Experimental study on the effect of H2O and O2 on the degradation of SF6 by pulsed dielectric barrier discharge
Yalong LI (李亞龍),Kun WAN (萬昆),Yufei WANG (王宇非),Xiaoxing ZHANG (張曉星),*,Zhaodi YANG (楊照迪),Mingli FU (傅明利),Ran ZHUO (卓然) and Dibo WANG (王邸博)
1 Hubei Key Laboratory for High-efficiency Utilization of Solar Energy and Operation Control of Energy Storage System,Hubei University of Technology,Wuhan 430068,People’s Republic of China
2 State Grid HuBei Direct Current Company,Yichang 443002,People’s Republic of China
3 CSG Electric Power Research Institute CO.Ltd,Guangzhou 510080,People’s Republic of China
Abstract SF6 has excellent insulation performance and arc extinguishing ability,and is widely used in the power industry.However,its global warming potential is about 23,500 times that of CO2,it can exist stably in the atmosphere,it is not easily degradable and is of great potential harm to the environment.Based on pulsed dielectric barrier discharge plasma technology,the effects of H2O and O2 on the degradation of SF6 were studied.Studies have shown that H2O can effectively promote the decomposition of SF6 and improve its degradation rate and energy efficiency of degradation.Under the action of a pulse input voltage and input frequency of 15 kV and 15 kHz,respectively,when H2O is added alone the effect of 1% H2O is the best,and the rate and energy efficiency of degradation of SF6 reach their maximum values,which are 91.9% and 8.25 g kWh-1,respectively.The synergistic effect of H2O and O2 on the degradation of SF6 was similar to that of H2O.When the concentration of H2O and O2 was 1%,the system obtained the best rate and energy efficiency of degradation,namely 89.7% and 8.05 g kWh-1,respectively.At the same time,different external gases exhibit different capabilities to regulate decomposition products.The addition of H2O can effectively improve the selectivity of SO2.Under the synergistic effect of H2O and O2,with increase in O2 concentration the degradation products gradually transformed into SO2F2.From the perspective of harmless treatment of the degradation products of SF6,the addition of O2 during the SF6 degradation process should be avoided.
Keywords: SF6,pulsed dielectric barrier discharge,degradation,discharge gas
1.Introduction
SF6is widely used in gas-insulated equipment because of its good insulation properties and thermal stability.At present,the use of SF6gas in the power industry accounts for about 70%of its total production,and the proportion of SF6in the exhaust gas produced exceeds 80% of the total exhaust[1,2].However,SF6is a strong greenhouse gas,its global warming potential is 23500 times that of CO2and its atmospheric lifespan is about 3200 years because its decomposition is difficult under natural conditions.Therefore,it is necessary to reduce SF6emissions [3].
In recent years,non-thermal plasma technology has achieved good results in the fields of waste gas treatment and material modification [4,5].Dielectric barrier discharge(DBD) technology has been widely used because of its simple device requirements and mild reaction conditions [6].Zhanget alstudied the degradation of SF6gas using a coaxial double dielectric barrier reactor,and the degradation rate of SF6reached 92% [7].Zhanget aland Xiaoet alstudied the static SF6degradation process using a coaxial double DBD reactor driven by an AC power supply,and found that Ar had the best degradation effect when it was used as the background gas [8,9].In addition,Donget alalso studied the effect of applied H2O and O2on the degradation of SF6exhaust gas by DBD under the condition of AC power supply.The results showed that when the initial SF6concentration was 2%,the OH radicals generated in a 1% concentration of H2O reacted with the primary decomposition products of SF6,which hindered the occurrence of the SF6recovery reaction thus improving the degradation rate; the SF6degradation rate reached 95.69% [10].The SF6degradation rate reached 98.2% under a concentration of 0.5% H2O and 2%O2[11].
However,most of the traditional DBD reactors are driven by an AC power supply,which will lead to continuous heating in the discharge channel,resulting in overheating or even thermal damage of the reactor [12,13].In contrast,a high-frequency repetitive pulse power supply triggers discontinuous discharge,which significantly reduces the heat loss between pulses and improves the discharge stability,while the narrow pulse rise time inhibits the generation of filamentary discharge and facilitates uniform discharge to promote chemical reactions [14].Wanget alstudied the effect of pulse power parameters on the dry reforming characteristics of methane (CH4),and pulsed discharges with short rise and fall times had better CH4conversion and energy efficiency [15].Yuanet alcompared the generation of reactive OH radicals in a DBD reactor driven by an AC power supply with one driven by a pulsed power supply,and found that the generation of OH radicals and energy efficiency were better under the condition of pulsed power supply than with an AC power supply [16].Liuet alstudied the effect of adding H2O on the discharge characteristics of an Ar DBD excited by a nanosecond pulsed supply,and found that the addition of an appropriate amount of H2O can effectively increase the OH radical content and improve the energy efficiency and plasma activity of DBD [17].
The addition of H2O can generate more OH radicals,which is beneficial to the degradation of SF6,and the addition of a mixture of H2O and O2further improves the degradation rate.Under the same conditions,more OH radicals are generated under a pulsed power supply than an AC power supply,and the addition of H2O can further increase the content of OH radicals.A pulsed discharge is also more stable and has a longer lifetime than an AC power discharge.Based on this,the effect of the addition of H2O and O2on SF6degradation under a pulsed power supply was studied in this work.
An experimental platform for SF6degradation by a pulsed DBD was built to study the effects of adding H2O and O2as active gases on the degradation rate,energy efficiency of degradation and degradation byproduct selectivity of SF6gas.The results will provide a reference for the efficient degradation of SF6exhaust gas by DBD.
2.Experiment
2.1.Experimental platform
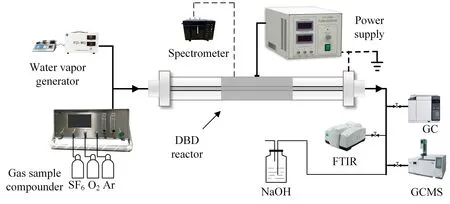
Figure 1.Diagram of the experimental platform.
Figure 1 shows the experimental platform used in this work.The experimental system comprises a power supply system,gas distribution system,reaction system and detection system.The gas distribution system includes a dynamic gas distribution instrument,a precision water and gas generator and standard gases.The precision water vapor generator is produced by Suzhou Furand Experimental Equipment Co.,Ltd.The instrument comprises a water supply device and humidity generator.The water supply device slowly injects pure water from the micro-sampling needle into the humidity generator to generate the required water vapor,and the water vapor content can be controlled by adjusting the corresponding water supply rate according to the gas flow rate.During the experiment,the gas pipeline between the water vapor generator and the DBD reactor was wrapped with a heating band heated to 110 °C to prevent the water vapor from liquefying in the gas pipeline.The inlet gas flow range of this instrument is 0-500 ml min-1and the accuracy is 1%full scale.The power supply system includes a microsecond pulse plasma power supply and a voltage regulator.The reaction system is a coaxial dual dielectric barrier reactor,and both the inner and outer tubes are made of quartz glass.The outer quartz tube has an inner diameter of 20 mm and a thickness of 2.5 mm,and the inner quartz tube has an outer diameter of 16 mm,a thickness of 2 mm and a gas gap width of 2 mm.The inner quartz tube is filled with aluminum powder as the inner ground electrode,and the stainless steel mesh wrapped around the outer quartz tube is used as the high-voltage electrode.The length of stainless steel mesh is 200 mm.The main components of the detection system are a gas chromatograph (GC),a Fourier transform infrared(FTIR) spectrometer,a gas chromatograph-mass spectrometer (GC-MS) and an optical emission spectroscope (OES).The GC was used to detect the SF6concentration,the FTIR spectrometer and GC-MS were used to detect the composition and content of the products and the OES was used to detect the emission spectra of energetic particles of the plasma reaction process.For the experiments,the system voltage and frequency were 15 kV and 15 kHz,respectively[18],the system input power was 45 W,the gas flow rate was 50 ml·min-1,the initial SF6concentration was 2%(volume concentration) and the background gas was Ar.
2.2.Measurement
In this study,the degradation rate (DR),energy efficiency(EY) of degradation and degradation byproduct selectivity of SF6were used as the main indicators for judging the effect on SF6degradation.
The degradation rate of SF6is calculated by equation (1)
whereCinis the SF6concentration before degradation in parts per million (ppm) andCoutis the concentration of SF6remaining after the degradation reaction in ppm.
The energy efficiency of SF6degradation is defined as the mass of SF6degraded per unit of input energy and is calculated as shown in equation (2)
wherePis the plasma discharge power,which is measured by the Lissajous figure method.The Lissajous figure can be obtained by measuring the voltageUand the chargeQduring the discharge process.The voltage is detected by a shunt capacitor divider,and the integral current during the discharge process of the reactor is obtained by a series sampling capacitorCm.The areaSof the Lissajous figure is proportional to the discharge powerP(P=fCmS) [18].Vgis the flow rate in ml min-1of the gas feed,and the unit of EY is g kWh-1.
The product selectivity of the main degradation products SO2,SOF2,SO2F2,SOF4and H2S is calculated by equation(3)
whereCKis the concentration of gasKafter the degradation reaction andSKrepresents the percentage concentration of gasKto degraded SF6.
3.Analysis of experimental results
3.1.Effect of H2O on SF6 degradation
The typical discharge voltage and current waveforms before and after the addition of H2O are shown in figure 2.It can be seen from figure 2 that the rising edge of the discharge voltage is 5μs and the peak voltage is about 15 kV.The discharge pulse period is about 66μs and the frequency is 15 kHz.Before and after adding H2O to the SF6gas mixture,the discharge voltage waveform did not change but the current waveform was greatly affected by H2O.Since the value of the power supply does not change during the discharge process,the discharge current amplitude of the SF6gas mixture containing H2O does not change much,but more burrs are generated near the current peak.The higher the concentration of H2O,the greater the number and amplitude of burrs,and the more serious the distortion of the current waveform,which will lead to an increase in filamentary discharge and a decrease in discharge uniformity.
The effects of different concentrations of H2O on the degradation rate and energy efficiency of SF6degradation are shown in figure 3.With increase in H2O concentration,the rate of SF6degradation increased first and then decreased,and the energy efficiency shows the same pattern of change.When the concentration of H2O is 1%,the maximum rate of degradation of SF6is 91.9% and the energy efficiency is 8.25 g kWh-1.With increase in the H2O concentration,the rate of SF6degradation and energy efficiency gradually decreased.When the concentration of H2O is 2.5%,the rate of degradation of SF6is 77.4%,and the energy efficiency is 6.95 g kWh-1.Therefore,in order to obtain a better degradation effect,it is necessary to add an appropriate concentration of H2O.The reason for this phenomenon may be that the appropriate amount of H2O can provide active substances such as OH radicals to react with the bond-breaking low-fluorine sulfides during the reaction process,promoting the rate of S-F bond breaking and separation and improving the rate of SF6degradation.However,since H2O is also electronegative,with the gradual increase in H2O concentration a large number of high-energy electrons are adsorbed by H2O,which leads to a reduction of active substances in the reaction system [17].At the same time,more discharge energy is used to ionize H2O molecules,which slows down the speed of S-F bond breaking and separation,thus reducing the rate of degradation of SF6.
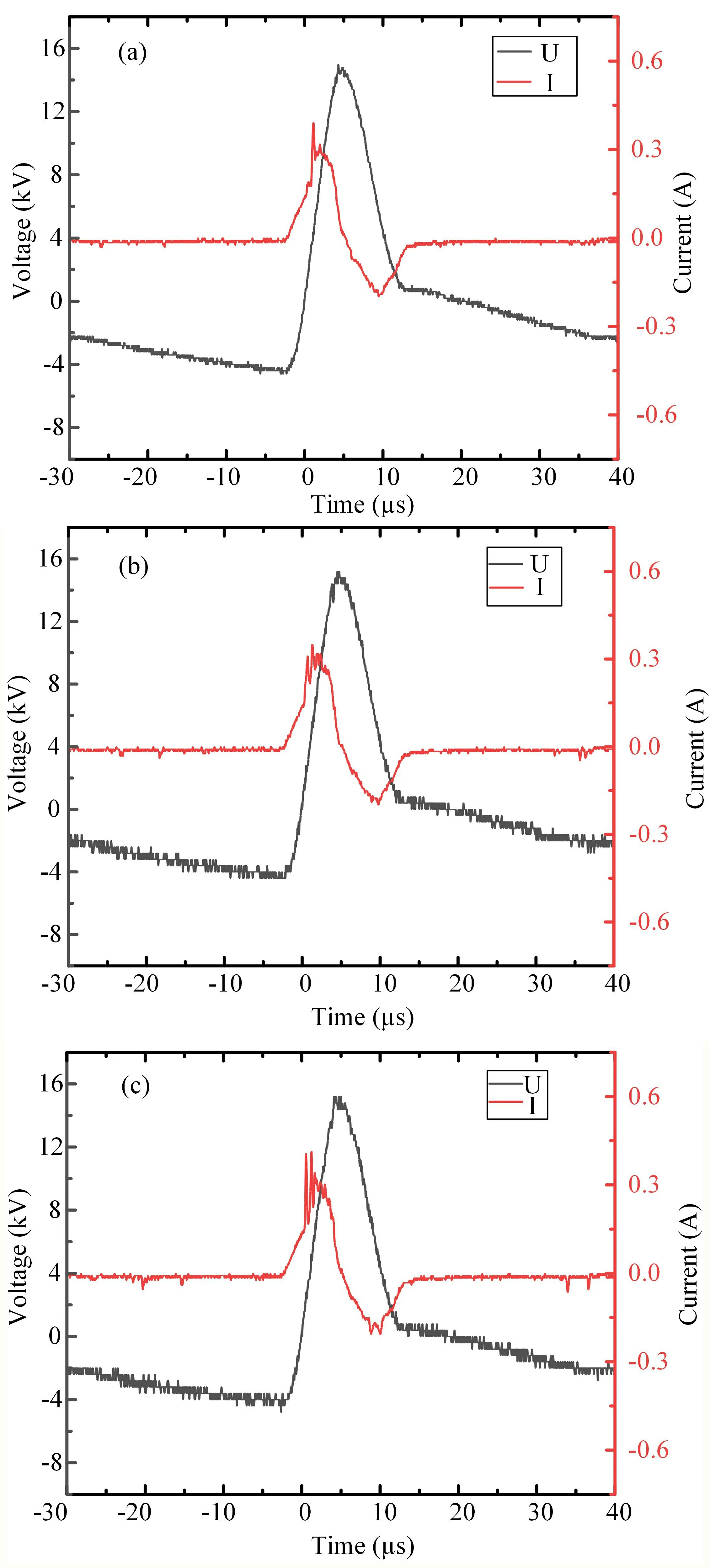
Figure 2.Voltage and current waveforms at different water concentrations: (a) H2O concentration 0%,(b) H2O concentration 1%,(c)H2O concentration 2.5%.
The DBD reaction process generates a large number of energetic electrons,free radicals,excited particles and other reactive substances,which promotes the bond-breaking separation of SF6gas molecules.After studying the effect of different concentrations of H2O on the rate and energy efficiency of SF6degradation,the active particles in the reaction process after the addition of H2O were analyzed.In order to understand the mechanism of action of active substances during the degradation of SF6gas by a pulsed DBD,the active particles generated during the discharge process were examined using an OES,which analyzed the types of active particles and plasma properties during the discharge process.Figure 4 shows the emission spectra in the band range from 300 to 850 nm under an H2O concentration of 1%.In the SF6/Ar discharge system,the characteristic spectral lines of the emission spectrum are mainly concentrated in the 300-400 nm band and the 650-850 nm band.Since the background gas used in the degradation system is Ar,there are mostly Ar spectral lines in the discharge system,including Ar 696.54 nm,Ar 750 nm,Ar 763.84 nm,Ar 772.78 and Ar 811.78 nm,which are all Ar I type spectral lines.The main process of formation of Ar spectral lines is shown in reaction (4).In addition,due to the bond breaking of SF6molecules,there are also free F atoms with a wavelength of 738.51 nm in the plasma system.The preliminary bond-breaking separation process of SF6is given in [19],and the bond-breaking process is shown in reactions(5)-(7)
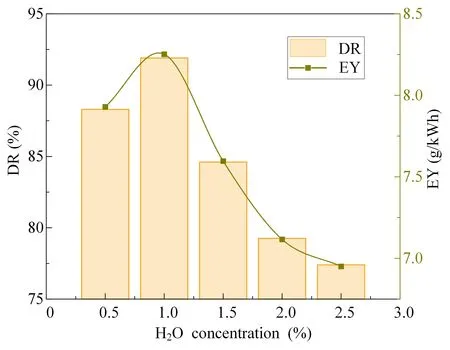
Figure 3.Variation of the rate of degradation of SF6 (DR) and energy efficiency of degradation (EY) under different H2O concentrations.
With the addition of H2O,the characteristic spectral lines of Ar and F in the 650-850 nm band are consistent with those without H2O.The spectra in the 300-400 nm band were expanded and analyzed,and the 308 nm spectrum of OH was detected.The OH radical is derived from the dissociation of H2O.The OH radical has strong chemical activity and can effectively promote the decomposition of SF6gas.In addition,the F atomic emission spectrum band was detected in the range of 300-400 nm,and the characteristic peak was not detected in the SF6gas mixture without water vapor,so is speculated to be the characteristic peak of HF gas.
The excited state Ar*in this experiment mainly comprises Ar(4S) metastable atoms and Ar(4P) excited atoms.The observed Ar elemental spectral lines in figure 4 are mainly formed by the spectra of deexcited Ar(4P) excited state atoms.It has been shown that OH and electrons are not generated and quenched in the same way at different H2O concentrations,and when the H2O concentration is low,OH radicals are mainly derived from reaction (8),while the electrons are generated through the graded dissociation offrom Ar(4S) substable atoms [17,20],as shown in reactions(9) and (10)
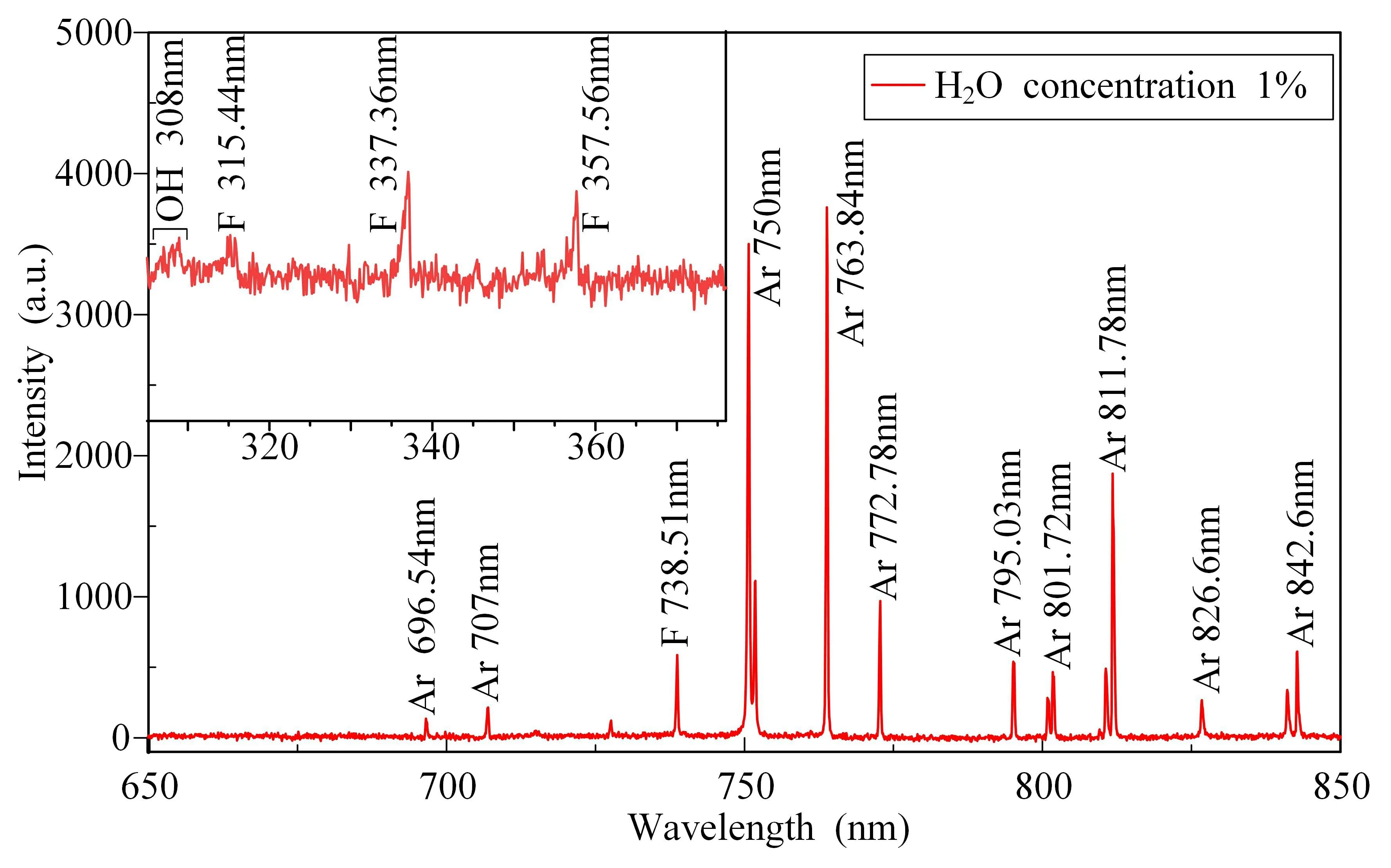
Figure 4.The emission spectra with 1% H2O.
When the H2O concentration is high,OH is mainly generated by dissociation through reactions (11) and (12),while the electrons in the reaction system are generated by direct ionization through reactions (13) and (14) [20],and HO2particles are generated and quenched [21,22]
The quenching process of OH radicals with electrons is shown in reactions (15)-(21).The electrons are mainly quenched by entering the anode at low H2O concentrations,while they are quenched by reactions (20) and (21) when the H2O concentration is too high [17]
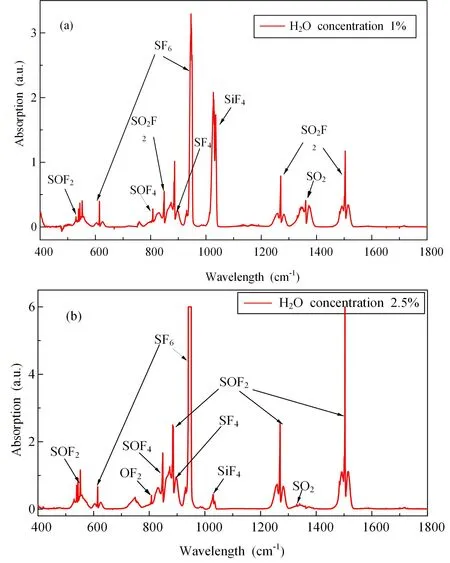
Figure 5.FTIR spectra of degradation products under different H2O concentrations: (a) H2O concentration of 1%; (b) H2O concentration of 2.5%.
When the concentration of H2O in the reaction system is excessive,a large number of electrons are quenched due to the adsorption of H2O molecules,which reduces the possibility of free electrons colliding with SF6gas molecules,resulting in a decrease in the SF6degradation rate.
Then,the effect on the degradation products of adding H2O was also studied.Figure 5 shows the FTIR spectra of the degradation products at H2O concentrations of 1% and 2.5%.After comparing and identifying the characteristic peaks,the main degradation products include SOF4,SO2F2,SOF2,SF4,OF2,SiF4and SO2,similar to the results in [23].Due to the large number of SF6degradation products and the absorption of SF6gas molecules in the infrared band,there may be overlapping peaks of some gas products,so only some easily identifiable components are listed in this paper.By comparing figures 5(a) and (b),it was found that the absorbance peak of SF6increased significantly with increase in the H2O concentration,and saturation occurred when the H2O concentration was 2.5%,indicating that the rate of degradation of SF6decreased and the amount of unreacted SF6gas increased.Meanwhile,SO2absorbance decreased significantly with increase in the H2O concentration,indicating that excessive H2O will inhibit the production of SO2gas.In addition,the characteristic absorption peak of SiF4was detected in the reaction system,indicating that SiO2in the reactor was involved in the reaction.The F ions generated during the SF6degradation process combine with H in H2O to form HF,which reacts with SiO2to etch the reactor and produce SiF4.Since SiF4is very soluble in water,with increasing H2O concentration a large amount of SiF4is dissolved in water,resulting in a decrease in its absorption peak intensity.
The formation pathways of SF6degradation products in the SF6/Ar/H2O system were inferred from the product detection results,as shown in reactions (22)-(28)
In order to further grasp the distribution of the main degradation products,the gases SO2,SOF2,SO2F2and SOF4,were quantitatively detected by GC-MS.Figure 6 shows the product selectivity under different H2O concentrations.With increase in the H2O concentration,the product selectivity of SO2shows an increasing and then decreasing trend,and reaches a maximum value of about 20% at an H2O concentration of 1%,when the SO2concentration was 3634.59 ppm.The selectivity of SOF2and SO2F2was 6.02% and 9.75%,respectively.When the H2O concentration was greater than 1%,the selectivity of SO2gradually decreased.From reactions (22)-(28),it can be seen that adding the appropriate amount of H2O can promote the whole reaction process and enhance the SF6degradation rate.It can be seen from reference [9] that reaction (27) is an endothermic reaction and must absorb a certain amount of energy before proceeding.When the H2O concentration is too high,a large number of electrons in the reaction system are quenched by H2O adsorption,and the reduction of energy in the system inhibits the occurrence of reaction (27),causing a decrease in SO2concentration.SOF2product selectivity remained relatively stable at different H2O concentrations.The content of SOF4in the reaction system is relatively low and,according to reactions (22) and (23),SOF4is a primary degradation product; when H2O is added the SF6degradation reaction by pulsed DBD is more thorough and SOF4is degraded into a more stable product.The selectivity of SO2F2increased with increase in the H2O concentration,and SO2F2selectivity reached 30% when the H2O concentration was 2.5% and the SO2F2concentration was 4799.35 ppm.Apparently,reaction(26) is promoted at high H2O concentrations and SOF4is hydrolyzed to SO2F2,while reaction (28) is inhibited at high H2O concentrations and a significant decrease in SO2production is observed.The degradation products such as SO2,SO2F2,SOF2and SOF4can be absorbed and transformed by sodium hydroxide (NaOH) and other alkali solutions (figure 1),thus avoiding the greenhouse effect caused by the direct emission of SF6.
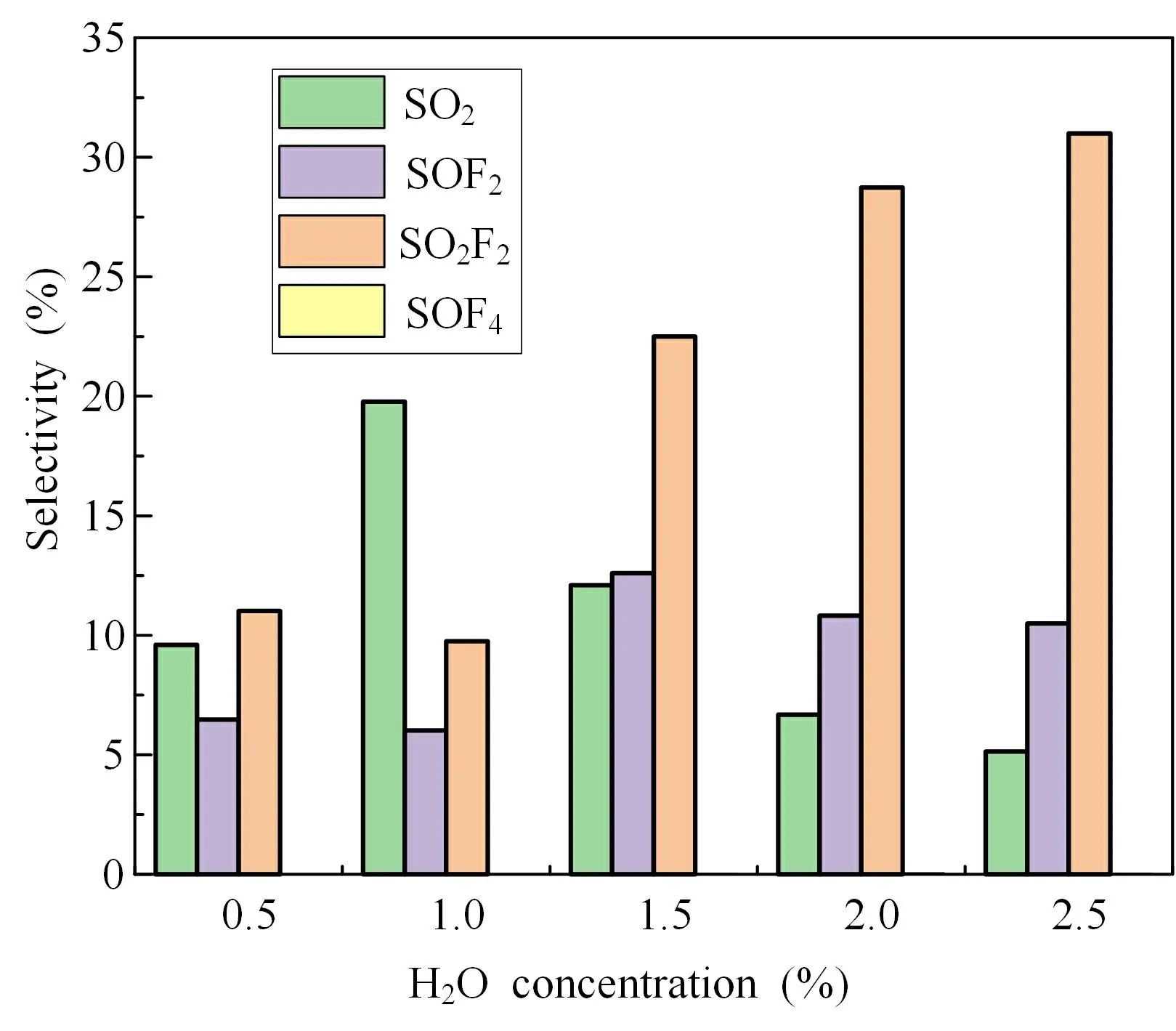
Figure 6.Selectivity of the main degradation products under different H2O concentrations.
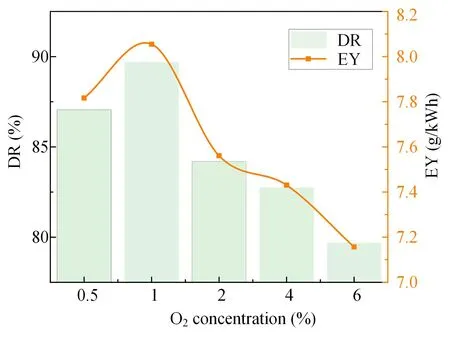
Figure 7.Effect of different concentrations of H2O and O2 on the rate and energy efficiency of SF6 degradation.
3.2.The synergistic effect of H2O and O2 on degradation of SF6
After studying the effect on SF6degradation of adding H2O alone,the synergistic effect of H2O and O2on the degradation of SF6was studied.When the concentration of H2O is fixed at the optimal concentration (1%),the effects of different concentrations of O2on the degradation of SF6by pulsed DBD are shown in figure 7.Under the synergistic effect of H2O and O2,the rate and energy efficiency of SF6degradation increased first and then decreased with increase in the O2concentration,similar to the change trend when just H2O was added.The rate and energy efficiency of SF6degradation were 89.7% and 8.05 g kWh-1,respectively,at 1% of both H2O and O2,similar to the experimental results with only 1% H2O,and the synergistic effect of H2O and O2did not significantly improve the SF6degradation rate.
Finally,the effect on the selectivity of degradation products when the two gases acted synergistically was studied,as shown in figure 8.When the concentration of H2O is 1%,the degradation products gradually produce SO2F2with a gradual increase in O2concentration.When the concentration of O2is 0.5%,the product selectivity of SO2,SO2F2and SOF2is 19.55%,21.36% and 6.67%,respectively.When the O2concentration was increased to 1%,the main product in the system was SO2F2,which was obviously promoted by the addition of O2.However,SO2F2is the most difficult gas to treat among the SF6degradation products.It reacts slowly with alkali solution and is difficult to be absorbed and removed by alkali solution.Since the addition of O2will promote the production of SO2F2,the addition of O2should be avoided in engineering applications; this will contribute to the harmless treatment of SF6degradation products.
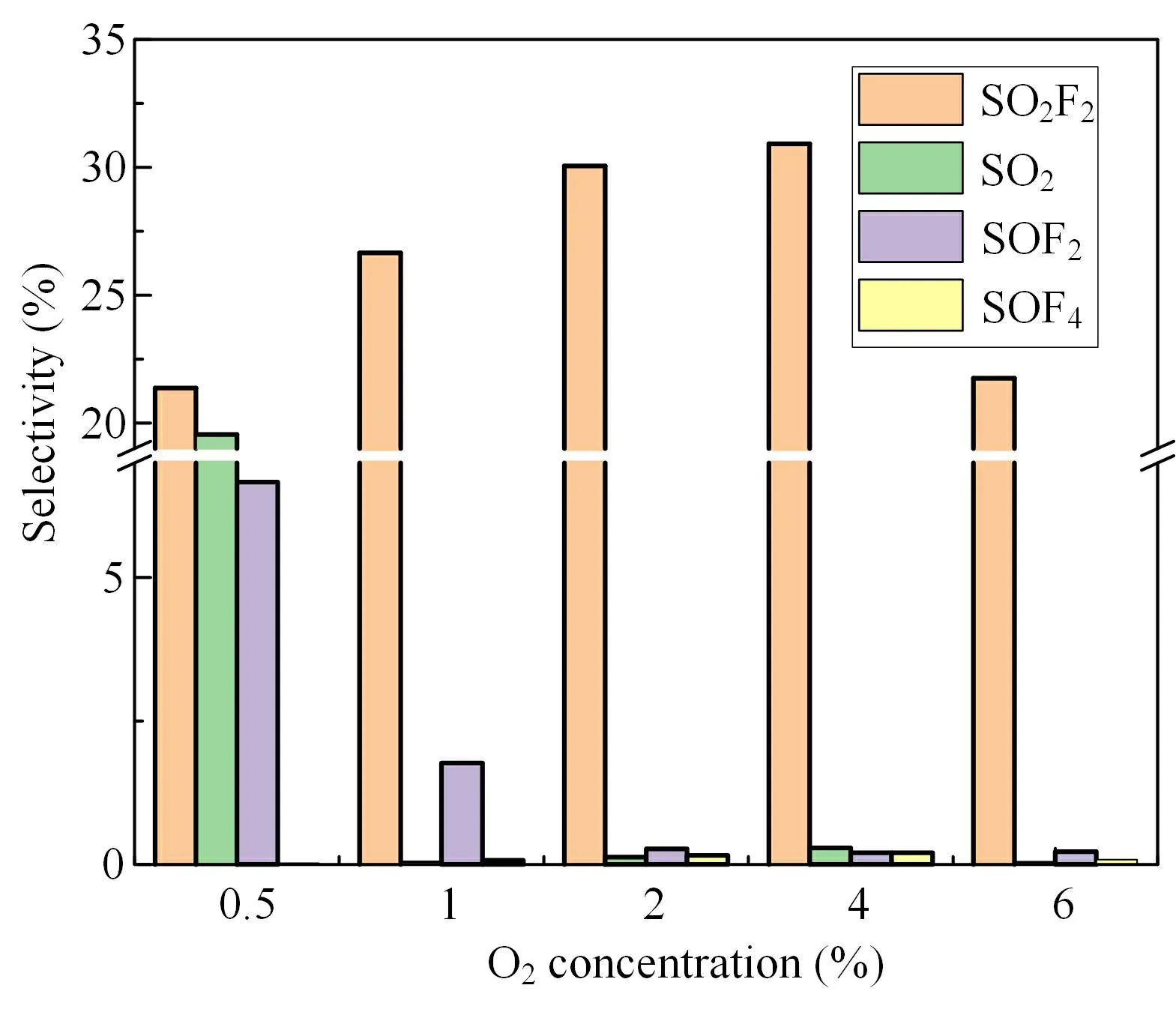
Figure 8.Effect of different concentrations of H2O and O2 on SF6 product selectivity.
4.Conclusion
In this work,the effects of H2O and O2on the rate and efficiency of degradation and the degradation products of SF6were studied by establishing a pulsed DBD degradation test platform.The following conclusions are obtained:
(1) In the SF6/Ar/H2O system,with increasing H2O concentration,the rate and energy efficiency of SF6degradation increased first and then decreased.When the concentration of H2O was 1%,the rate and energy efficiency of SF6degradation reached their maximum values,namely 91.9%and 8.25 g kWh-1,respectively.
(2) In the SF6/Ar/H2O/O2system,the overall degradation effect was similar to that of the SF6/Ar/H2O system,and the synergistic effect of H2O and O2did not significantly improve SF6degradation.When the concentrations of H2O and O2were both 1%,the rate and energy efficiency of SF6degradation reached maximum values of 89.7% and 8.05 g kWh-1,respectively.
(3) In the SF6/Ar/H2O/O2system,the main degradation products of SF6are SO2,SOF2,SO2F2,SOF4,OF2,which are the same as for the SF6/Ar/H2O system.However,with increase in the O2concentration,the degradation products gradually tended toward SO2F2.When the O2concentration was raised to 1%,the main product in the system was SO2F2,and the addition of O2obviously promoted the production of SO2F2.
Acknowledgments
The work was supported by Guizhou Province (Ceneral),grant/award number QianKeHeZhiCheng [2022] General 207,National Natural Science Foundation of China (No.52307170)and Natural Science Foundation of Hubei Province,China(No.2023AFB382).
 Plasma Science and Technology2024年2期
Plasma Science and Technology2024年2期
- Plasma Science and Technology的其它文章
- Non-thermal atmospheric-pressure positive pulsating corona discharge in degradation of textile dye Reactive Blue 19 enhanced by Bi2O3 catalyst
- The characteristics of negative corona discharge and radio interference at different altitudes based on coaxial wire-cylinder gap
- Characteristics of laser-induced breakdown spectroscopy of liquid slag
- Airfoil friction drag reduction based on grid-type and super-dense array plasma actuators
- Multi-layer phenomena in petawatt laserdriven acceleration of heavy ions
- Phase field model for electric-thermal coupled discharge breakdown of polyimide nanocomposites under high frequency electrical stress
