Zeolite-Coated Anti-Biofouling Mesh Film for Efficient Oil-Water Separation
Baixian Wang, Qifei Wang, Jiancheng Di,*, Jihong Yu,2,*
1 State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012,P.R.China.
2 International Center of Future Science, Jilin University, Changchun 130012, P.R.China.
Abstract:The development of the global economy has been accompanied by frequent oil spills caused by accidental leaks and industrial manufacturing, which have seriously threatened the aquatic environment and human health.Traditional methods for the treatment of oily wastewater include centrifugation, skimming,flotation, oil-absorbing technology, etc., which are limited by low separation efficiency as well as secondary pollution during the post-processing of oil absorption materials.Recently, separation technologies utilizing the special wettabilities of filtration membranes have been developed to enrich and recycle oils from wastewater.Among these, the fabrication of superhydrophilic/underwater superhydrophobic membranes have attracted intensive research interest, which can selectively allow the passage of water through the membrane while blocking the oils.However, microorganisms are more likely to breed on these hydrophilic surfaces, eventually leading to the blockage of the membranes.In this study, ZSM-5 zeolite crystals (MFI topological structure) were coated onto the stainless-steel meshes by means of seeding and secondary hydrothermal growth.Then,70% of the total Na+ ions in the zeolite channels were substituted by Ag+ ions via an ion exchange process.The resultant membranes (Ag@ZCMFs) were superamphiphilic in air, with both water contact angle and oil contact angle of approximately 0°.However, they became superoleophobic when immersed in water, and the underwater oil contact angle reached 151.27° ± 4.34°.In terms of special wettability, Ag@ZCMF achieved efficient separation for various oil-water mixtures with separation efficiencies above 99%.The water flux and intrusion pressure of Ag@ZCMF depended on the diameter of pinholes in the membrane, which could be modulated by altering the time of secondary hydrothermal growth.For instance, the average diameter of pinholes in Ag@ZCMF with optimum secondary growth time of 14 h (Ag@ZCMF-14) reached approximately 21 μm, giving rise to the water flux and intrusion pressure of 54720 L·m-2·h-1 and 4357 Pa,respectively.The anti-corrosion test and rubbing test confirmed the high chemical and mechanical stability of Ag@ZCMF-14, respectively.The separation efficiency of Ag@ZCMF-14 remained stable during ten purification-regeneration cycles,and no obvious attenuation was observed, proving the high separation stability of Ag@ZCMF-14.Furthermore, the loaded Ag+ ions afforded the membrane excellent anti-biofouling activity, which could effectively inhibit the growth of both alga and bacteria in the operating environment, thus preventing membrane blockage during the oil-water separation process.In particular, the bacteriostatic rate of Ag@ZCMF-14 to Escherichia coli reached to 99.6%.These results demonstrate that Ag@ZCMFs with anti-biofouling activity has promising potential future applications in the removal of oil slicks from oily wastewater.
Key Words:Zeolite;Wettability;Oil-water separation;Ion exchange;Anti-biofouling activity

1 Introduction
Oil pollutions triggered by the industrial manufactures and oil leaks not only are the waste of resources1-3, but also cause significant negative impact on the ecological environment4-8.Therefore, the enrichment and recovery of the oil slicks from oily wastewater are of great importance in the field of environmental protection9,10.Traditional oil-water separation methods, including centrifugation, skimming, flotation, and oil absorbing technology, suffer from the limitation of low separation efficiency, high cost, and secondary pollutants11-16.To overcome these drawbacks, the filtration technique by using the separation membrane with special wettability has been developed to separate the oil-water mixtures owing to its high efficiency and low power consumption17-24.Because of the higher density of water than most oils, the fabrication of membranes with superhydrophilicity/underwater superoleophobicity has attracted intensive research interest, which are prone to interact with water and form a barrier to repel the immiscible oils19,25-34.Moreover, the oil contaminations on the membrane can be easily rinsed off by water.However, the aquatic organisms,such as bacteria and algae, are more likely to adhere on these hydrophilic surfaces, which will decrease the separation capacity and eventually result in the blockage of the membranes35,36.Till now, several polymer membranes have been reported, which are capable to simultaneously achieve the functions of oilwater separation and anti-biofouling activity5,37,38.However,because of the inherent nature of polymers, the stability of these membranes is poor in harsh conditions, which may limit the practical applications in oil-water separation.
Zeolites are a kind of crystalline aluminosilicate materials with regular molecule-sized pores, which have been widely used in the fields of catalysis, adsorption, and ion exchange,etc.39-46.In our previous work, we successfully developed a pure-silica zeolite-coated stainless-steel mesh film with superhydrophilicity in air and underwater superoleophobicity, which could realize the highly efficient separation for various oil-water mixtures32.But the framework of the pure-silica zeolite is constructed entirely by silicon-oxygen tetrahedra, which is neutral and restricts the further functionalization by ion exchange.To this end, the trivalent Al element is introduced into the skeletons of zeolites, which can result in the anionic frameworks of zeolites,affording them excellent ion exchange ability46-50.Taking account of the broad-spectrum antibacterial performance of silver5,51,52, it is expected that the Ag+loaded zeolite films can integrate the abilities of oil-water separation and anti-biofouling activity.
Herein, aluminosilicate ZSM-5 (MFI zeotype) zeolite crystals are coated over the stainless-steel meshes through secondary hydrothermal growth process, which are further functionalized with Ag+ions by means of ion exchange.The resultant membranes (Ag@ZCMF) exhibit superamphiphilicity in air/underwater superoleophobicity, which facilitate the gravitydriven separation of various oil-water mixtures with high separation efficiency and stability.Moreover, the loaded Ag+ions in zeolite afford Ag@ZCMF superior inhibitory effect on the growth of chlorella andEscherichia coli, exhibiting excellent anti-biofouling performance.
2 Experimental
2.1 Materials
Tetrapropylammonium hydroxide (TPAOH, 25%),tetraethylorthosilicate (TEOS), aqueous ammonia (28%),aluminum isopropoxide, silver nitrate (AgNO3), cyclohexane,methylbenzene, n-octane, petroleum ether, and n-hexane were of analytical grade.Sodium hydroxide (NaOH) and sodium chloride (NaCl) were of guarantee grade.Stainless-steel mesh(360 mesh) was obtained from Xinxiang Kairui Co.Ultrapure deionized water was generated by using a Millipore Milli-Q plus system (US).Chlorella was purchased from Guangda Tech Co.Escherichia coli(E.coli) (ATCC 25922) was from the Guangdong culture collection center.All chemicals were used as received without further purification.
2.2 Preparation of silicalite-1 crystal seeds
The nano-sized pure silica silicalite-1 (MFI) zeolite crystals were used as the seeds for the preparation of ZSM-5 zeolite coatings on stainless-steel meshes.The precursor solution was composed of TPAOH, TEOS, water, and EtOH with the molar ratio of 9 : 25 : 480 : 100.After the continuous stirring for 12 h,the clear precursor solution was sealed in the polypropylene bottle (50 mL), which was placed in an oven at 90 °C for 4 d.The collected product was washed by repeating the centrifugation/ultrasonic dispersion process to wash away the residual alkalis.Finally, the product was dried in the oven(60 °C) overnight.
2.3 Preparation of ZSM-5 zeolite coatings
The ZSM-5 zeolite coatings on the stainless-steel meshes were prepared by seeding and secondary growth process.To prepare the seed solution, the as-prepared silicalite-1 nanocrystals were evenly dispersed in diluted aqueous ammonia(pH value was about 10 and the concentration was 20 g·L-1).Then the stainless-steel mesh was cut into the size of 3.5 cm ×3.5 cm and then immersed into the seed solution under ultrasonic condition for 10 min.The seeded substrate was dried in the oven at 120 °C for 2 h.The molar ratio of the reaction solution for the secondary growth of zeolite coating was fixed at TPAOH :Al2O3: Na2O : TEOS : H2O : EtOH = 3: 0.1 : 0.4 : 25 : 1534 :100.Then the vertically placed seeded mesh and 60 g reaction solution were sealed in a 100 mL autoclave, and the reaction was conducted at 170 °C forxh (x= 4, 8, 12, 14, 16, 18, 20, 22, and 24, respectively).The as-prepared ZSM-5 coated mesh films(ZCMFs) and the ZSM-5 powders along with the ZCMFs were calcined at 550 °C for 4 h in air with the heating rate of 1 °C·min-1.
2.4 Ag+ ion exchange process
The Ag+ion exchange process was operated by immersing the one piece of ZCMFs or 800 mg ZSM-5 powder into 100 mL AgNO3solution (0.05 mol·L-1) and stirring slowly at 50 °C for 24 h.Then the Ag+ion exchanged ZCMFs (Ag@ZCMFs) or ZSM-5 powder (Ag@ZSM-5) were washed with deionized water for three times to remove the excessive Ag+ions, and then dried with nitrogen gas at room temperature.
2.5 Separation of oil-water mixtures
One piece of the as-prepared Ag@ZCMF was squeezed in the stainless-steel flanges, on which two glass tubes were connected.Water and oil were mixed with the volume ratio of 1 : 1, and the filtrate through the water pre-wetted film was collected in a jar.During the separation process, no external driving force was applied on the system, which was only its own gravity.Water flux was evaluated under the constant intrusion pressure of about 230.3 Pa, and then calculated according to the formula:F=V/St,whereVwas the water volume (L) through the membrane inttime (h),Swas the effective area of the membrane (m2).
2.6 Anti-biofouling activity test of Ag@ZCMF
The anti-biofouling activity of Ag@ZCMF was tested by evaluating the inhibitory performance of the membrane to the growth assay of chlorella andE.coli, respectively.For instance,one piece of Ag@ZCMF or ZCMF was placed in a 100 mL chlorella suspension, which was incubated in a biochemical incubator at 25 °C for different days, such as 7 and 14 d.The optical density (OD) of the chlorella suspension at 540 nm was measured to evaluate the inhibiting effect of the membrane to the growth of chlorella.
The anti-bacterial property of membranes was measured by the zone inhibition method and the bacterial dynamics curve.In the zone inhibition method, theE.colibacteria was inoculated into the Luria-Bertani (LB) solid medium, on which the UV-sterilized Ag@ZCMF-14 or ZCMF-14 membrane with diameter of around 1.0 cm was placed.The width of the inhibition zone was measured after culturing for 24 h at 37 °C.In the bacterial dynamics curves, a volume of 100 μLE.colibacteria suspensions with the concentration of 3 × 108colony forming units per mL (CFU·mL-1) was added into LB liquid medium.The UV-sterilizedAg@ZCMF-14 and ZCMF-14 membrane were placed into LB liquid medium and incubated at 37 °C for 90 min, respectively.Then, the optical density was measured at 600 nm to evaluate the anti-bacterial efficacy of the membrane.
2.7 Release behavior of Ag+ ions
The release behavior of Ag+ions was measured by adding 500 mg Ag@ZSM-5 powders into 100 mL water.The Ag+ions concentrations were measured after the release time for 2, 4, 6,and 8 d, respectively.The average concentration of Ag+ions at each time point was obtained by three repeated measurements.
2.8 Characterizations
SEM images were performed using JEOL JSM-6510 microscopy (Japan).The energy dispersive spectrum (EDS)mappings were recorded on JEOL FE-SEM 6700 F (Japan).Powder X-ray diffraction analysis was conducted using a Rigaku D-Max 2550 diffractometer with CuKα radiation (λ= 0.15418 nm, 50 kV) (Japan).Inductively coupled plasma (ICP) analysis was carried out on an iCAP 7000 SERIES ICP spectrometer(US).Contact angle measurement was recorded using the Data-Physics OCA20 machine at ambient temperature, and five different positions were measured for each sample.The residual oil content in the collected water was examined on an OIL480 infrared spectrometer oil content analyzer.The optical density value was measured using Shimadzu UV-visible spectrophotometer UV-1700 (Japan).A digital differential pressure gauge (AS510, Smart Sensor) was used to measure the intrusion pressure.
3 Results and discussion
3.1 Preparation of Ag@ZCMFs
The ZSM-5 coated mesh films (ZCMFs) were prepared on the stainless-steel meshes by means of a secondary hydrothermal growth process for different time.Chemical composition analysis by ICP measurement indicates that the ZCMFs contain Si, Al, and Na elements.Fig.1a presents the scanning electron microscope (SEM) image of the ZCMF after the secondary growth of 14 h (ZCMF-14), revealing that the stainless-steel fibers have been uniformly coated by the zeolite crystals.The pin-holes are intentionally kept tofacilitate the passage of liquid during the oil-water separation process.The enlarged SEM image (Fig.1b) exhibits the rough surface of ZCMF-14 containing micro- and nano-scale geometries.The X-ray diffraction (XRD) pattern of ZCMF-14 (red line in Fig.1c) fits well with that of the simulated the MFI structure (blue line in Fig.1c).The ICP measurement reveals that the Si/Al molar ratio of ZCMF-14 reaches to 120, which is closed to that in the precursor solution.In addition, 70% of the dissociative Na+ions in the channels of ZCMF-14 have been substituted by Ag+ions after ion exchange process.Fig.2 gives the energy dispersive spectrum (EDS) mappings of the resultant membrane(Ag@ZCMF-14).It can be seen that silver element (green) is homogeneously dispersed in the zeolite coating.

Fig.1 (a, b) SEM images of ZCMF-14.Scale bar: (a) 50 μm;(b) 5 μm.(c) XRD patterns of ZCMF-14 (red line) and simulated MFI structure (blue line).

Fig.2 EDS mapping analysis of Ag@ZCMF-14.(a) Ag (blue color),(b) Si (green), (c) Al (purple), and (d) O (red).Scale bar: 25 μm.
3.2 Wettability of Ag@ZCMF-14
The wettability of the ZCMF-14 and Ag@ZCMF-14 were measured on a contact angle meter and the cyclohexane was used as the detecting oil.In Fig.3, ZCMF-14 and Ag@ZCMF-14 are all superamphiphilic in air, and the water contact angle (WCA)and oil contact angle (OCA) of them are close to 0°.When immersed in aqueous media, both of ZCMF-14 and Ag@ZCMF-14 exhibit superior repellence to the oil droplet, and the underwater oil contact angles (θo/w) reach to 149.77° ± 1.16° and 151.27° ± 4.34°, respectively (Fig.3c, f).These results indicate that the loaded Ag+ions in the zeolite channels has no influence on the wettability of the membrane.Moreover, the measuredθo/wvalues of Ag@ZCMF-14 for a selection of oils are all above 130°, proving the extraordinary underwater oleophobicity of the membrane (Fig.3g).

Fig.3 The WCAs of (a) ZCMF-14 and (d) Ag@ZCMF-14 in air.The OCAs of (b) ZCMF-14 and (e) Ag@ZCMF-14 in air.The θo/w of (c)ZCMF-14 and (f) Ag@ZCMF-14, in which cyclohexane (3 μL) was used as the detecting oil.(g) The θo/w of Ag@ZCMF-14 for a selection of oils.
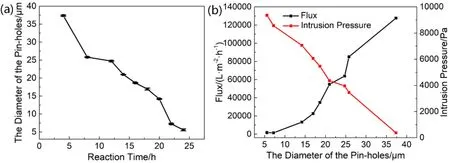
Fig.4 (a) The relationship between the average diameter of the pin-holes and the crystallization time of Ag@ZCMFs.(b) Influence of the diameter of the pin-holes of Ag@ZCMFs on water flux and intrusion pressure.Black line: water flux; Red line: intrusion pressure of cyclohexane.

Table 1 The separation parameter of Ag@ZCMF-14 compared with other oil/water separation membranes.
3.3 Separation of oil-water mixtures
As is well-known that the water flux and intrusion pressure are the key factors that determine the separation capacity of the membranes, which can be adjusted by turning the diameter of the pin-holes in the membrane.Fig.4a gives the linear relation between the average diameter of the pin-holes in Ag@ZCMFs and the crystallization time.As seen that the diameter of the pinholes decreases from 35 to 5 μm with the extension of crystallization time from 4 to 24 h, leading to the increase of intrusion pressure from around 376.9 to 9356.7 Pa (red line in Fig.4b).Meanwhile, the water flux sharply decreases from 127656 to 1411 L·m-2·h-1(black line Fig.4b).To achieve the balance between water flux and intrusion pressure, thesecondary hydrothermal growth of 14 h was considered to be optimum crystallization time, giving rise to the average diameter of the pin-holes in the membrane (Ag@ZCMF-14) of about 21 μm.Compared with the separation parameters of the lately reported membranes with superhydrophilicity/underwater superoleophobicity(Table 1), Ag@ZCMF-14 exhibits higher separation efficiency(99.98%), water flux (54720 L·m-2·h-1), and intrusion pressure(4356.7 Pa) than most reported membranes12,25,32,33,53-58.
The cyclohexane/water mixture was employed to demonstrate the oil-water separation process, which was conducted on the equipment in Fig.5a.The Ag@ZCMF-14 squeezed in the flanges was pre-wetted by a small amount of water.Then, a mixture of cyclohexane (red, dyed by Sudan III) and water with volume ratio of 1 : 1 was poured onto the membrane.The filtrate was collected in the jar, in which there was no visible oil (red),suggesting the successful separation of oil-water mixture by Ag@ZCMF-14 (Fig.5b).

Fig.5 Separation process of cyclohexane/water mixture.(a) The oilwater mixture was poured onto the water pre-wetted Ag@ZCMF-14.(b) Water selectively permeated through the membrane and the cyclohexane (red color dyed by Sudan III) was retained.

Fig.6 (a) The separation efficiencies of Ag@ZCMF-14 for a selection oils.(b) The separation efficiencies of Ag@ZCMF-14 for the cyclohexane/water mixture during ten purification-regeneration cycles.
The separation efficiency of Ag@ZCMF-14 was evaluated by measuring the residual oil content in the filtrate.Based on the underwater oleophobicity, only a trace amount of oil was detected after the separation processes and the corresponding separation efficiencies for various oil-water mixtures were all above 99% (Fig.6a), indicating the good separation capability of Ag@ZCMF-14.The recyclability of Ag@ZCMF-14 was also tested by repeating separation process for ten times.After each separation cycle, the membrane was heated at 150 °C for 0.5 h to remove the residual liquid.The separation efficiency kept stable and no obvious attenuation was observed during the 10 cycles of testing (Fig.6b), proving a stable performance of Ag@ZCMF-14 on oil-water separation.The chemical stability of Ag@ZCMF-14 was investigated by immersing the membrane into corrosive medium of 1 mol·L-1HCl and 1 mol·L-1NaCl for 48 h, respectively.Compared with the pristine Ag@ZCMF-14,the zeolite structure (Fig.7a), surface morphology (Fig.7b, c),and underwater wettability (Fig.7e, f) of the membrane were almost unchanged after the corrosion test, ascertaining the high chemical stability.The mechanical durability of the Ag@ZCMF-14 was evaluated by rubbing the membrane using the sand paper(grit No.800) under the pressure of 2800 Pa59.After the rubbing test, the Ag@ZCMF-14 was polished and the peak intensity in the XRD pattern decreased (Fig.7a).But the membrane remained underwater superhydrophobic (Fig.7g), and no defects were observed on the membrane (Fig.7d), indicating the high mechanical durability.

Fig.7 (a) The XRD patterns of Ag@ZCMF-14 after the corrosion test and the rubbing test.(b-d) SEM images and(e-g) θo/w of Ag@ZCMF-14 after the corrosion test and the rubbing test, respectively.Scale bar: 5 μm.
3.4 Anti-biofouling activity of Ag@ZCMFs
As is known that the breeding of the biological contaminants,such as alga and bacteria, in operating environments may lead to the blockage of the oil-water separation membranes.To test the anti-biofouling activity, the inhibition performance of Ag@ZCMF-14 toward the growth of chlorella was first evaluated.The OD value at 540 nm was measured to ascertain the concentration of chlorella in the solution.After the incubation for 7 d, the difference on the OD values of the chlorella solution, the solution with ZCMF-14 or Ag@ZCMF-14 was not significant (Fig.8a).When prolonging the incubation time to 14 d, the OD values of the chlorella solution and the solution with ZCMF-14 sharply increased.In contrary, the almost unchanged OD value of the solution with Ag@ZCMF-14 indicated the excellent inhibiting ability of the membrane to the breeding of chlorella.To test the stability of Ag+ions in zeolites,we measured the time-dependent release behavior of Ag+ions in the solutions (Fig.8b).The concentration of Ag+ions in the solution sharply increased in the first two days, and then kept stable.The released quantity was about 5.0% of the total Ag+ions in zeolites.

Fig.8 (a) The OD values of chlorella solutions incubated after 7 and 14 d, respectively.(b) The release behavior of Ag+ ions.

Fig.9 Digital images of inhibition zones of (a) ZCMF-14 and(b) Ag@ZCMF-14.(c) The bacterial dynamics curve in liquid medium of E.coli.
The agar diffusion method was employed to test the bacteriostatic effect of the Ag@ZCMF-14, and theE.coliwas selected as the representative target bacteria.Compared with ZCMF-14, a clear circular inhibition zone was observed around Ag@ZCMF-14 with diameter of about 0.15 cm (Fig.9a, b).Fig.9c gives the bacterial dynamics curves of Ag@ZCMF-14 and ZCMF-14, which obviously demonstrate the inhibition effect of Ag@ZCMF-14 on the growth of bacteria, giving the bacteriostatic rate of 99.6%.It is because that the Ag+ions can disrupt the cell membrane integrity, generate reactive oxygen species (ROS), which will cause cell damage and eventually limit the growth of bacteria5,60-62.These results confirm the high anti-biofouling activity of the Ag@ZCMF-14.
4 Conclusions
In summary, the ZSM-5 zeolite coated mesh films have been fabricated by a seeding and secondary hydrothermal growth process, and then the Ag+ions are loaded in the membranes by means of ion exchange.The resultant membrane with secondary growth time of 14 h (Ag@ZCMF-14) can achieve the efficient separation of various oil-water mixtures in terms of their superhydrophilicity/underwater superoleophobicity.The Ag@ZCMF-14 exhibits excellent chemical and mechanical stability.The separation efficiency remains stable during ten purification-regeneration cycles, proving superior separation stability of Ag@ZCMF-14.Furthermore, Ag@ZCMFs can effectively inhibit the growth of alga and bacteria in the operating environments, and prevent the membrane from being blocked by the biological contaminants.These results demonstrate that Ag@ZCMFs with anti-biofouling activity hold promising potentials in future practical applications for the removal of oil slicks from the oily wastewater.

