Anti-N-methyl-D-aspartate receptor-associated encephalitis: A review of clinicopathologic hallmarks and multimodal imaging manifestations
Bryce David Beutler,Alastair E Moody,Jerry Mathew Thomas,Benjamin Phillip Sugar,Mark B Ulanja,Daniel Antwi-Amoabeng,Lucas Anthony Tsikitas
Abstract Anti-N-methyl-D-aspartate receptor-associated encephalitis (NMDARE) is a rare immune-mediated neuroinflammatory condition characterized by the rapid onset of neuropsychiatric symptoms and autonomic dysfunction.The mechanism of pathogenesis remains incompletely understood,but is thought to be related to antibodies targeting the GluN1 subunit of the NMDA receptor with resultant downstream dysregulation of dopaminergic pathways.Young adults are most frequently affected;the median age at diagnosis is 21 years.There is a strong female predilection with a female sex predominance of 4:1.NMDARE often develops as a paraneoplastic process and is most commonly associated with ovarian teratoma.However,NMDARE has also been described in patients with small cell lung cancer,clear cell renal carcinoma,and other benign and malignant neoplasms.Diagnosis is based on correlation of the clinical presentation,electroencephalography,laboratory studies,and imaging.Computed tomography,positron emission tomography,and magnetic resonance imaging are essential to identify an underlying tumor,exclude clinicopathologic mimics,and predict the likelihood of long-term functional impairment.Nuclear imaging may be of value for prognostication and to assess the response to therapy.Treatment may involve high-dose corticosteroids,intravenous immunoglobulin,and plasma exchange.Herein,we review the hallmark clinicopathologic features and imaging findings of this rare but potentially devastating condition and summarize diagnostic criteria,treatment regimens,and proposed pathogenetic mechanisms.
Key Words: Anti-N-methyl-D-aspartate receptor-associated encephalitis;Autoimmune encephalitis;Encephalitis;Ovarian teratoma;Paraneoplastic syndrome;Teratoma
INTRODUCTION
Anti-N-methyl-D-aspartate receptor-associated encephalitis (NMDARE) is a rare immune-mediated neuroinflammatory condition characterized by the rapid onset of neuropsychiatric symptoms and autonomic dysfunction.NMDARE may be idiopathic,but often occurs as a paraneoplastic process in the setting of small cell lung carcinoma,ovarian teratoma,and other benign and malignant neoplasms[1,2].A significant majority of patients diagnosed with NMDARE are young adults ranging in age from 18 years to 42 years[3].The mechanism of pathogenesis remains incompletely understood[4].
The clinical presentation of NMDARE is variable and may include vague prodromal symptoms,such as headache and nausea,followed by the rapid development of cognitive dysfunction,behavioral changes,and central hypoventilation[5].Careful correlation of clinical history,electroencephalography (EEG),and imaging is required to establish a presumptive diagnosis;serology or cerebrospinal fluid analysis is the gold standard for definitive diagnosis,with the presence of anti-GluN IgG antibodies constituting a positive result.Management may involve high-dose corticosteroids,intravenous immunoglobulin,and immunotherapy[6].
Herein,we review the clinicopathologic and imaging hallmarks of NMDARE and discuss management strategies for this rare but potentially devastating syndrome.
HISTORY OF NMDARE
The first cases of NMDARE were reported by Dalmauetal[7] in 2007,who described a small group of patients who presented with neuropsychiatric symptoms and were subsequently found to have antibodies to the NMDA receptor in blood or cerebrospinal fluid.One year later,Dalmauetal[7] launched a case-control study in which they detailed the clinical characteristics -including symptoms,management,and outcomes -of 100 patients with antibody-positive NMDARE[8].The syndrome was subsequently thrust into the mainstream consciousness when a prominent New York Post journalist,Susannah Cahalan,was diagnosed with NMDARE;her experience as a patient is detailed in the bestselling memoirBrainonFire[9].The following years were defined by an explosion of NMDARE research,culminating in the establishment of validated diagnostic criteria and consensus practice guidelines.
DIAGNOSTIC CRITERIA
The hallmark clinical features,therapeutic regimens,and outcomes of paraneoplastic and non-paraneoplastic NMDARE were described in a multi-institutional observational study conducted by Titulaeretal[3] in 2013.Graus,Titulaer,and colleagues subsequently proposed three diagnostic criteria that could be used to establish a diagnosis of probable NMDARE: (1) Rapid onset of at least four of six classic symptoms;(2) an abnormal EEG or cerebrospinal fluid analysis showing pleocytosis or oligoclonal bands;and (3) reasonable exclusion of other disorders[5].Graus and Titulaer proposed that a definitive diagnosis could be established with positive IgG anti-GluN1 antibodies in the presence of the aforementioned clinical criteria.
The diagnostic criteria introduced by Graus and Titulaer has served as a foundational guide for the assessment of suspected NMDARE.However,two key clinical features are not included in the criteria: (1) A history of benign or malignant neoplasm and (2) imaging features.NMDARE develops as a paraneoplastic process in up to 60% of cases[8].Mature or immature ovarian teratomas are by far the most common underlying tumors and a known ovarian teratoma is included as a modifier within the Graus diagnostic criteria.However,NMDARE has also been described in the setting of small cell lung cancer,clear cell renal carcinoma,chronic myelogenous leukemia,pancreatic neuroendocrine tumor,and many other benign and malignant neoplasms[2,10].The presence of a neoplasm or history of cancer therefore represents an important clinical finding that favors NMDARE over other encephalitides or neuropsychiatric disorders.
IMAGING FEATURES OF NMDARE
Imaging,including computed tomography (CT),positron emission tomography (PET),and magnetic resonance imaging (MRI),plays a central role in the evaluation of NMDARE.The utility of imaging is two-fold: (1) To identify an underlying primary neoplasm and (2) to exclude clinicopathologic mimics of NMDARE.Common causes of neuropsychiatric symptoms in young adults include herpes encephalitis,drug intoxication,and central nervous system vasculitides,all of which demonstrate imaging features that are distinct from those of NMDARE[11].For example,herpes encephalitis is classically characterized by asymmetric T2/FLAIR hyperintensity within the medial temporal lobes whereas opioid intoxication may show symmetric T2/FLAIR hyperintensity within the posterior limb of the internal capsule.The imaging differential diagnosis for NMDARE is further detailed in Table 1.
The magnetic resonance imaging manifestations of NMDARE within the central nervous system are variable.Zhanget al[12] introduced a classification schema that can be used to evaluate T2/FLAIR hyperintense lesions in patients with NMDARE,which categorizes patients into four distinct categories based on distribution: Type 1 -normal brain MRI;type 2 -lesions within the hippocampus;type 3 -lesions involving structures other than the hippocampus;and type 4 -lesions within the hippocampus and other structures within the supratentorial or infratentorial brain parenchyma.A normal brain MRI,or a type 1 pattern,is present in approximately half of patients with a serologically confirmed diagnosis and typically portends a favorable outcome.The type 4 pattern is the second most common and has been associated with poor functional outcomes.The type 2 pattern is seen with intermediate frequency and is associated with relatively poor outcomes.Type 3 patterns are seen with intermediate frequency and are most often associated with positive outcomes,although some degree of long-term functional impairment may occur in some individuals.
Supratentorial and infratentorial T2/FLAIR hyperintense brain lesions with a slight hippocampal predilection represent the imaging hallmark of NMDARE.However,other central nervous system manifestations have been described,including myelitis,optic neuritis,and isolated meningitis[13,14].The prodromal and neuropsychiatric symptoms are similar even in the setting of atypical lesions and the presence of spinal cord or cranial nerve lesions does not exclude an NMDARE diagnosis.Lesions in unusual locations can affect symptomatology,and patients may present with visual disturbances,hemiparesis,and other neurologic deficits superimposed upon the classic psychotic symptoms that typify NMDARE.Correlation of clinical history,laboratory studies,and comprehensive neuraxis imaging is therefore essential to establish a diagnosis.
Magnetic resonance spectroscopy (MRS) may also be of value for the assessment of suspected NMDARE.In a case report by Kataokaetal[15],authors described a reducedN-acetylasparate (NAA) peak with a decreased NAA/creatine ratio and a slightly increased choline peak within the basal ganglia,suggestive of diminished neuronal activity in the setting of neuroinflammation;the abnormal MRS findings improved following treatment of the underlying NMDARE.Splendianietal[16] described similar findings in a subsequent report.The underlying cause of metabolic dysfunction and the prognostic value of abnormal MRS findings remain to be established.
NUCLEAR MEDICINE AND MOLECULAR IMAGING IN NMDARE
Nuclear imaging can play an important role in the evaluation and management of NMDARE[17].Brain18F-fluorodeoxyglucose (FDG) PET has emerged as a valuable modality to distinguish NMDARE from other autoimmune encephalopathies.In a systematic review by Morbellietal[18],authors described several distinct patterns of cerebral hyper-and hypometabolism correlating with different autoantibodies.NDMARE was characterized by normal or increased metabolic activity within the frontal lobes with marked parieto-occipital hypometabolism.Limbic encephalitis with anti-LGI-1 antibodies,in contrast,was associated with temporal hypermetabolism and fronto-occipital hypometabolism.A subsequent study by Jhaetal[19] revealed other cerebral metabolic patterns unique to specific autoimmune encephalopathies,including frontal lobe and basal ganglia hypermetabolism in anti-CASPR2 encephalitis and basal ganglia hypermetabolism with concurrent temporal lobe hypometabolism in anti-GAD encephalitis (Table 2)[19,20].The degree of cerebral hypo-or hypermetabolism may correlate with disease severity and outcomes,although further research is necessary to clarify the prognostic value of brain FDG PET in autoimmune encephalopathies[21,22].
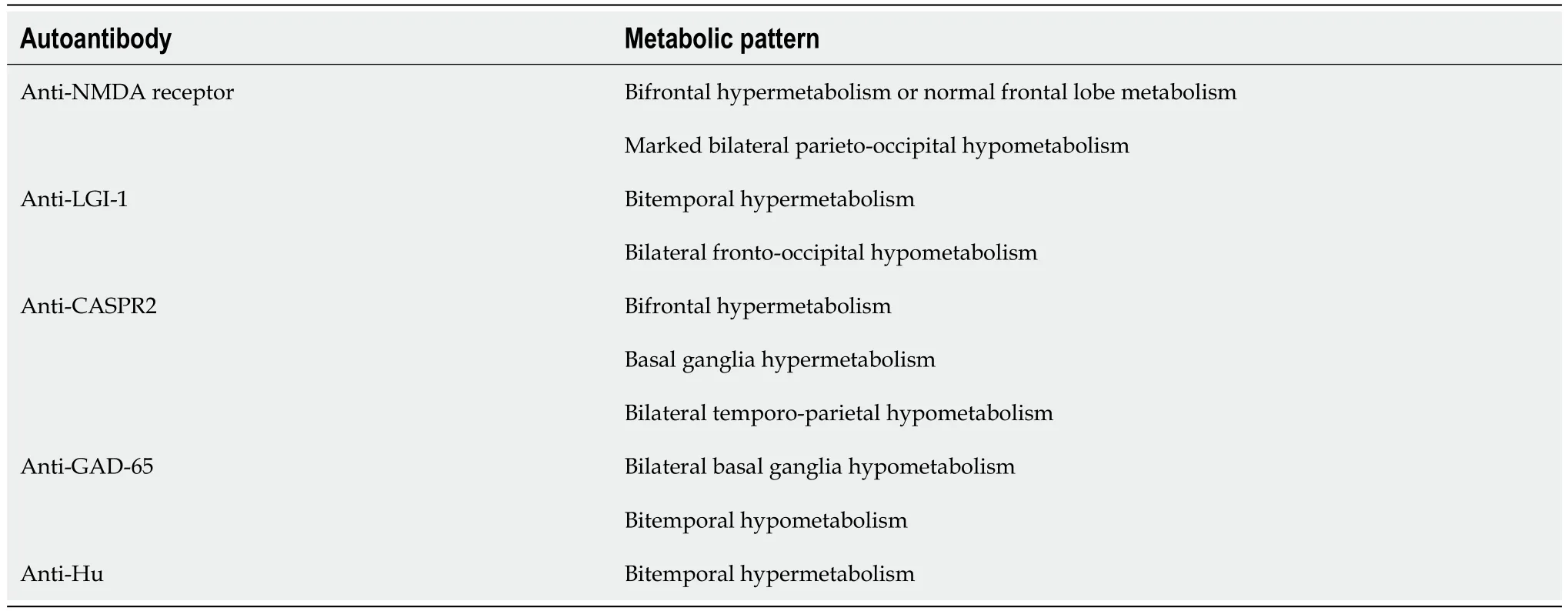
Table 2 Cerebral metabolic patterns of autoimmune encephalopathies[18-20]
Other nuclear imaging studies that may be of value for the assessment of suspected NMDARE include whole-body FDG PET/CT scan,which is highly sensitive for the detection of occult malignancies,including ovarian teratoma and other neoplasms that have been associated with NMDARE[17].Single photon emission computed tomography (SPECT) with technetium-99 hexamethyl propylenamine oxamine (HMPAO) and N-isopropyl-p-123-I-iodoampheatmine (I-123-IMP) have also been used to help diagnose NMDARE and may help identify cerebral metabolic abnormalities in the setting of a normal brain MRI and FDG PET[23,24].The multimodal imaging features of NMDARE are further detailed inTable 3.

Table 3 Multimodal imaging of anti-N-methyl-D-aspartate receptor-associated encephalitis[12-24]
MECHANISM OF PATHOGENESIS
The mechanism of pathogenesis for NMDARE remains to be established.Antibodies targeting the GluN1 subunit of the NMDA receptor are present in both paraneoplastic and non-paraneoplastic NMDARE;a juxtaposed T-cell mediated response is thought to occur only in paraneoplastic NMDARE[25].The neuropsychiatric symptoms of NMDARE may be related to antibody-mediated blockade of NMDA receptors in the presynaptic gamma-aminobutyric acid ergic neurons of the thalamus and frontal cortex with resultant downstream dysregulation of dopaminergic pathways[26].Indeed,the clinical hallmarks of NMDARE,including confusion,paranoia,and delusions,mirror those of psychosis;thus,hyperactive dopaminergic signal transduction may represent a shared mechanism underlying both conditions.Seizures are also common in NMDARE and may be related to excessive extrasynaptic NMDA receptor signaling.In a recent EEG study by Symmondsetal[27],authors observed aberrant NMDA signaling predominantly affecting NMDA receptors of excitatory neurons.However,despite the increasingly robust clinical data,the complex interplay between anti-NMDA antibodies,NMDA receptors,and dopaminergic pathways remains incompletely understood.Further research is necessary to establish a unifying model to account for the unique constellation of neuropsychiatric and autonomic symptoms that characterize NMDARE.
MANAGEMENT OF NMDARE
There are no established clinical practice guidelines for the management of NMDARE.Supportive care is essential for most patients and may include benzodiazepines,anti-epileptic drugs,beta-blockers,anticholinergics,and close monitoring in the intensive care unit[28].High-dose corticosteroids,intravenous immunoglobulin,and/or plasma exchange represent the mainstay of management for the underlying autoimmune dysfunction[29].Immunotherapy,such as rituximab and cyclophosphamide,may serve as effective second-line agents.Agitation,hallucinations,and delusions may be challenging to manage in NMDARE patients and benzodiazepines often do not provide adequate sedation;ketamine or propofol may be required for some individuals.Catatonia may occur in some patients and may improve with high-dose benzodiazepines or electroconvulsive therapy[30].Pregnant patients represent a special population occasionally affected by NMDARE.The limited existing data suggest that high-dose corticosteroids are safe and effective,but second-line agents -including rituximab and cyclophosphamide -should be avoided in pregnancy due to the risk of teratogenicity.
Clinical monitoring and follow-up of NMDARE is distinct from that of many other encephalitides.Acute disseminated encephalomyelitis,herpes encephalitis,and other similar neuroinflammatory conditions typically resolve or improve within days of treatment initiation.However,clinical resolution of NMDARE may require many weeks or months;functional improvements have been observed over 2 years after resolution of the acute phase of illness[3].The current consensus guidelines suggest that treatment with a first-or second-line agent should be continued for at least 6 wk before clinical re-evaluation and escalation or discontinuation of therapy[31].Dose escalation or transition from a first-to second-line agent may be considered before 6 wk in the setting of severe illness with autonomic dysfunction.Physical and occupational therapy -including mobility training,gait training,and speech-language therapy -play a key role in improving long-term functional outcomes and is recommended for nearly all patients with an NMDARE diagnosis[32].
CONCLUSION
NMDARE represents a rare immune-mediated clinical entity that presents with a unique constellation of neuropsychiatric and autonomic symptoms.Early diagnosis and management is essential to prevent catastrophic outcomes or death.A presumptive diagnosis can be established through careful correlation of clinical history,EEG,and imaging studies.However,cerebrospinal fluid analysis is the gold standard diagnostic test,with the presence of IgG anti-GluN1 antibodies allowing for definitive diagnosis.
High-dose corticosteroids,intravenous immunoglobulin,and plasma exchange are first-line therapies for NMDARE.Rituximab or cyclophosphamide may be required for some individuals.Nearly all patients with NMDARE will require supportive care,which may include sedatives,airway protection,and close monitoring in the intensive care unit.The prognosis for patients with NMDARE is variable;the existing data suggest that patients presenting without hippocampal lesions on MRI tend to experience relatively favorable outcomes.Nuclear imaging may also be of value for prognostication,as emerging evidence indicates that cerebral metabolic gradients on FDG PET may help predict functional outcomes.
The underlying mechanism of pathogenesis for NMDARE remains to be established,although most authors agree that dopaminergic pathways are implicated in the neuropsychiatric symptoms.Neuroinflammation may also play an important role in the pathogenesis of NMDARE but cannot yet be diagnosed by imaging or by routine laboratory studies.
Imaging will undoubtedly play a central role in NMDARE diagnosis in the future.Widespread adoption of the MRI classification schema introduced by Zhangetal[12] may improve diagnostic accuracy and provide important prognostic information to help guide clinical management.In addition,brain FDG PET can currently be used to identify patterns of cerebral metabolism suggestive of underlying NMDARE.However,advancements in molecular imaging and the development of novel radiotracers may allow for detection of aberrant proteins that are expressed early in the disease process.It has been definitively established that early intervention portends better patient outcomes;multimodal imaging will be vital to ensure timely and accurate diagnosis and expedited management.Future research is needed to develop targeted therapies and improve clinical outcomes for patients who develop this rare but potentially devastating immunemediated condition.
FOOTNOTES
Author contributions:Beutler BD performed the majority of the writing;Moody AE prepared the tables;Thomas JM and Sugar BP assisted with the literature review;Ulanja MB and Antwi-Amoabeng D contributed to the sections on pathogenesis and management;Tsikitas LA designed the outline and coordinated the writing of the paper.
Conflict-of-interest statement:None of the authors have real or potential conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Bryce David Beutler 0000-0002-5071-1826;Alastair E Moody 0000-0002-5232-7705;Mark B Ulanja 0000-0001-5966-3966;Daniel Antwi-Amoabeng 0000-0001-8594-004X.
S-Editor:Liu JH
L-Editor:Filipodia
P-Editor:Zhao S
 World Journal of Radiology2024年1期
World Journal of Radiology2024年1期
- World Journal of Radiology的其它文章
- Computed tomography-based nomogram of Siewert type II/III adenocarcinoma of esophagogastric junction to predict response to docetaxel,oxaliplatin and S-1
- From strength to precision: A systematic review exploring the clinical utility of 7-Tesla magnetic resonance imaging in abdominal imaging
