Role of intravascular ultrasound and optical coherence tomography in intracoronary imaging for coronary artery disease:a systematic review
Maruf Sarwar,Stephen Adedokun,Mahesh Anantha Narayanan,3,?
1. Department of Cardiovascular Sciences,White River Health,Batesville,AR,USA;2. Division of Cardiology,University of Tennessee at Memphis,TN,USA;3. University of Arkansas Medical Sciences,Little Rock,AR,USA
ABSTRACT Coronary angiography has long been the standard for coronary imaging,but it has limitations in assessing vessel wall anatomy and guiding percutaneous coronary intervention (PCI).Intracoronary imaging techniques like intravascular ultrasound (IVUS) and optical coherence tomography (OCT) can overcome these limitations.IVUS uses ultrasound and OCT uses near-infrared light to visualize coronary pathology in unique ways due to differences in temporal and spatial resolution.These techniques have evolved to offer clinical utility in plaque characterization and vessel assessment during PCI.Meta-analyses and adjusted observational studies suggest that both IVUS and OCT-guided PCI correlate with reduced cardiovascular risks compared to angiographic guidance alone.While IVUS demonstrates consistent clinical outcome benefits,OCT evidence is less robust.IVUS has progressed from early motion detection to high-resolution systems,with smaller compatible catheters.OCT utilizes near infrared light to achieve unparalleled resolutions,but requires temporary blood clearance for optimal imaging.Enhanced visualization and guidance make IVUS and OCT well-suited for higher risk PCI in patients with diabetes and chronic kidney disease by allowing detailed visualization of complex lesions and ensuring optimal stent deployment and positioning in PCI for patients with type 2 diabetes and chronic kidney disease,improving outcomes.IVUS and recent advancements in zero-and low-contrast OCT techniques can reduce nephrotoxic contrast exposure,thus helping to minimize PCI complications in these high-risk patient groups.IVUS and OCT provide valuable insights into coronary pathophysiology and guide interventions precisely compared to angiography alone.Both have comparable clinical outcomes,emphasizing the need for tailored imaging choices based on clinical scenarios.Continued refinement and integration of intravascular imaging will likely play a pivotal role in optimizing coronary interventions and outcomes.This systematic review aims to delve into the nuances of IVUS and OCT,highlighting their strengths and limitations as PCI adjuncts.
Two-dimensional coronary angiography has been the gold standard for diagnostic and interventional coronary imaging,but it has substantial limitations.Coronary angiography is limited in its ability to assess coronary vessel wall anatomy including luminal dimensions,plaque length,morphology,and to efficiently guide percutaneous coronary intervention (PCI)-e.g.,assess for presence of dissections,stent apposition and expansion.Multiple studies have shown that even something as fundamental as estimating the severity of coronary disease is sub optimal with angiography,interventional cardiologists tend to overestimate degree of coronary stenosis before intervention[1]and underestimate after intervention.There is already extensive plaque in the vessel wall by the time the lumen gets compromised (Glagov phenomenon) on angiography and therefore is a major limitation while using angiography alone for decision making.[2]Furthermore,it is difficult to estimate the physiologic significance of a coronary artery stenosis by angiography alone,correlation between stenosis estimated on angiography and coronary physiology estimated by fractional flow reserve (FFR) is highly sub-optimal[3]and revascularization decisions made on the basis of coronary stenosis had inferior results in the FAME II study.[4]Visualizing stenosis with other modalities may overcome some of these limitations and intracoronary imaging might be one such option to improve decision making.[5]
In current day clinical practice,two major imaging modalities are being increasingly utilized for intra coronary imaging in patients with coronary artery disease (CAD)-intravascular ultrasound (IVUS) and optical coherence tomography (OCT).IVUS,as the name implies,is based on ultrasound properties,while OCT uses near-infrared light to generate an image.Both modalities carry their own advantages and limitations and so it is prudent to understand the nuances of different imaging technologies while choosing the right imaging technique to answer the question.For practical purposes,it is important to understand some of the crucial differences between the two techniques including differences in temporal and spatial resolution and the depth at which imaging is possible.This in fact influences the question that each technique can answer—plaque characterization vs.whole vessel characterization and how a stenosis is measured—lumen based in OCT vs.EEM based sizing in IVUS.
A general summary of the field seems to be that use of intravascular imaging guidance (IVUS or OCT)for PCI reduces the risk of cardiovascular death and major adverse cardiovascular events (MACE) when compared to angiographic guidance alone.The major evidence comes from meta-analyses of randomized clinical trials (RCTs) and adjusted observational studies but the effect was less robust in individual RCTs,primarily due to the limitations of the individual trials.Whereas it has been shown that IVUS guidance reduces the risks of all-cause death,myocardial infarction,target lesion revascularization and stent thrombosis,there is lesser evidence for OCT use in guiding PCIs.IVUS and OCT both seem to be comparable in terms of clinical outcomes.
IVUS and OCT are gaining popularity among interventional cardiologists performing PCI on higher risk patients with T2DM and CKD.These patients are prone to procedural complications like stent thrombosis or restenosis.IVUS and OCT allow thorough vessel examination to optimize stent placement and minimize complications.They also permit better assessment of stent expansion and wall apposition compared to angiography alone,reducing thrombosis/restenosis risk.Patients with diabetes develop complex lesions that IVUS and OCT visualize in detail to guide treatment.They also enhance lumen and stenosis visualization versus angiography.Zero and low contrast PCI techniques utilizing IVUS and OCT guidance are being explored to reduce contrastinduced nephropathy (CIN) risk.By enabling optimal stent deployment,IVUS and OCT improve outcomes and reduce major adverse cardiovascular events and CIN in these high risk T2DM and CKD patients undergoing PCI.
The frequency of utilization of IVUS and OCT as an important adjunct to PCI remains unclear and there have been significant regional trends.[6]The National Inpatient Sample (NIS) database showed that these imaging modalities were used infrequently in the US (1.0% of all diagnostic angiography and 4.8% of all PCIs),but its use has been steadily increasing from 2.1% in 2004-2005 to almost 6.6% in 2013-2014.There appears to be a wide variability among the centers (only 13% of facilities used intravascular imaging in > 15% of PCIs) and many of them are IVUS guided PCIs (99%).This systematic review elucidates the intricacies of IVUS and OCT,underscoring their assets and shortcomings as ancillary tools for PCI.
INTRAVASCULAR ULTRASOUND-BASIC BACKGROUND
It was back in 1953 when Edler and Hertz recorded motion of cardiac structures using M-Mode ultrasound.[7]Yock,et al.[8]developed a miniaturized model in 1991 for deploying a mechanically rotated device into an intravascular catheter.This paved way for rapid development of devices based on a mechanically rotated single element system or the phased array synthetic aperture system steered electronically.[8]These devices were further refined to have better speed and resolution.Mechanical systems had frequencies ranging between 20 to 40 MHz with an axial and lateral resolution of about 120 μm and 200 μm respectively.[9]Recently,60 MHz mechanical devices are being used as they provide a higher resolution but are associated with an increase in blood speckle.This can affect optimal imaging and thus requires better filtering and image processing.[10]Device size has also been optimized and IVUS catheters can now be used with a 5 Fr guide catheter.
The use of IVUS imaging has significantly increased over the last decade,especially after the availability of data supporting its role in certain conditions.In some observational studies,IVUS has shown to improve clinical outcomes.However,there is wide variation in usage with a relatively higher use in the Far East when compared to some European centers including France.
The normal appearance of a coronary artery on IVUS depends on the ultrasound scatter from the different components in the arterial wall.Collagen reflects ultrasound the most and therefore the adventitia,with its high collagen,appears as a very bright structure.The external elastic lamina (EEL) represents the media-adventitia interface and typically is not echo-lucent.The typical three-layered appearance on IVUS is due to the echo-lucent media.The intimal and medial layers affect ultrasound variably,and form part of the three-layered image.Atherosclerotic plaque typically starts as intimal thickening and then plaque growth obscures the differentiation of inner intimal-internal elastic lamina (IEL) from the media.Thus,these two components are measured together as plaque volume in IVUS.The EEL,representing the media-adventitia interface,is an important landmark for plaque measurement and vessel sizing in IVUS imaging.
OPTICAL COHERENCE TOMOGRAPHY -BASIC BACKGROUND
Intravascular OCT uses light instead of ultrasound to image the artery and it currently is the most usable technique to obtain the highest resolution imaging of the coronaries.OCT has undergone major technological advancements since its first demonstration in 1991 by Fujimoto,et al.[11]at MIT,where the concept of using low-coherence interferometry to obtain cross-sectional images of biological tissue was introduced.It originally started as a way to exquisitely image the retina and quickly evolved into multiple other applications including a catheter based form for vascular imaging.[11]The first-generation OCT system was based on a time-domain OCT (TDOCT) imaging method that relied on a moving reference mirror to scan each depth position in the image pixel.[12]In 1993,the firstin vivoOCT images of the human retina were published by Swanson,et al.,[13]demonstrating its potential for non-invasive optical biopsy in ophthalmology.While the images produced were high resolution,this mechanical scanning process limited the rate at which images could be acquired.
The introduction of further innovations like the development of Fourier-domain OCT in 1996 led to improved imaging speeds and sensitivities.FD-OCT has enabled faster image acquisition,reducing motion artifacts and improving the overall efficiency of the procedure.[14,15,16]The first commercial OCT system designed specifically for ophthalmic imaging was launched by Carl Zeiss Meditec in 2001,enabling widespread clinical use.OCT imaging was subsequently introduced in cardiology as well,with the first OCT images of coronary arteries in patients published in 2002 by Tearney,et al.[18]The first OCT system for cardiology and intracoronary imaging was launched in 2004 by LightLab/St.Jude Medical.Since then,newer OCT modalities like Doppler OCT for blood flow imaging in 2006 and swept-source OCT(SS-OCT) for faster imaging in 2007 have expanded its capabilities.The utilization of SS-OCT has resulted in enhanced penetration depth and higher resolution,enabling detailed assessment of vessel layers and plaque morphology.[12,19]
Current day OCT systems incorporate advanced near infrared light sources and optical components operating at a wavelength of 1310 nm and a low-coherence interferometer is used to measure the backscattered light.Tissue composition affects light reflection and propagation time for reflected energy and this is used to construct a 2-dimensional image of the optical scattering from tissue architecture.The optical resolution of OCT is 10 times higher than that of IVUS (both longitudinal and temporal resolution).[18]A limitation of OCT is that it requires temporary clearance of blood from the imaging field as the penetration depth of OCT is only 0.5 to 2 mm and near infrared penetrates only for a short distance through blood.[20]Early systems thus required balloon occlusion,but modern systems accomplish clearance through simple flushing.The use of OCT has been slower than that of IVUS due to a number of reasons including cost,a longer learning curve and less robust data about its clinical advantage over IVUS.
Overall,innovations in light sources,scanning methods,and processing over the past 30 years have transformed OCT from an interferometry-based microscopy technique into a clinically valuable diagnostic imaging modality.
METHODS
This systematic review was conducted following PRISMA guidelines to assess the utility of IVUS and OCT in guiding PCI for coronary disease.Evidence selected for this review was based on selection guidelines from the Journal of Clinical Medicine (JCM).The review aims to provide an in-depth evaluation of the safety and efficacy of IVUS and OCT in guiding coronary interventions,and their utility in specific patient populations.
A systematic search was performed in PubMed,Embase,and Cochrane Library databases for studies published until July 2023 (Figure 1).The search strategy included the following Medical Subject Headings (MeSH) terms with various combinations:"Intravascular Ultrasonography," "Intravascular Ultrasound," "IVUS," "Optical Coherence Tomography," "OCT," "coronary artery disease," "coronary disease," "myocardial ischemia," "angina," "diabetes mellitus," "diabetes mellitus,type 2," "diabetic,""kidney failure,chronic," "renal insufficiency," "percutaneous coronary intervention," "stents," "catheterization," "angiography," "intravascular imaging,"and related entry terms.Outcomes of interest included "contrast-induced nephropathy","CIN","acute kidney injury","AKI","stent thrombosis","restenosis","target lesion revascularization","TLR","major adverse cardiac events","MACE." The search was limited to human studies in English.

Figure 1 PRISMA flow diagram for identification and screening of studies included in the analysis.
Original research articles,randomized controlled trials,observational studies,systematic reviews,meta-analyses,and retrospective analyses evaluating the use of IVUS and/or OCT during PCI in patients with coronary disease were included.Conference abstracts,case reports,editorials,and non-English studies were excluded.Reference lists of eligible studies were hand-searched.
Three independent reviewers performed study selection and data extraction using predefined criteria.Extracted data included study design,patient demographics,sample size,procedural techniques,clinical outcomes,and safety parameters.Study quality was assessed using standardized critical appraisal tools.
Results were synthesized narratively and tabulated to evaluate the efficacy and safety of IVUS-guided and OCT-guided PCI (Table 1).The safety and efficacy of these imaging modalities were also specifically evaluated in patients with DM and CKD.Key outcomes analyzed included procedural success,contrast usage,stent optimization,post-PCI complications,contrast-induced nephropathy,and major adverse cardiovascular events.

Table 1 Summary of Clinical Studies on IVUS and OCT.
The objective of the quality assessment was to guarantee the reliability and validity of the evidence presented in this review.Every effort was made to include all relevant studies.As with any review,potential bias can exist in the selection and interpretation of studies,and the findings should be interpreted considering these limitations.
ADVANTAGES OF USING IVUS OVER PLAIN ANGIOGRAPHY
There is significant evidence from randomized trials and meta-analyses demonstrating concrete benefits of IVUS-guided PCI compared to angiography guidance alone—this is true with the older bare metal stents as well as the newer drug coated stents.A meta-analysis by Parise,et al.[21]of over 5,000 patients in 16 randomized trials found IVUS-guided bare metal stent implantation reduced restenosis (OR=0.27,95% CI: 0.18-0.41) and repeat revascularization (OR=0.53,95% CI: 0.37-0.76) versus angiography guidance.However,no mortality benefit was seen.[21]A meta-analysis by Wang,et al.[22]including over 4,500 patients across 6 trials showed IVUS guidance with drug-eluting stents lowered risk of MACE (RR=0.60,95% CI: 0.41-0.88),stent thrombosis (RR=0.51,95% CI: 0.30-0.87) and repeat revascularization (RR=0.26,95% CI: 0.15-0.46) compared to angiography guidance.The ADAPT-DES study of over 8,500 patients found IVUS-guided PCI significantly lowered 2-year rates of stent thrombosis (0.7% vs.1.0%),MACE (8.4% vs.11.2%) and MI(2.9% vs.4.6%) compared to angiography guidance.[23]
Key advantages of IVUS include enhanced vessel sizing for proper stent dimensions,identification of high-risk plaque features,and ensuring optimal stent expansion and apposition.IVUS guidance has changed both the soft and hard end points (MACE or stent thrombosis,etc.) even though the major benefit has been a reduction of the rate of restenosis and target vessel revascularization.While RCTs have probably been underpowered,evidence from collective studies like meta-analyses have been very encouraging.Though it requires further study,IVUS may be very cost effective particularly in high-risk groups.[24]Patients requiring complex interventions,including distal left main coronary artery (LMCA)lesions,diabetics,and acute coronary syndromes (ACS)appear to derive particular benefit from IVUS guidance during PCI.
ADVANTAGES OF USING OCT OVER PLAIN ANGIOGRAPHY
Unlike IVUS,there is a scarcity of data comparing OCTvs.Angiography for optimizing PCI and all the available studies are either observational or registry based.There is data to suggest that OCT gives a better definition of PCI related complications and in detecting sub optimal stenting results (edge dissection,mal-apposition,etc).There is also some evidence to suggest OCT allows better stent expansion.[25]A growing body of evidence supports potential benefits of OCT guidance over angiography alone.
The ILUMIEN IV randomized controlled trial(RCT) of over 2,500 patients found that OCT-guided PCI significantly reduced 1-year rates of the composite endpoint of major adverse cardiovascular events(MACE) compared to angiography guidance alone(6.8%vs.8.1%,HR=0.83,95% CI: 0.70-0.99;P=0.04).This benefit was driven by lower rates of mortality and repeat revascularization.[26]The ILUMIEN III RCT also demonstrated that OCT guidance led to larger post-stent minimal lumen area (6.64 ±1.43 mm2vs.5.80 ± 1.64 mm2,P< 0.001) and lower stent malapposition (8.4%vs.20.1%,P< 0.001) compared to angiography guidance.[27,28]
Additional observational studies like CLI-OPCI II have shown OCT resulted in altered procedural strategy in 34.5% of patients,though the exact clinical impact of these changes is still uncertain.It appears that use of OCT changes the clinical strategy in a significant number of subjects but whether this has any clinical implication is hard to prove.[29]Registry studies like OPINION have also observed benefits of OCT in optimizing stent placement parameters such as lower post-PCI percent diameter stenosis.[30]
The higher resolution imaging provided by OCT enables superior definition of the coronary lumen and wall structures.This allows more accurate detection of procedural complications like edge dissections,stent under-expansion,and malapposition compared to angiography.OCT offers detailed visualization of plaque characteristics and optimization of stent sizing and positioning.One area of future interest is to see if OCT guided intervention allows for better stent coverage and lowers neo atheroma which in turn could help predict the duration of DAPT.Early studies seem to suggest that this may be possible.[31]
While further RCT data is warranted,the totality of current evidence indicates OCT guidance confers advantages in optimizing PCI results.This in turn may translate into tangible improvements in clinical outcomes,as suggested by reduced MACE rates in ILUMIEN IV.However,broader adoption of OCT remains limited by the need for a learning curve as well as increased procedural time and cost compared to reliance on angiography alone.Finally,even if it does not show immediate benefits,intravascular imaging with OCT helps to understand the mechanism of stent failure and very late stent thrombosis.[32]It is reasonable to conclude that the bulk of the collective evidence would suggest that intravascular imaging has outcome advantages when compared to angiography.[5]
IVUS VERSUS OCT: GENERAL DIFFERENCES
While both IVUS and OCT provide vessel and plaque information,they differ in some important respects (Figure 2).One big difference in their clinical use is in vessel sizing—both modalities use different boundaries in a vessel for stent sizing.IVUS usually visualizes intima and EEL without being able to delineate the media and thus uses EEL for stent sizing while OCT uses the lumen edge for sizing.In the OPUS-CLASS study,Kuboet al.[30]showed that IVUS overestimated vessel size compared to OCT(8.03 ± 0.58 vs.7.45 ± 0.17 mm2,P< 0.0001) and IVUS-derived minimal lumen area was larger than OCT by 1.01 ± 0.78 mm2(P< 0.0001).For the same vessel in a phantom,IVUS overestimated vessel size by about 8% but OCT measurements were accurate.[30]The same was seen in a clinical study by Gerbaud,et al.[33]in which mean minimum lumen diameter was lowest with QCA (1.64 ± 0.48 mm),while that by IVUS was larger than that measured by FD-OCT(2.09 ± 0.60 vs.1.91 ± 0.69 mm;P< 0.001).The minimum lumen area behaved similarly and both imaging techniques were very reproducible,but OCT was still better.Mean lumen area by IVUS was 3.47± 1.52 mm2compared to 2.96 ± 1.56 mm2by OCT (P<0.001).Interobserver reproducibility for lumen diameter was better for OCT (intraclass correlation 0.980)than IVUS (0.928).[33]These results demonstrate automated OCT measurements are possible and are accurate while those for IVUS readings often require human correction.
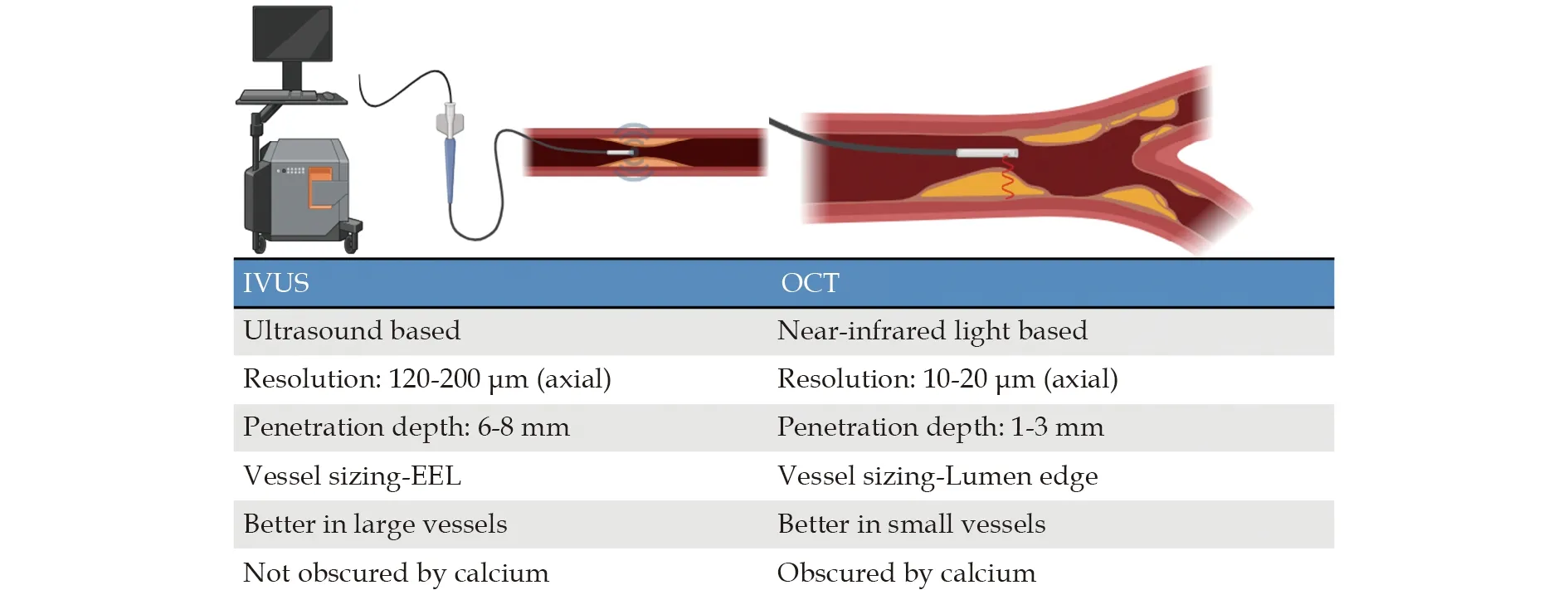
Figure 2 Distinguishing Attributes of IVUS and OCT for Intravascular Imaging. IVUS: intravascular ultrasound;EEL: external elastic lamina; OCT: optical coherence tomography.
On the other hand,OCT has shorter depth penetration in imaging the vessel wall and lipid plaque can obscure any morphology behind it.Clinicians are more familiar with IVUS patterns and have used the imaging modality for a longer period,[2]while OCT requires a learning curve and has been in use for a much lesser time.While image patterns are being standardized,[10]there are still some gray areas like detecting macrophage infiltration,and this makes OCT difficult to have consistent definitions.[34]For example,in the study by Kini,et al.,[34]OCT-derived cap thickness had a significant association with plaque features like macrophages,showing quantification is possible but standard definitions are still needed.The mean cap thickness was 119 ±62 μm for fibroatheromas compared to 62 ± 19 μm for TCFAs (P< 0.001),with 97% of TCFAs having a cap thickness < 65 μm by OCT.However,consistent standardized criteria for features like macrophage infiltration are still lacking.[34]
One limitation of IVUS is the vessel calcification(a reflector of ultrasound) and so it is challenging to obtain good images.[35]True necrosis is often ambiguously misinterpreted along with shadowing or severe signal dropout in IVUS.Sheet,et al.[35]analyzed 53 arterial segments with varying degrees of calcification by IVUS and histology.Heavy calcification led to full signal attenuation behind calcium in IVUS,obscuring tissue morphology.While IVUS had a sensitivity of 96%,heavy calcification still posed challenges for tissue characterization,with only 77% sensitivity for fibrotic tissue behind calcium.In summary,key quantitative results were that heavy calcification caused severe acoustic shadowing in IVUS,leading to only low sensitivity in detecting calcium and obscuring tissue morphology behind it.The data illustrates limitations posed by calcification for obtaining diagnostic quality IVUS images.[35]
Recent studies show the importance of preparing a vessel bed in the face of extensive calcification in a vessel before stenting.This might be better with OCT since light travels through calcium with little backscatter unlike in lipid rich or necrotic core,which usually tend to produce signal poor regions with a diffuse border on OCT.In OCT analysis of lipidrich atherosclerotic segments,we found a strong correlation (r=0.84,P< 0.0001) with fibrous cap macrophage density.Using OCT thresholds of 6.15%-6.35%,we achieved excellent diagnostic accuracy for > 10% macrophage marker staining.In another study,ACS patients had higher macrophage density in fibrous caps than stable angina patients,and plaque rupture sites had significantly greater macrophage density (6.95% ± 1.60%vs.0.29% ± 1.17%;P=0.002).[36]
In chronic total occlusions,IVUS helps in both antegrade (resolves proximal cap ambiguity and facilitates reentry into true lumen from the sub intimal layer) and retrograde (reentry from false to true lumen) approaches but there is less data for OCT in this regard.One area where OCT will reign superior is estimating the cap thickness for thin cap fibroatheromas (TCFA) which is thought to be the sine qua non for a vulnerable plaque.A cap thickness of less than 65 microns is thought to denote a high-risk plaque and OCT is currently the best method to define this in vivo,provided careful quality control is established.[34]
PERFORMANCE OF OCT-GUIDED INTERVENTION COMPARED TO IVUSGUIDED PCI
OCT has better resolution,both lateral and axial,than IVUS and thus provides better imaging-OCT can thus be better for detecting dissections,mal-apposition,thrombus,neoatheroma and TCFA cap.On the other hand,IVUS can see farther out into the vessel (8 mm compared to about 2-3 mm for OCT)and is better for imaging larger vessels including LMCA ostium and calcified plaques.This led to great enthusiasm in exploring the appropriate imaging technique.Some early studies including RCTs have looked at this question,however,with somewhat disappointing results-the initial enthusiasm that the beautiful pictures obtained on OCT would translate into clinical superiority has not panned out as expected.The ILUMIEN II study did not find a difference in stent sizing between IVUS and OCT despite different approaches to sizing.[37]The ILUMIEN-III study showed no advantage of OCT-guided PCI over IVUS-guided PCI in stent size and expansion but showed lesser rates of mal-apposition.[28]OCT was better than angiography-guided PCI as far as stent expansion and mal-apposition were concerned.Surprisingly,the incidence of dissection was similar to angiography guided PCI but less than with IVUS guided PCI.The other major RCT,however,did not show major differences between the two forms of intravascular imaging in terms of target vessel failure or stent size or rate of restenosis.[38]
ILUMIEN IV,a recent comprehensive RCT,provides valuable insights into OCT-guided PCI.It sheds light on clinical outcomes related to stent sizing and placement.In terms of minimum stent area post-PCI,the OCT group achieved 5.72 ± 2.04 mm2,whereas the angiography group attained 5.36 ± 1.87 mm2(mean difference,0.36 mm2;95% CI: 0.21-0.51;P<0.001).Over a two-year period,target-vessel failure occurred in 88 patients in the OCT group and 99 patients in the angiography group (Kaplan-Meier estimates,7.4% and 8.2%,respectively;hazard ratio,0.90;95% CI: 0.67-1.19;P=0.45).While OCT guidance resulted in a larger minimum stent area compared to angiography guidance,no significant difference in the percentage of patients with targetvessel failure at 2 years was observed between the two groups.[26]Another large RCT is underway(OCTOBER Trial) and will hopefully add to this overall picture.
In summary,OCT provides a better definition of procedural complications and shows sub optimal stent deployment better.This might help in detecting predictors of stent-related adverse events better than with IVUS.It appears that under-expansion and edge disease remain major predictors of suboptimal outcomes with both techniques.Better detection of acute stent mal-apposition with OCT does not seem to have any advantage over IVUS in predicting or averting early or late restenosis or thrombosis.
SAFETY WITH IVUS AND OCT
Intravascular images are obtained in both techniques after engaging the coronary ostium with the guiding catheter and insertion of a guidewire,which is then followed by the insertion of IVUS or OCT catheter over the wire.There are a few complications associated with using both IVUS and OCT,with higher rates of complications with ACS compared to non-ACS patients with stable angina pectoris and in asymptomatic patients (2.1% in ACS compared to 0.8% and 0.4% in stable angina and asymptomatic patients).[39]Like any other intravascular procedure,these catheters require use of anticoagulation with heparin.Nitroglycerine is routinely administered considering 3% reported incidence of coronary spasm associated with these imaging techniques.[39,40]Complications other than the spasms include embolism,dissection,acute vessel occlusion,thrombus formation and usually present as acute myocardial infarction or requiring emergency coronary artery bypass surgery.In the IVUS PROSPECT trial which included 697 ACS patients receiving threevessel coronary imaging,complication rate was 1.6% including 10 dissections and 1 perforation.[40,41]In the more recent Thoraxcenter study from Rotterdam,NL,in which 1000 OCTs and 2500 IVUS were performed,the complication rates were much lower.While using OCT,there was 0.6% complication rate that occurred during image acquisition,compared to a 0.5% complication rate using IVUS (P=0.6).[42]As with any procedure,the complications are lowest in centers using this on a regular basis than those doing fewer studies.
A recent cohort study by Jones,et al.[43]with a large sample size (n=87,166) aimed to determine the effect on long-term survival of patients who underwent OCT-or IVUS-guided PCI compared to angiography-guided PCI.They found that OCT-guided procedures had greater procedural success and reduced in-hospital major adverse cardiac events (MACE).Significantly lower mortality was observed for patients undergoing OCT-guided PCI (7.7%) versus IVUS-guided (12.2%) or angiography-guided PCI(15.7%,P< 0.0001).Overall,both advanced intravascular imaging modalities (OCT and IVUS)predicted better survival than angiography guidance alone.This study demonstrated not only the safety,but also the clinical benefit of using these newer imaging techniques as part of the PCI diagnostic process.[43]
IVUS enables precise measurements of the vessel size and plaque burden,as it can penetrate to the external elastic membrane.This facilitates mid-wall or true vessel stent sizing,optimizing stent dimensions,and identifying areas with the smallest plaque burden to minimize geographical miss.Proper stent positioning and expansion are crucial factors in reducing the incidence of restenosis and stent thrombosis,both of which can have significant long-term implications for patients.[44]IVUS has established predictors of stent failure,such as stent under-expansion,major edge dissections,and geographical miss.
Several meta-analyses have confirmed the benefits of IVUS guidance.One meta-analysis of randomized trials comparing IVUS-guided bare metal stent implantation with angiography-guided procedures revealed a reduction in restenosis and repeat revascularization,without impacting death or myocardial infarction.[21]A total of 19 studies met the inclusion criteria,comprising 27,610 patients divided into IVUS (n=11,513) and CA (n=16,097).Compared with standard CA-guided PCI,we found that the risks of cardiovascular death (relative ratio (RR)=0.63;95% CI: 0.54-0.73),myocardial infarction (RR=0.71;95% CI: 0.58-0.86),target lesion revascularization (RR=0.81;95% CI: 0.70-0.94),and stent thrombosis (RR=0.57;95% CI: 0.41-0.79) were all significantly lower using IVUS guidance.[21]
Two recent meta-analyses of studies comparing IVUSguided drug-eluting stent procedures with angiography-guided interventions showed a decreased incidence of death,major adverse cardiac events,and stent thrombosis.[22,45]The first study showed IVUS guidance was associated with a significant reduction in major adverse cardiac events (RR=0.61;95% CI:0.53-0.70;P< 0.001),all-cause death (RR=0.55;95% CI: 0.42-0.71;P< 0.001),cardiac death (RR=0.45;95% CI: 0.32-0.62;P< 0.001),myocardial infarction(RR=0.66;95% CI: 0.55-0.80;P< 0.001),and stent thrombosis (RR=0.48;95% CI: 0.27-0.84;P=0.01)compared with angiographic guidance.[22]The second study showed MI and all-cause death at 3 years between IVUSvs.angiography guidance.IVUS guidance provided a significantly lower incidence of the composite of MI and all-cause death (hazard ratio=0.82,95% CI: 0.49-1.37;P=0.001).[45]These findings are supported by additional clinical studies,including the RESET trial from South Korea and the ADAPTDES observational study involving over 8500 patients.[23,46]Comparing IVUS guidance to angiography guidance,the 1-year rates of MACE were 3.2%vs.4.8%,respectively (number needed to treat,64;95% CI: 42-137;hazard ratio=0.67;95% CI: 0.53-0.84).Between 1 and 2 years,MACE occurred in 1.9% of patients treated with IVUS guidance and 3.0% treated with angiography guidance (hazard ratio=0.64;95% CI: 0.47-0.86).Thus,the 2-year rates of MACE were 4.9% with IVUS guidance versus 7.5% with angiography guidance (number needed to treat,41;95% CI: 29-69;hazard ratio=0.65;95% CI: 0.54-0.78).Comparing IVUS guidance to angiography guidance,the 2-year rates of cardiac death were 1.7%vs.2.4%,P=0.03,and the trend was consistent within 1 year(0.9%vs.1.2%;P=0.11) and between years 1 and 2(0.9%vs.1.2%;P=0.13).[46]Collectively,these advantages demonstrate the significant role of IVUS in improving outcomes and safety in coronary interventions.
CLINICAL USES OF INTRAVASCULAR IMAGING
Stenosis Severity: Left Main Coronary Artery Lesions
Assessment of the LMCA with angiography can be challenging.IVUS of LMCA has been shown to correlate more with FFR measurement of the LMCA.[47]It is important to remember the difference in LMCA sizes between the various ethnic groups.In general,Asian population have a smaller vessel diameter,and so MLA when compared to the western population.In Koreans,an IVUS LMCA MLA of 4.5-5.8 mm2results in negative FFR (FFR ≥ 0.8) while in the western population,LMCA MLA of less than 6 mm2has been shown to be associated with an FFR of <0.75.[47]In a Spanish registry,[48]patients with an MLA > 6 mm2(179/186),who did not get revascularization,had similar cardiac death free survival (P=0.5) when compared to patients who had MLA <6 mm2(152/168) and who had revascularization.Also,even free survival in both the groups were similar(P=0.3).The study also showed that people who had MLA between 5 to 6 mm2did worse when treated only with medical therapy without revascularization.In a study of 122 patients with LMCA lesion not treated at the time of IVUS assessment,the annual event rate was about 14% and minimal luminal diameter on IVUS was the most important predictor of cardiovascular events.[49]Ideally,IVUS should be done from both the left anterior descending and left circumflex artery to evaluate LMCA dimensions.OCT has a few limitations including the requirement of contrast and inability to measure ostial lesions.But OCT is ideal to measure distal left main lesions which is prone to form complex plaques extending left anterior descending and left circumflex arteries.[50]As a result,OCT is a valuable tool to help guide management of angiographically intermediate lesions.It allows for more information to be gained giving the operator the ability to accurately decide between revascularization vs conservative management.This point is further demonstrated by a study by Datoet al.[50],which used OCT data to help guide decision for revascularization or conservative management.Target vessel failure free survival was not different between patients undergoing conservative management vs.revascularization (HR=0.40,95% CI: 0.10-1.61,P=0.20).[50]
Stenosis Severity: Non-Left Main Coronary Artery Lesions
In non-LMCA lesions,MLA of 4 mm2has been shown as the cut off to differentiate between vessels with and without ischemia.In a study by Abizaid,et al.,[51]MLA > 4 mm2was found to be associated with a coronary reserve of ≥ 2.When revascularization was deferred based on MLA > 4 mm2,IVUS MLA and area stenosis (AS) were the only independent predictors of MACE at follow up.[52]FFR is known to be the gold standard for evaluation of ischemia in three randomized controlled trials (RCTs)and FFR based revascularization for patients with a value of < 0.8 has been shown to improve patient outcomes and prognosis.[4,53-56]Comparison between FFR and IVUS in patients with intermediate coronary lesions was performed in a recent study.[57]FFR of < 0.8 and MLA on IVUS < 4 mm2were used as criteria for revascularization.94 lesions were treated with IVUS whereas 83 were treated with FFR.A significant number of people in the IVUS arm underwent revascularization therapy when compared to FFR guided therapy (92% and 34% respectively,P<0.001) but there was no significant difference in MACE at one year follow up (3.6% in FFR guided PCI versus 3.2% in IVUS guided PCI).In a few published studies,the range of IVUS measurements guiding ischemia ranged from 2.1 mm2to 4.4 mm2suggesting inconsistency.One needs to be aware of the difference in vessel dimensions between the western population (higher MLA) and the Asian population (typically lower MLA) while interpreting IVUS MLA.The sensitivity of IVUS MLA (< 4 mm2) compared to FFR was only 92% with a 56% specificity,while a longer lesion length of > 10 mm had a sensitivity of only 41% with higher specificity of 80%.[58]Based on the various studies,it can be concluded that an IVUS MLA of > 4 mm2could be treated conservatively(higher negative predictive value) but treating all lesions < 4 mm2MLA on IVUS leads to treating almost 50% of lesions without ischemia (lower positive predictive value).
There is a good correlation between IVUS and OCT measured MLA with nearly similar sensitivity and specificity.[59]OCT guided MLAs are typically smaller in the range of 1.6 mm2to 2.4 mm2.In an OCT based study treating 62 intermediate lesions in 59 patients,an OCT derived MLA of < 1.91 mm2and percentage lumen AS of > 70% were shown to correlate well with an FFR of <0.75.A large metaanalysis published in 2020 did show that OCT had a had a higher specificity in terms of diagnosing flow limiting stenosis compared to IVUS (0.763vs.0.665,P<0.001).[60]OCT however suffers similar limitations like IVUS with a higher negative predictive value but a lower positive predictive value.There are no RCTs comparing IVUS to OCT similar to FFR and iFR studies.It is important to remember that both IVUS and OCT,do not take into account,the subtended viable myocardial mass,or information on antegrade or retrograde flow through collaterals.
Monitoring Plaque Morphology and Changes with Therapy
A number of studies were done to evaluate the effect of medical therapy on plaque volumes and IVUS has been the preferred mode of imaging in most of these studies.This has helped us understand plaque biology and effect of treatments including statins and more recently,the PCSK-9 inhibitors.[61]For example,von Birglelen,et al.[62]followed mild LMCA atherosclerosis over a period of 18 ± 9 months and showed that LDL levels correlated with plaque progression whereas HDL levels had an inverse correlation.The REVERSAL (Reversal of atherosclerosis with aggressive lipid lowering) is a double blinded RCT that demonstrated IVUS derived atheroma volume showed a significantly lower progression of atherosclerotic plaque in patients treated with atorvastatin (P=0.02).[63]Progression of plaque did not occur in the atorvastatin group whereas the pravastatin group had plaque progression.The ESTABLISH trial also showed regression of plaque volume in the atorvastatin group.[64]In ASTEROID,the mean percentage atheroma volume(PAV),the most reliable IVUS measure of disease progression and regression,showed significant regression in patients treated with high dose rosuvastatin.[65]However,it is essential to standardize the methods for plaque assessment,and this in fact comes with significant challenges.
Some would argue that it is important to identify vulnerable plaques,but this view is controversial.[66]The most common finding of the so-called vulnerable plaque is the TCFA,a precursor for thrombotic plaque rupture.Grayscale IVUS has suggested four different morphologies consistent with a TCFA including large plaque burden,shallow echolucent zones,spotty calcifications and attenuated plaque(shadowing without calcification) though only the former three (except attenuated plaque) have been shown to be associated with adverse outcomes including myocardial infarction,hospital admission and need for PCI.[41,67,68]The largest data comes from the virtual histology IVUS that has shown to predict future adverse events in non-culprit lesions.Both gray scale IVUS and radiofrequency (RF) IVUS (integrated backscatter IVUS and virtual history IVUS)have shown to predict cardiac events.The difference between OCT and radiofrequency IVUS is that RF IVUS can only suggest the presence of TCFA by identifying the necrotic core along the lumen whereas OCT is able to define the thickness of a thin fibrous cap less than 65 μm,macrophages in the fibrous cap and also presence of underlying lipid core.Studies have shown that the presence of a higher plaque burden of > 70% can be used to predict a TCFA.OCT is able to differentiate between ruptured and nonruptured atheroma and to identify the culprit versus non-culprit plaque ruptures.Another OCT based study showed that the rapid angiographic lumen loss of > 0.4 mm in 7 months was associated with increased frequency of intimal laceration,microvessels,TCFA,macrophages,lipid accumulation and intraluminal thrombi.[69]
Near infrared spectroscopy intravascular ultrasound (NIRS) is a special type of IVUS imaging that can detect lipid rich plaques.There are a few studies evaluating using the NIRS technique to identify vulnerable plaques,including the LRP (NCT02033 694),PREDICT (NCT02792075),and PROSPECT-2(NCT02171065) trials.In the LRP study that had 1563 patients enrolled,a patient whose maxLCBI4mm exceeds 400 faced an 87 percent higher risk compared to a patient with a lower maxLCBI4mm.In analyses focused on individual plaque levels,there is a 45% increased risk of experiencing an event within a vulnerable coronary segment within 24 months for every 100 unit increase in maxLCBI4mm.Furthermore,the probability of an event occurring in a coronary segment with a maxLCBI4mm greater than 400 is 411 percent higher than in a segment with a lower maxLCBI4mm.NIRS-intravascular ultrasound device-related events were seen in six (0.4%)patients.This supports the idea that it is a safe tool that can be used for additional information.The PREDICT trial which is still in the recruitment phase plans to evaluate target vessel failure rates in different imaging modalities including,OCT,NIRS,and IVUS.PROSPECT-2 study demonstrated that highly lipidic lesions (851 (24%) of 3500 lesions,present in 520 (59%) of 884 patients) were an independent predictor of patient-level non-culprit lesion-related MACEs (adjusted odds ratio=2.27,95% CI: 1.25-4.13) and non-culprit lesion-specific MACEs (adjusted odds ratio=7.83,95% CI: 4.12-14.89) (NCT0217 1065).These studies in total show promise for the use of NIRS IVUS in identification of lipid rich plaques.
The efficacy of serial intravascular imaging remains has not fully been established.Serial intravascular imaging has shown that plaque morphology could rapidly change over a period of few months without actual rupture,especially in patients treated with high dose statins.As intravascular imaging is invasive,it is important to evaluate the appropriateness of imaging to identify vulnerable plaques,which again depends on the prevalence of TCFAs,their frequency,stability,pharmacotherapy needed during the procedure and complications associated with intravascular imaging.It is essential to understand that there are a lot of gray areas in this space -we don’t have a consistent technique or definition that best predicts outcomes in vulnerable plaques -studies have shown wide disagreement between the different imaging modalities,including gray scale IVUS,RF IVUS,angioscopy,OCT and NIRS,often in the range of 25%-30%.These techniques suffer from biased populations and high sensitivity at the expense of low specificity.[41]As a result the frequency and type of evaluation used on lesion is still very operator dependent.
Patients with Chronic Kidney Disease (CKD)
Conventional PCIs often lead to severe complications in patients with CKD.However,both low-tozero contrast IVUS and low molecular weight dextran (LMWD) OCT-guided PCIs offer a promising alternative for CKD patients.Minimal contrast and contrast-free IVUS-guided procedures demonstrate safety in both cardiac and renal outcomes,comparable to conventional PCIs,even for complex atherosclerotic lesions.The OCT intravascular imaging modality offers a higher axial resolution than IVUS,but its increased use of contrast volume is linked to CIN development.Recent development of zero and LMWD contrast OCT-guided PCI produces safe,effective outcomes comparable to both traditional intervention and IVUS-guided PCI.These techniques either avoid or reduce nephrotoxic contrast agents and thus may allow OCT-guided PCI to be suited for patients with CKD.
Burlacu,et al.[70]performed a systematic review to include studies evaluating the efficacy and safety of PCI using IVUS and minimal or zero-contrast in chronic kidney disease patients.Of the 238 patients,none of the patients in the studies experienced renal function deterioration or required renal replacement therapy following the zero-contrast IVUSguided procedures.Moreover,from a cardiovascular standpoint,this technique has proven to be safe with favorable cardiovascular outcomes.[70-76]
The CONSaVE-AKI study was a prospective,randomized study that assessed short-term outcomes and safety of ULC-PCI compared to conventional PCI in patients with ACS who were at high risk for contrast induced acute kidney injury (CI-AKI).Patients who had an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2 with a moderate to very high Maioli score for pre-procedural risk of developing CI-AKI were included in the study.All patients (n=82) were randomized and equally divided into two equal groups: ULC-PCI and conventional PCI.IVUS was used in 17% of patients within the UCL-PCI group.The development of CIAKI occurred significantly more in patients who underwent conventional PCI (17.1%) than in the ULC-PCI group (0) (P=0.012).Contrast volume was also significantly lower in the ULC-PCI group(41.02 ± 9.8vs.112.54 ± 25.18 mL;P< 0.0001).The use of IVUS guided imaging helped to reduce the contrast utilization drastically and to effectively reduce the incidence of CI-AKI.Intervention by ULCPCI with IVUS guidance is feasible and can be safely used in patients with renal dysfunction to reduce the incidence of CI-AKI.[77]
Sakai,et al.[71]examined the use of IVUS-guided minimum-contrast (MINICON) PCI (n=98) compared to standard angiography-guided PCI (n=86)in patients with advanced CKD (n=184).The IVUSguided MINICON PCI group showed significantly reduced contrast volume and a lower incidence of CI-AKI compared to the angiography-guided standard PCI group (CI-AKI;2%vs.15%;P=0.001).The success rate of the PCI procedure was similarly high in both groups (100%vs.99%;P=0.35).At the one-year follow-up,the IVUS-guided MINICON PCI group had a lower rate of renal replacement therapy(RRT) induction (2.7%vs.13.6%;P=0.01),but there were no significant differences in all-cause mortality or myocardial infarction between the groups.[71]These findings suggest that IVUS-guided MINICON PCI produce high success rates and effectively reduce CI-AKI and RRT induction in patients with advanced CKD.
Sacha,et al.[72]performed a retrospective analysis on the safety and efficacy of zero-contrast IVUS PCI in patients with severe renal impairment and hemodialysis subjects.Zero-contrast IVUS PCI was performed within 29 coronary arteries in 20 patients with advanced CKD.This group included four patients who were treated with hemodialysis but presented preserved residual renal function.The estimated median probability of AKI in non-dialysis patients undergoing standard PCI using 100-200 mL of contrast medium was 57%.This median risk estimation reduced to 26% when using the assumption that contrast dye was not used.With the zerocontrast IVUS approach,the factual risk of AKI was reduced to 10% and no patient required renal replacement therapy.The technique also yielded good clinical outcomes in mid-term observations.During the median follow-up of 3.2 (1.2-5.3) months no patient experienced an acute coronary event or required revascularization.IVUS identified the lesion length,determined balloon and stent diameters,and landing zones for stent implantation,as well as verified and documented the final PCI effect.Since the procedure was based on IVUS imaging,it allowed optimization of stent implantation with good procedural results,and consequently no stent thrombosis occurred during the follow-up.[72]The study’s results indicate if the patient is at a high risk of AKI,zero-contrast-IVUS PCI should be considered as a preventative solution,even for dialysis patients with residual renal function.
Kumar,et al.[74]sought to assess the safety and short-term outcomes of 'absolute' zero-contrast PCI under IVUS guidance in CKD patients.There were 42 CKD patients who underwent absolute zero-contrast PCI under IVUS guidance.A total of 66 coronary vessels underwent intervention,including 14(21.2%) LMCA PCI (seven of which were LMCA bifurcation PCI) and three chronic total occlusion(CTO) PCI procedures.Even with the inclusion of these complex PCIs,technical success was 92.4% without any major complications.Two patients died of non-cardiac causes on follow up (3-12 months),and all the remaining were symptom free.[74]IVUSguided 'absolute' zero-contrast PCI is feasible and safe for CKD patients,allowing successful completion of the procedure without any contrast or complications,even in complex lesion morphologies.
Ultra-low contrast PCI guided by zero-contrast OCT can be safely performed without major adverse events in non-STEMI patients with high-risk CKD who require revascularization.Liu,et al.[78]evaluated the safety and efficacy of zero contrast OCT-guided PCI in CKD patients with NSTEMI.The study involved 29 NSTEMI patients with highrisk CKD (median Cr=2.1) undergoing revascularization.There were no significant changes in creatinine level or eGFR in the short-or long-term.OCT's high-resolution imaging capabilities enabled precise assessment of coronary lesions and better guided interventional procedures with greater accuracy.During the PCI procedure,OCT revealed 15 (52%)cases of abnormalities post-dilation.The main approach,zero-contrast OCT,resulted in safe and successful PCI without AKI,CIN,and the need for renal replacement therapy.Furthermore,no patients experienced post-interventional complications and no MACEs were observed.[78]
Azzalini,et al.[79]also reported that it is feasible to use dextran as a substitute for contrast in OCT guidance.The study evaluated the feasibility of an ultralow contrast volume percutaneous coronary intervention (ULC-PCI) protocol in patients with severe CKD.Extensive intravascular dextran-based OCT guidance was used to compare the outcomes of the ULC-PCI protocolvs.conventional angiographybased PCI in patients with eGFR < 30 mL/min per 1.73 m2.Technical success was achieved in all ULCPCI procedures,and the incidence of CI-AKI was 0%vs.15.5% in the ULC-PCI and conventional groups,respectively (P=0.28).The implementation of an ULC-PCI protocol with OCT guidance in patients with advanced CKD is both achievable and safe.Furthermore,this approach shows promise in reducing the occurrence of CI-AKI when compared to the conventional angiographic guidance used alone.
Though OCT's 10-fold higher axial resolution than IVUS allows precise plaque and lumen characterization,its contrast flushing requirement increases total volume used,risking CIN.Kurogi,et al.[80]evaluated OCT-guided PCI using LMWD in patients with CKD.This single-center retrospective study found that OCT-guided PCI using LMWD as a flushing medium did not negatively affect renal function compared to IVUS-guided PCI using contrast media alone.There were no significant differences between 133 matched pairs of OCT and IVUS patients in changes in serum creatinine or eGFR after the procedure.The incidence of CIN was also similar between groups (1.5% OCTvs.2.3% IVUS).When stratified by CKD stage,there were again no differences between OCT with LMWD and IVUS with contrast in renal function changes or contrast volumes used.The study suggests OCT imaging with LMWD is a feasible approach that does not impair renal function relative to IVUS imaging with contrast alone,supporting its use as an alternative flushing medium in patients with CKD undergoing PCI.[80]
Recently,saline has been explored as a replacement for contrast to primarily avoid nephrotoxicity risks and expand FD-OCT to more patients safely.Several studies have aimed to evaluate if saline could provide adequate image quality in coronary FDOCT.[75,76]The SOCT-PCI study demonstrated the feasibility of using heparinized saline as a flushing medium for FD-OCT image acquisition during PCI optimization.In 27 patients undergoing FD-OCTguided PCI,heparinized saline was used for blood clearance during 118 OCT runs.Overall,61% of runs were good quality,27% were clinically usable,and 88% were clinically effective for PCI optimization.There were no significant hemodynamic or arrhythmic changes.Saline FD-OCT enabled visualization of coronary lesions and post-PCI optimization comparably to contrast FD-OCT in one case.[81]This suggests saline could replace contrast as the flushing medium for FD-OCT during PCI in patients at risk of CIN.
Gupta,et al.[82]explored saline as an alternative to radio-contrast for OCT-guided PCI.This prospective study analyzed 13 pairs of OCT runs using both contrast and heparinized saline in the same coronary arteries during PCI.The primary endpoints were quantitative measurements of minimal lumen area,reference diameters,and area stenosis.There were no significant differences in these primary endpoints between saline and contrast OCT.Bland-Altman analysis demonstrated good agreement without proportional bias between the two-flushing media for vessel measurements.All plaque morphologies and post-PCI optimization parameters were clearly visualized in the saline runs similar to contrast.The results indicate heparinized saline can yield comparable dimensional measurements and adequate image quality versus contrast for coronary OCT during PCI optimization.Using saline could not only eliminate the risk of CIN,but also produce high quality images in high risk patients.[82]While both of these studies show promising results for the use of saline in OCT,further large scale randomized studies directly comparing saline and contrast OCT are still needed to validate these findings.
Patients with Diabetes Mellitus
CAD remains a significant health concern,particularly in patients with diabetes mellitus,who often present with more complex and diffuse lesions.It has been previously demonstrated that coronary remodeling enables patients to develop large atherosclerotic plaques without a reduction in lumen size,leaving diabetic patients at high risk for developing myocardial infarction.[2]IVUS guidance during PCI has emerged as a valuable tool in optimizing outcomes for these high-risk patients.IVUS revealed that the prevalence of asymptomatic CAD in T2D patients is high,suggesting a need for a broader residual CV risk management using alternative approaches.[83]
Numerous studies have reported the benefits of IVUS guidance in diabetic patients undergoing coronary interventions.The ABCD trial is the first trial to date to perform invasive IVUS imaging,a gold-standard technique for evaluation of CAD,in asymptomatic patients with T2DM and revealed a CAD prevalence of 84%,indicating a significantly higher burden of disease than previously assumed.[83,84]IVUS provides accurate measurements of coronary vessels,enabling precise stent sizing and better stent deployment.Specific coronary lesions associated with diabetes are mainly confined to coronary small-vessels.IVUS allows physicians to analyze and diagnose small-vessel lesions to improve the therapeutic effect of available interventional approaches more accurately.Arora,et al.[83]demonstrated that the addition of IVUS guidance was associated with larger post-PCI minimum lumen diameter and more post-dilatation,which translated into clinically and statistically significant lower rates of target lesion revascularization or in-stent restenosis after drug eluting stent (DES) implantation.IVUS revealed mean maximal intimal thickness,percent atheroma volume and total atheroma volume in the T2DM population were 0.75 ± 0.27 mm,33.8 ± 9.8% and 277.0 ± 137.3 mm3as compared to 0.41 ± 0.19 mm,17.8 ± 7.3% and 134.9 ±100.6 mm3in the reference population,indicating a higher atheroma and plaque burden in T2DM patients with CAD.[83]By characterizing plaque morphology,IVUS helps identify vulnerable lesions in high risk populations,leading to a more tailored and effective treatment approach.
Regarding safety,the reviewed studies generally reported no significant increase in adverse events associated with IVUS-guided PCI in diabetic patients.Rahman’s retrospective observational study demonstrated a significant reduction in MACE in diabetic patients (n=73/134,54.5%) who underwent IVUS-guided PCI.MACEs occurred in 9.7% of patients,where 3% of patients experienced heart failure.Furthermore,iatrogenic coronary dissection was zero.[85]The use of IVUS resulted in a significant decrease in MACE.IVUS appeared to contribute to a reduction in procedural complications,such as stent thrombosis and restenosis.
IVUS provides detailed visualization of coronary vessels,enabling accurate stent sizing,better stent deployment,and identification of vulnerable plaques.By characterizing plaque morphology,IVUS helps identify high-risk lesions,leading to tailored and effective treatment strategies.IVUS has shown a consistent safety profile in diabetic patients,with low rates of procedural complications.Its ability to optimize stent deployment contributes to improved long-term patency and decreased adverse events,making it a valuable tool in the management of coronary disease in this high-risk patient population.The precise stent placement achieved with IVUS may contribute to improved long-term patency and decreased incidence of adverse events.These findings underscore the potential long-term benefits and procedural safety of IVUS guidance in this high-risk population.
The use of OCT guidance in coronary disease management for patients with diabetes has demonstrated promising efficacy and safety.Patients with diabetes often have more complex and diffuse coronary lesions.[41,86,87]OCT provides high-resolution,detailed imaging of coronary arteries,allowing precise assessment of plaque morphology,stent positioning,and stent apposition.
The efficacy of OCT in patients with diabetes lies in its ability to identify vulnerable plaques and guide interventional procedures,leading to better treatment outcomes in cases with complex plaque morphology and coronary lesions.Fabris,et al.studied the natural history of OCT-detected TCFA,ThCFA,and non-LRP in patients enrolled in the prospective multicenter COMBINE FFR-OCT trial.[87,88]In the COMBINE FFR-OCT trial,a total of 390 diabetic patients underwent OCT evaluation and were clinically followed for 18 months with a primary endpoint of primary end point of cardiac death,target vessel-related myocardial infarction,target-lesion revascularization,and hospitalization for unstable angina.[86,88]The high resolution of OCT enabled precise differentiation between TCFA,ThCFA,and non-LRP within coronary artery lesions.After 18 month follow-up,TCFA lesions were associated with a much higher risk of future cardiovascular events compared with patients with ThCFA (13.3%vs.3.8%,P< 0.01) and non-LRP(13.3%vs.1.9%,P< 0.01) which portend similar and benign outcomes (3.8%vs.1.9%,P=0.38).[88]OCT allows for more accurate lesion characterization,leading to tailored treatment strategies and improved stent deployment,which can help reduce the risk of future adverse cardiovascular events.By leveraging the OCT criteria,it becomes possible to significantly reduce the pool of patients eligible for novel approaches,thereby opening the pathway to evaluating the efficacy of personalized therapeutic strategies in this vulnerable population.
Regarding safety,OCT is generally well-tolerated in patients with diabetes.The procedure carries similar risks to other intravascular imaging techniques,such as IVUS.[55]Potential complications include vessel dissection,perforation,or thrombus formation,but these are relatively rare and occur at low rates.Khurwolah,et al.[89]explored the safety of FD-OCT in the treatment of angiographically-intermediate coronary lesions (ICL).Ten diabetic patients underwent OCT-guided treatment(PCI,n=7;optimized medical therapy (OMT),n=3)and were monitored for 30-day major adverse cardiac events (MACE).The incidence of 30-day MACE was zero in both the OCT-guided PCI and OCT-guided OMT groups.[89]This study established FD-OCT was safe in the evaluation and treatment of ICL in diabetic patients.
OCT has emerged as a valuable and effective tool for guiding coronary interventions in patients with diabetes.Its exceptional resolution capabilities enable precise assessment of coronary lesions,including vulnerable plaques,which are common in diabetic individuals.By providing intricate visualization of plaque morphology,OCT allows for accurate stent sizing,optimal stent deployment,and identification of potential complications.Despite the complexity of coronary disease in diabetes,studies have shown that OCT guidance is safe and well-tolerated in this patient population.Moreover,the ability to differentiate between thin-cap fibroatheroma,thick-cap fibroatheroma,and non-lipid rich plaque aids in tailored therapeutic strategies,ultimately leading to improved treatment outcomes.As the field of intravascular imaging continues to advance,OCT's role in optimizing coronary interventions for patients with diabetes is likely to become even more critical,reaffirming its position as a valuable tool for enhancing the safety and efficacy of coronary interventions in this high-risk group.
CONCLUSIONS
In conclusion,intravascular imaging,specifically IVUS and OCT,has demonstrated significant benefits in accurately assessing vessel dimensions,plaque burden,and vulnerable lesions.IVUS is valuable in guiding optimal stent sizing and positioning,leading to improved procedural outcomes and long-term patient benefits.It also offers a low contrast usage advantage,reducing the risk of CI-AKI in CKD patients.On the other hand,OCT's high-resolution imaging allows for detailed visualization of coronary arteries,aiding in precise stent positioning and identifying vulnerable plaques.Both modalities have their unique strengths,and their use may depend on patient characteristics and lesion complexities.
Despite the proven benefits,the adoption of intravascular imaging has been limited,partly due to the reliance on collated studies and underpowered RCTs for hard clinical outcomes.Cost and a steep learning curve are also contributing factors.However,increasing use is observed in complex lesions and high-risk patients,and advancements in technology such as heartbeat OCT,high frame IVUS,and automated analysis are expected to further promote its incorporation into clinical practice.Wellconducted RCTs may be necessary to determine the appropriate use of these techniques.
 Journal of Geriatric Cardiology2024年1期
Journal of Geriatric Cardiology2024年1期
- Journal of Geriatric Cardiology的其它文章
- Influencing Factors on Cardiovascular Health in China
- Development and validation of a model integrating clinical and coronary lesion-based functional assessment for longterm risk prediction in PCI patients
- Interaction between systemic iron parameters and left ventricular structure and function in the preserved ejection fraction population: a two-sample bidirectional Mendelian randomization study
