to open surgery,etc.) compared to the group who only had surgery.To calculate the odds ratio for the primary and secondary outcomes,a crude logistic regression was performed.RESULTS Of the 452 patients,52 patients received NACT prior to surgery.There were no statistically significant differences in the odds of morbidity between the two groups,including bile leak [odds ratio (OR),0.69;95% confidence interval (95%CI): 0.16-2.10;P=0.55],superficial wound infection (OR,0.58;95%CI: 0.03-3.02;P=0.61),and organ space wound infection (OR,0.63;95%CI: 0.18-1.63;P=0.61).CONCLUSION There was no significant difference in the risk of 30-day post-operative morbidity between the NACT and surgery group and the surgery only group.
Key Words: Gallbladder cancer;Neoadjuvant chemotherapy;Radical cholecystectomy;National Surgery Quality Improvement Program;Postoperative outcome
lNTRODUCTlON
Gallbladder cancer is the sixth most common gastrointestinal malignancy in the United States with an incidence of 1.13 cases per 100000[1].Current guidelines recommend cholecystectomy for stage T1a and radical cholecystectomy (cholecystectomy,segment IVb and V liver resection,regional lymphadenectomy) for T1b or greater.Neoadjuvant chemotherapy (NACT) may be considered for locoregional advanced disease to prevent rapid progression of the cancer and improve rates of R0 and R1 resection[2].There are currently no consensus guidelines in regards to neoadjuvant therapy for gallbladder cancer.
There have been few clinical studies that have looked into the effects of neoadjuvant therapy,including chemotherapy and radiation,for gallbladder cancer to determine survival benefit and rate for curative resections[3-6].A 2019 review of six retrospective and two prospective studies showed that of the 40% (approximately 189 out of 474 patients) of patients who had received neoadjuvant therapy,92.5% had R0 resections and the median overall survival for those patients ranged from 18.5 to 50.1 months[6].Since the studies that were reviewed lacked comparison between the treatments,the authors of the review concluded that there was not sufficient data to support the use of neoadjuvant therapy for gallbladder carcinoma[1].The debate whether NACT is beneficial is ongoing and there is a current trial[7] in place to further study the overall survival benefit of NACT for gallbladder carcinoma.Although,post-operative complications are not the focus of these studies and the prevalence of these complications are not fully documented,it would be beneficial for surgeons to be aware of the possible complications in the post-operative setting and whether NACT impacts the patients’ overall recovery.From the clinical studies that have been reviewed,the most commonly documented postoperative complication is bile leak[4,8].
The goal of this study was to use the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) data to identify post-operative morbidities in the setting of NACT as opposed to those who had undergone surgery upfront and determine if there is a significant risk difference between the two groups.This data may assist surgeons in determining whether NACT would be beneficial for their patients prior to undergoing surgery with regards to perioperative morbidity.
MATERlALS AND METHODS
A retrospective analysis of the ACS NSQIP participant use data files was performed.These files include data from participating institutions across the United States based on a robust sampling strategy described previously[9].Procedure-targeted hepatectomy data files for 2015-2019 were obtained.We included all patients 18 years and older with a diagnosis of gallbladder cancer and excluded patients that underwent emergent surgery,had viral hepatitis B and/or C,or unknown hepatitis status.
The primary outcome for this study was bile leakage within 30 d of surgery.Secondary outcomes were blood transfusion,on ventilator greater than 48 h,length of intensive care unit stay,readmission within 30 d,superficial incisional wound infection,organ space wound infection,secondary intervention,conversion rate to open,and need for biliary reconstruction.
Numerous covariates and potential confounders were included in our analyses.We included demographic variables including age,sex,race,and ethnicity.The following comorbidities: diabetes,history of smoking within one year of surgery,history of hypertension requiring medication,steroid use for chronic condition,and greater than 10 percent loss of body weight in the last six months.
We also included procedure-specific variables including placement of a biliary stent prior to surgery and cancer staging.Pre-operative laboratory values for serum albumin,total bilirubin,blood urea nitrogen,serum creatinine,and international normalized ratio were also examined.
Statistical methods
Statistical analyses were performed using R (R Foundation for Statistical Computing,Vienna,Austria) and StataSE version 16 (StataCorps LLC,College Station,Texas).Missing data was accommodated with listwise deletion in crude and multivariable analyses.We calculated descriptive statistics using mean ± SD for continuous variables and number with percentage for categorical variables.To determine group differences given exposure group,we used Fisher’s exact test (cell counts less than 5),Pearson’sχ2test (cell counts greater than 5),and Wilcoxon rank sum test for non-normally distributed continuous variables.
Model-building strategies were used to determine the difference in the odds of bile leakage given exposure NACT while adjusting for potential confounders.We performed crude logistic regression for all variables with bile leakage as the outcome,only including variables that were statistically significantly associated with the outcome in the final multivariable model (P< 0.05 establisheda priori).Crude logistic regression was performed for all secondary dichotomous outcomes and Poisson regression for length of stay.There were no statistically significant differences in secondary outcomes given exposure to NACT.Thus,we did not perform any further testing on these outcomes.
Post hoc analyses
Bivariate analyses were performed to further explore the association between NACT and selected outcomes by tumor stage.We independently assessed differences in outcomes given exposure to NACT in stage T2 patients,then again in stage T3/T4 patients.We used Pearson’s chi-squared test and Fisher’s exact test for dichotomous outcomes and Wilcoxon rank sum test for length of stay.Again,we selected α=0.05 for these analyses.
RESULTS
After exclusions,we included 452 patients in our sample (Table 1).Seventy percent of patients were tumor stage II,III,or IV though approximately 17% had unknown T stage.Nodal stage was equally distributed across all categories,and the majority were either M0/Mx or had unknown metastasis.The majority of patients did not undergo NACT (88.5%),and there were no statistically significant differences across exposure group for any of the covariates except pre-operative total bilirubin (P< 0.01),which is not clinically meaningful.

Table 1 Descriptive statistics for patients in the National Surgical Quality lmprovement Program hepatectomy targeted dataset diagnosed gallbladder cancer,2015-2019,n (%)
Ten percent of patients had bile leakage with only three of those patients having had NACT.On univariate logistic regression,the odds of bile leakage were not statistically significantly different given age,sex,race,ethnicity,diabetes status,smoking,steroid use for a chronic condition,or > 10% loss of body weight in the last six months (P> 0.05).
There was a statistically significant difference in the odds of bile leakage among patients with a biliary stent placed preoperatively [odds ratio (OR)=3.66,95% confidence interval (95%CI)=1.73,7.41,P< 0.01].There was no statistically significant difference in the odds of bile leakage for nodal stages 1/2 or Nx/unknown compared to N0 (P> 0.05),nor was there a difference for metastasis stage 1 or unknown compared to M0/Mx (P> 0.05).
Based on these results,we included NACT and pre-operative placement of a biliary stent in our multivariable logistic regression model.We also included race and ethnicity,as they are commonly hypothesized confounders,and preoperative bilirubin,which was different across exposure groups.We found there was not a statistically significant difference in the odds of bile leakage among patients who received NACT after controlling for potential confounders (OR=0.69,95%CI=0.16,2.10,P=0.55).
The median length of stay for all patients regardless of exposure group was five hospital days.Few patients required post-operative mechanical ventilation greater than 48 h (1.3%),while the most common outcome was biliary reconstruction (19.2%).Of note,21.2% of patients who had NACT required a blood transfusion within 72 h of surgery.
Secondary outcomes and Post hoc analyses
On univariate logistic regression,there were no statistically significant differences in the odds of any of the secondary outcomes given exposure to NACT (Table 2).The hospital length of stay also did not differ significantly on Poisson regression (P=0.12).Patients with stage T2 cancer that underwent NACT did not experience a bile leakage following surgery.There were no significant differences in the other outcomes given NACT among patients with T2 cancer either (P> 0.05).
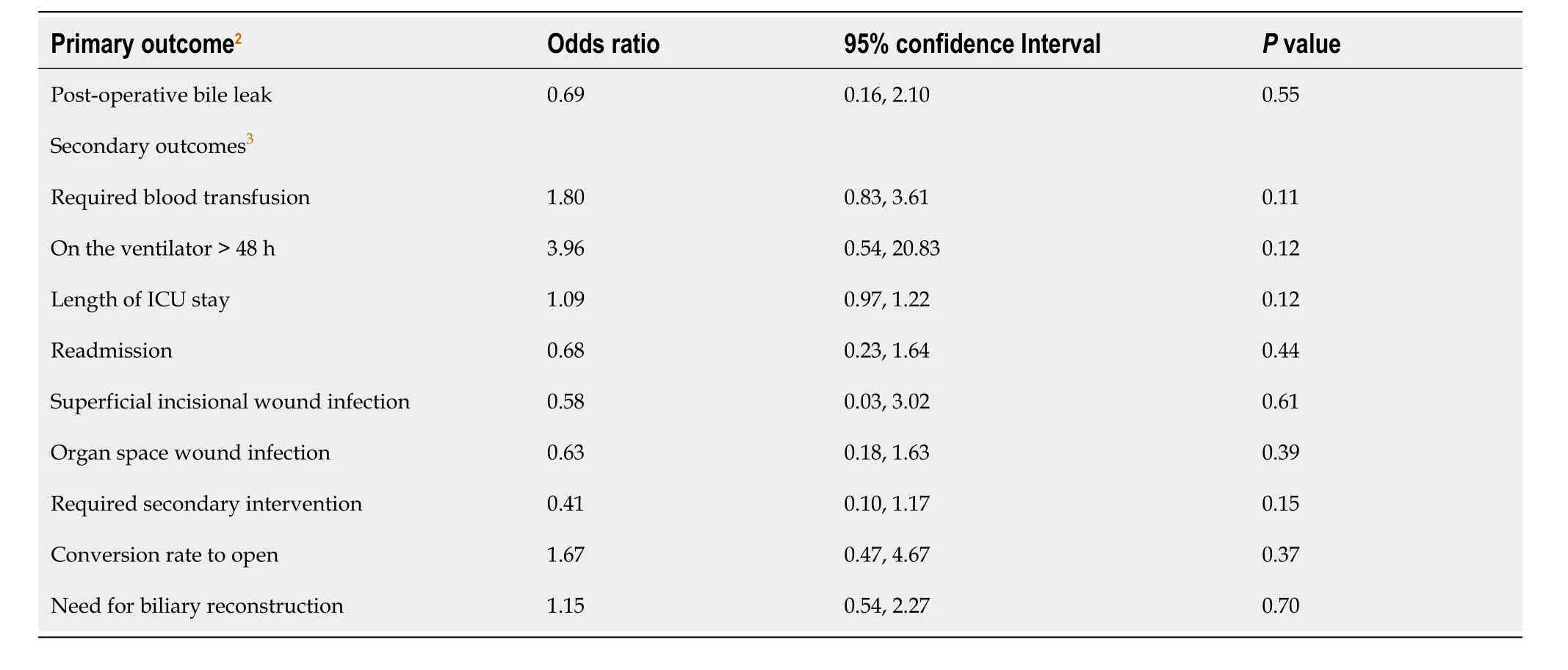
Table 2 Odds ratios for 30-day postoperative complications among patients with gallbladder cancer who underwent neoadjuvant chemotherapy (n=4111)
Approximately 20% of patients with stage T3/T4 gallbladder cancer experienced a bile leakage following surgery.However,there was not a statistically significant difference given exposure to NACT (Fisher’s exact test,P=0.37).Only two patients in that group experienced a bile leakage.Similar to stage T2,there were no statistically significant differences in any of the other outcomes among stage T3/T4 patients given exposure to NACT.
DlSCUSSlON
According the to the National Comprehensive Cancer Network guidelines,NACT is considered in patients with gallbladder cancer if there is locoregional advanced disease or if a patient has an unresectable disease.There is no preferred regimen for NACT since there is limited clinical data to define a standard regimen[2].Patients who undergo NACT have commonly received gemcitabine and cisplatin.This regimen proved to have significant survival benefit in advanced biliary cancer and therefore has been implemented in patients with gallbladder cancer[10].
The goal of surgery for gallbladder cancer is to obtain R0 resection for potential curative treatment[11].Clinical trials have shown that NACT improves rates of R0 resection in locally advanced gallbladder cancer[1,4,5].Compared to R0 resection,R1 resection has worse survival[12,13].Patients with R1 resection may undergo adjuvant therapy for improved survival benefit[11,14-16].
De Savornin Lohmanet al[17] evaluated the survival benefit of re-resection after incidentally found gallbladder cancer.They found that there was overall survival benefit with re-resection;however,prognosis was affected by the presence of residual disease and lymph node metastasis despite clear resection margins.Lundgrenet al[18] also found similar results in improved survival in re-resection for pT2 and pT3 incidental gallbladder cancer and residual disease impaired survival.With residual disease,surgeons must consider if additional surgery should be performed.Further resection may not have added benefit since residual disease can be clinical equivalent to distant metastatic disease[19] and patients are at risk for further peri-operative morbidity with major hepatectomy and pancreatoduodenectomy[20-22].The intended benefit of NACT is to improve overall survival by achieving R0 resection and to avoid further resection.However,NACT does have its own risks and complications.Aside from the direct side effects of chemotherapy,there are concerns chemotherapy can complicate surgery and increase risks for peri-operative morbidities.NSQIP allows us to evaluate potential peri-operative complications within thirty days of surgery.
The primary outcome evaluated in our study was post-operative bile leak as this was a well-documented complication in clinical studies that evaluated survival impact of NACT in gallbladder cancer[4,8].The treatment for the bile leaks included maintaining the drain placed during surgery or percutaneous drainage.The secondary outcomes that were evaluated were readmission within thirty days of discharge,superficial incisional wound infection,organ space wound infect,and the need for secondary intervention.In our study,we found that there was no statistical significance of any of these complications between the NACT and upfront surgery group.
Although our data may provide reassurance that NACT is safe to use for the appropriate patient population without having concerns for complications in the immediate post-operative period,this data is limited by the power of the study.The power of our study is low,as there were 452 patients diagnosed with gallbladder cancer and 52 patients had undergone NACT.With a larger study sample,there could be a statistically significant difference between the NACT group and upfront surgery group indicating that NACT could increase post-operative complications.Another limitation is the definition of a bile leak.NSQIP defines a bile leak as clinical diagnosis or persistent drainage that may have required maintenance of drain on or after post operative day 3,requiring percutaneous or operative intervention,or spontaneous wound drainage.The definition does not indicate if bilirubin levels were measured to prove a bile leak.A third limitation of this study are that the ACS NSQIP Targeted Hepatectomy dataset does not capture the specific details in regards to timing of chemotherapy,the chemotherapy regimen,duration of treatment,or if patients completed a full course of treatment.The data also only captures perioperative outcomes thirty days from the index operation.
Despite these limitations,our study provides additional information and insight into the use of NACT.As further clinical trials evaluate the effect of NACT,this study should be re-evaluated to determine potential significant complications of the use of NACT in gallbladder cancer and within the post-operative period.
CONCLUSlON
Gallbladder cancer is a rare and aggressive cancer when it is diagnosed late.Randomized controlled clinical trials are needed to validate the routine use of NACT in gallbladder cancer irrespective of their stage at presentation.Although our study shows that NACT does not increase post-operative morbidity,additional data on NACT for gallbladder cancer is needed to better understand the effect of NACT on 30-day post-operative morbidity.Until further information is available,surgeons will need to carefully evaluate the benefit and risks of NACT for patients undergoing surgical intervention.
ARTlCLE HlGHLlGHTS
Research background
Gallbladder cancer is the most common malignancy of the biliary tract.There are no consensus guidelines in regards to the use of neoadjuvant chemotherapy (NACT) for gallbladder cancer.Until a standardized regimen and guidelines are implemented,surgeons need to be aware of the potential effects of NACT on post-operative outcomes.
Research motivation
NACT is recommended based on clinical and pathological findings.Physicians need to carefully tailor the management of gallbladder cancer to the individual patient.By being aware of the benefits and risks of NACT both pre-operative and post-operatively,physicians can make informed decisions regarding its use in gallbladder cancer.
Research objectives
The objective of the study was to investigate the 30-day post-operative morbidities associated with NACT in gallbladder cancer.
Research methods
We performed a retrospective analysis using the National Surgery Quality Improvement Program database between 2015 and 2019.Patients with gallbladder cancer were identified and divided the patients into two cohorts based on their NACT status.
Research results
Compared to the upfront surgery group,patients who underwent chemotherapy and surgery for gallbladder cancer did not experience worse outcome.There were no statistically significant post-operative morbidities.
Research conclusions
While there were no differences in the 30-day post-operative morbidities between the two cohorts,the benefits and risks of NACT should be carefully considered for patients,taking into account the potential side effects of chemotherapy.
Research perspectives
Further research on the effects of NACT for gallbladder cancer needs to be conducted.When more clinical data is available,the post-operative morbidities associated with NACT can be further evaluated.
FOOTNOTES
Author contributions:Kim,M,Aploks K,Ostapenko A,Dong X,and Seshadri R contributed to the conceptualization of the project;Kim M,Stroever S,Aploks K,Ostapenko A,Dong X,and Seshadri R contributed to the methodology and validation of the data;Stroever S conducted the formal statistical analyses;Kim,M,Aploks K,Ostapenko A prepared the original manuscript;Kim,M,Aploks K,Ostapenko A,Dong X,and Seshadri R contributed to the final draft revision and edition;Dong X,and Seshadri R supervised the project.
lnstitutional review board statement:Ethical review and approval was not required for this study since the data used was de-identified and obtained from a participant use file.
lnformed consent statement:This study is a retrospective review that utilized only de-identified patient data from the American College of Surgeons National Surgical Quality Improvement Program.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Data sharing statement:Data was obtained with the permission from the American College of Surgeons NSQIP database.NSQIP data can be obtained at https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Minha Kim 0000-0003-3280-7426;Krist Aploks 0000-0003-3775-1775;Xiang Da Dong 0000-0001-9324-1281;Ramanathan Seshadri 0000-0003-0136-4562.
S-Editor:Lin C
L-Editor:A
P-Editor:Zhang YL
 World Journal of Gastrointestinal Surgery2024年1期
World Journal of Gastrointestinal Surgery2024年1期
- World Journal of Gastrointestinal Surgery的其它文章
- Prospects in the application of ultrasensitive chromosomal aneuploidy detection in precancerous lesions of gastric cancer
- Prognostic value of ultrasound in early arterial complications post liver transplant
- Added value of ratio of cross diameters of the appendix in ultrasound diagnosis of acute appendicitis
- Single-incision laparoscopic transabdominal preperitoneal repair in the treatment of adult female patients with inguinal hernia
- Predictive value of machine learning models for lymph node metastasis in gastric cancer: A two-center study
- Micro-power negative pressure wound technique reduces risk of incision infection following loop ileostomy closure
