High-Entropy Spinel Oxide Nanofibers as Catalytic Sulfur Hosts Promise the High Gravimetric and Volumetric Capacities for Lithium–Sulfur Batteries
Liyuan Tian, Ze Zhang* , Sheng Liu, Guoran Li, and Xueping Gao*
1. Introduction
Lithium–sulfur (Li-S) batteries offer a great potential to achieve high energy density because of high theoretical gravimetric/volumetric energy densities(2600 Wh kg?1and 2800 Wh L?1).[1,2]In principle,the sulfur cathode undergoes a complex conversion process from solid S8molecules to soluble lithium polysulfides (LiPS), and fully to insoluble Li2S2/Li2S.[3,4]Such a multi-electron reaction is favorable for the high theoretical capacity of 1675 mAh g?1of sulfur. However, the shuttle effect originated from soluble LiPS, and sluggish redox kinetics of both insulating sulfur and Li2S2/Li2S are harmful to achieve the high utilization of active sulfur(capacity)and stable cycle life of Li-S batteries.[5–7]
Considerable efforts have been made to address the shuttle effect by confining sulfur within various host materials,which can physically or/and chemically trap soluble LiPS for improving the battery performance.[8–11]However,such passive entrapping strategies are incapable of fully eliminating the LiPS dissolution into electrolyte. In this regard, a positive electrocatalytic strategy that accelerating the conversion of soluble LiPS to final product Li2S by using transition metal compounds as catalytic materials is suggested recently.[12–17]Despite their relatively inferior conductivity to carbon nanomaterials, these polar electrocatalysts can enhance the electrochemical kinetics of sulfur redox reactions, and suppress the shuttle effect due to the strong chemical confinement of sulfur species, thus realizing the high utilization and cycle stability of sulfur cathode.[18–21]In addition, a high-efficiency electrocatalyst is favorable for realizing the high electrochemical performance of sulfur cathodes even under high sulfur loading and low electrolyte occupation, which is fundamentally essential for the development of high energy density Li-S batteries.[22–26]Furthermore,kinds of strategies have been developed to enhance the activity of these electrocatalysts, including defect engineering,[27,28]heterojunction construction,[29]and single-atom catalysis.[30]The core technology among all strategies is to explore new materials for achieving favorable adsorption catalysis on the conversion of soluble LiPS.
In particular, the catalytic activity is closely related to metal cation centers, and recently, multicomponent metal oxides are proved to be the effective regulator of soluble LiPS because of the synergistic effect among multiple metal cations.[31,32]Specifically,these multicomponent oxides have been employed as additive such as Mg0.6Ni0.4O[33]and Mg0.8Cu0.2O,[34]or the host such as spinel NiFe2O4[35]and NiCo2O4[36]oxides,and could effectively enhance the chemical adsorption of soluble LiPS,as compared to the single metal ones.[37,38]Zhang et al.[39]further demonstrated Ba0.5Sr0.5Co0.8Fe0.2O3?δperovskite nanoparticles as a bifunctional promoter for LiPS conversion and Li2S deposition. In consideration of these attributes of multicomponent oxides, high-entropy materials (HEMs) such as high-entropy alloys,[40]oxides,[41]and sulfides[42]feature homogeneously mixed and tunable five or more principal elements in a single crystal phase and are favorable to the pursuit of high adsorption ability of reaction intermediates and the catalytic activity for energy storage and conversion.[43,44]In particular, high-entropy oxides (HEOs) are recognized as single-phase oxide systems with 5 or more cations, which benefits for providing strong polar surface to chemically adsorb the sulfur species. For instance, Qiao et al. prepared a cubic rock salt type HEO containing Ni, Mg, Cu, Zu, and Co elements for Li-S batteries via a mechanical ball milling method combining with the thermal treatment at 1000 °C.[45]The as-prepared HEO exhibited an irregular bulk shape and extremely low surface area, which might not be favorable for high-efficiency loading of sulfur. In view of these issues, it is desirable to develop HEO materials with regular nanostructures as efficient catalysts for Li-S batteries.

Figure 1. Structural characterization. a) SEM image, b) TEM image, c) the corresponding SEAD pattern, d) high-resolution TEM image of HEO nanofibers, e)XRD patterns, f) TG curve, and g) N2 adsorption/desorption isotherms. h) TEM image, i) HAADF-STEM image, and j) the corresponding EDS elemental maps of S/HEO composite.
In this contribution, for the first time, high-entropy spinel oxide nanofibers are prepared via electrospinning method and used as catalytic host of sulfur to facilitate the conversion of soluble LiPS, which is the rate-determining step in the electrochemical reaction of sulfur cathode. The synergistic concept of well-designed porous 1D nanostructure and multiple metal cations in a single spinel structure not only can provide desired paths for facilitating Li-ion diffusion,but also can afford abundant active sites for chemically anchoring LiPS and catalyzing the conversion of LiPS. Moreover, the heavy HEO host is helpful to the high tap density of S/HEO composite (1.92 g cm?3).As a result, fast kinetics and excellent cycle stability can be realized in S/HEO composite to fulfill the demand of both high gravimetric and volumetric capacities.
2. Results and Discussion

Figure 2. Electrochemical evaluation. a) CV curve at a scan rate of 0.1 mV s?1, b) rate capability, c) the initial discharge/charge curves at 0.1 C rate, cycle stability at d) 0.1 C and e) 1 C, f) areal capacity at 0.1 C of S/HEO electrode with different sulfur loadings, g) cycle stability of S/HEO electrode with different E/S ratios.
The high-entropy oxide (denoted as HEO, (Mg0.2Mn0.2Co0.2-Ni0.2Zn0.2)Fe2O4) nanofibers were prepared by electrospinning the mixture solution of polyacrylonitrile (PAN) and principal metal salts followed by a calcination process in air. The as-prepared HEO nanofibers are about tens of micrometers in length with diameter range of 120–200 nm (Figure 1a). Transmission electron microscope (TEM)image in Figure 1b shows that the HEO nanofiber with a ~160 nm diameter is composed of abundant nanocrystals. The polycrystalline characteristic is identified by the diffraction rings in the selected area electron diffraction(SAED)pattern(Figure 1c).As further confirmed in the X-ray diffraction (XRD) pattern (Figure 1e), the HEO sample presents a single spinel structure(JCPDS 89-4927)without any impurities,similar to that of NiFe2O4.[46]It is evidently demonstrated that the extra multiple metal cations (Mg, Mn, Co, and Zn) occupy the same lattice to Ni cation in a stabilized single phase,thus leading to a high configurational entropy. Accordingly, all the diffraction rings (Figure 1c) can be ascribed to the different crystalline planes of cubic spinel structure.High-resolution TEM image in Figure 1d presents the clear lattice spacing of ~0.24 nm, corresponding to the (311) plane of HEO sample.After sulfur incorporation,the S/HEO composite shows a clear appearance of orthorhombic sulfur due to the high sulfur content of 79.6 wt% (Figure 1f). Interestingly, two-step weight loss is observed in the thermogravimetric(TG)curve,which is because of the strong chemical interaction between HEO host and sulfur,[47]thus leading to the postponed evaporation of sulfur (3.4 wt%). The morphology of S/HEO composite was further characterized using scanning electron microscope(SEM),TEM,and high-angle annular dark-field scanning electron microscopy (HAADF-STEM). SEM image (Figure S1, Supporting Information),TEM,and HAADF-STEM images(Figure 1h,i)show the wellretained 1D nanofiber morphology of S/HEO, indicating the uniform distribution of sulfur among HEO nanofibers.The uniform distribution of sulfur can be also confirmed by the corresponding energy-dispersive spectroscopy (EDS) elemental mapping (Figure 1j). In addition, the incorporation of sulfur into HEO decreases the specific surface area from 384.4 to 154.7 m2g?1(Figure 1g, and Table S1, Supporting Information) due to the filling of sulfur inside the pore structure or covering on the surface of HEO nanofibers. As a comparison, porous carbon nanofibers (CNFs) with abundant micro-/mesopores are also prepared and used as host of sulfur (Figure S2 and S3, Supporting Information).

Figure 3. Adsorption of LiPS on HEO. a) UV-vis results of the Li2S6 solution after full adsorption of different sorbents.Inset shows the photographs of static adsorption test.XPS spectra of b)Co 2p3/2,c)Ni 2p3/2,and d)S 2p of HEO before and after adsorbing Li2S6.
The electrochemical performance of S/HEO and S/CNF(sulfur content of 78.7 wt%, Figure S4, Supporting Information) is evaluated in CR2032 coin cells. For regular Li-S batteries, the sulfur loading of~1.4 g cm?2and electrolyte/sulfur (E/S) ratio of 20 μL mg?1were applied. Cyclic voltammetry (CV) curves in Figure 2a of the S/HEO and S/CNF composites demonstrate that two cathodic peaks are in accord with the two-step reduction of sulfur to soluble LiPS and further to insoluble Li2S2/Li2S, and two overlapped anodic peaks are corresponding to the opposite conversion.The lower value of the peak separation in S/HEO implies the good catalytic effect of HEO on the polysulfide-involving reversible conversion reactions. The improved kinetics results in better rate capability of the S/HEO electrode. As shown in Figure 2b, specific discharge capacities of 1368.7, 1100.8,982.2, 885.8, and 752.3 mAh g?1can be achieved for S/HEO at 0.1,0.2,0.5,1,and 2 C rates,respectively.Even at 5 C rate,the high reversible capacity of 632.1 mAh g?1is still realized,and both the high and low discharge potential plateaus maintain well for S/HEO (Figure S5a,Supporting Information). However, the S/CNF composite delivers much lower capacity of 297.4 mAh g?1with severe shrinkage of both the two discharge plateaus(Figure S5b,Supporting Information).More impressively,the superiority of S/HEO is overwhelmingly expressed in terms of volumetric capacity (2627.9 mAh cm?3at 0.1 C rate), about 2.5 times that of S/CNF(1039.8 mAh cm?3),which is because of the significant difference in tap density of S/HEO (1.92 g cm?3) and S/CNF (0.84 g cm?3). The results clearly demonstrate the great potential of heavy HEO to realize both high gravimetric and volumetric capacities compared with lightweight carbon hosts.Figure 2c,d shows the cycle stability of the S/HEO and S/CNF composites at various rates.The S/HEO composite displays much better cycle stability than that of S/CNF over 100 cycles at 0.1 C rate.Even at 1 C rate,the gravimetric capacity can reach up to 879.6 mAh g?1for S/HEO(Figure 2e), corresponding to the volumetric capacity of 1688.8 mAh cm?3, exceeding that of S/CNF (544.2 mAh g?1and 457.1 mAh cm?3). The high discharge capacity can be remained at 558.4 mAh g?1after 500 cycles with a low capacity fading rate of ~0.073% per cycle, as well as a high average Coulombic efficiency of ~99.4%. It demonstrates that the shuttle effect of LiPS is greatly restricted due to the strong chemical binding of sulfur species on HEO catalytic host. By contrast, S/CNF composite suffers lower specific capacity and rapid capacity decay because of the weak entrapment of LiPS on CNF host.
High sulfur loading and low E/S ratio are indispensably required for achieving high energy density of Li-S batteries. However,the redox kinetics of sulfur cathode is greatly impeded under such harsh conditions.[48]In this aspect, HEO catalytic host with multiple cation composition can accelerate the redox kinetics of sulfur cathode, thereby benefiting for satisfying performance under high sulfur loading and low E/S condition. In Figure 2f, with high sulfur loading of 2.8, and 4.6 mg cm?2,the S/HEO electrode can offer maximum discharge capacities of 1107.1 and 956.5 mAh g?1at 0.1 C rate with an E/S ratio of 15 μL mg?1, corresponding the areal capacities of 3.1 and 4.4 mAh cm?2, respectively. The high areal capacities of 2.6 and 3.8 mAh cm?2can be obtained after 100 cycles. Figure 2g shows the cycle stability of S/HEO composite with different E/S ratios.When the E/S ratio is set to be 10 and 5 μL mg?1, S/HEO composite displays high specific capacities of 896.8 and 809.3 mAh g?1at 0.1 C rate,respectively. In the meantime, the good cycle performance can be also realized upon 100 cycles. It is commonly stated that larger potential polarization occurs under low E/S ratio,resulting in a severe shrinkage of discharge potential plateaus.[49]As for the S/HEO composite, the slight decrease in potential plateaus appears (Figure S6, Supporting Information)owing to the high-efficiency catalysis of HEO on the conversion of soluble LiPS. Therefore,the as-prepared HEO with sufficient catalytic sites holds the great potential as alternative to carbon hosts in constructing high-performance sulfur cathodes.
To understand the adsorption catalysis performance, the adsorption of soluble LiPS on HEO host was firstly investigated. Based on the results of the visual adsorption tests and UV-vis spectra (Figure 3a),HEO exhibits much stronger adsorption of Li2S6solution than CNF in spite of the lower surface area.It indicates that the chemical interaction is the dominating factor for the efficient entrapment of LiPS,instead of surface area and physical adsorption.[50]The interaction mechanism is further verified by evidence of X-ray photoelectron spectra (XPS) of HEO sample before and after adsorbing Li2S6. Figure 3b shows the high-resolution Co 2p3/2spectrum with the Co3+/Co2+couple at 780.3 and 783.1 eV, respectively. After adsorbing Li2S6, the decreased fraction of Co3+from 64.6%to 44.7%can be observed,and the peaks shift toward lower binding energies due to the electron transfer from Li2S6.Similarly,the Ni 2p3/2spectrum(Figure 3c)shows the decreased fraction of Ni3+and the peaks of Ni3+/Ni2+couple shift to lower binding energies. These results demonstrate the strong chemical interaction between Co/Ni cations and LiPS,which benefits for effectively alleviating the shuttle effect of soluble LiPS. The important shifts can be also observed in the high resolution of Mg 1s, Mn 2p, Zn 2p, and Fe 2p spectra (Figure S7, Supporting Information). Therefore, HEO nanofibers could provide preferable chemical adsorption of LiPS owing to the synergistic effect of multi-metal cations.[45]Moreover,in the S 2p core level(Figure 3d),the peak at 162.1 eV is attributed to the bridging sulfur (S0B), and the peak at 160.5 eV is indexed as the terminal sulfur()in Li2S6and sulfdies.Notably,the dominating S0Bspecies in pristine Li2S6became weak, while the S-1Tspecies became strong in terms of peak area, which should be assigned to the strong sulfur–metal(S-M)chemical interaction between LiPS and HEO host.[51]In addition,the signals at higher binding energies are ascribed to thiosulfate and polythionate complex, and these sulfur species at high oxidation state indicate the oxidation of Li2S6upon contacting with HEO nanofibers,which is in accord with the reduction of high valence metal cations.The chemical interaction on HEO host is favorable for adsorbing LiPS and further catalyzing the conversion of LiPS,thus leading to the significant improvement of cycle stability of sulfur cathodes.
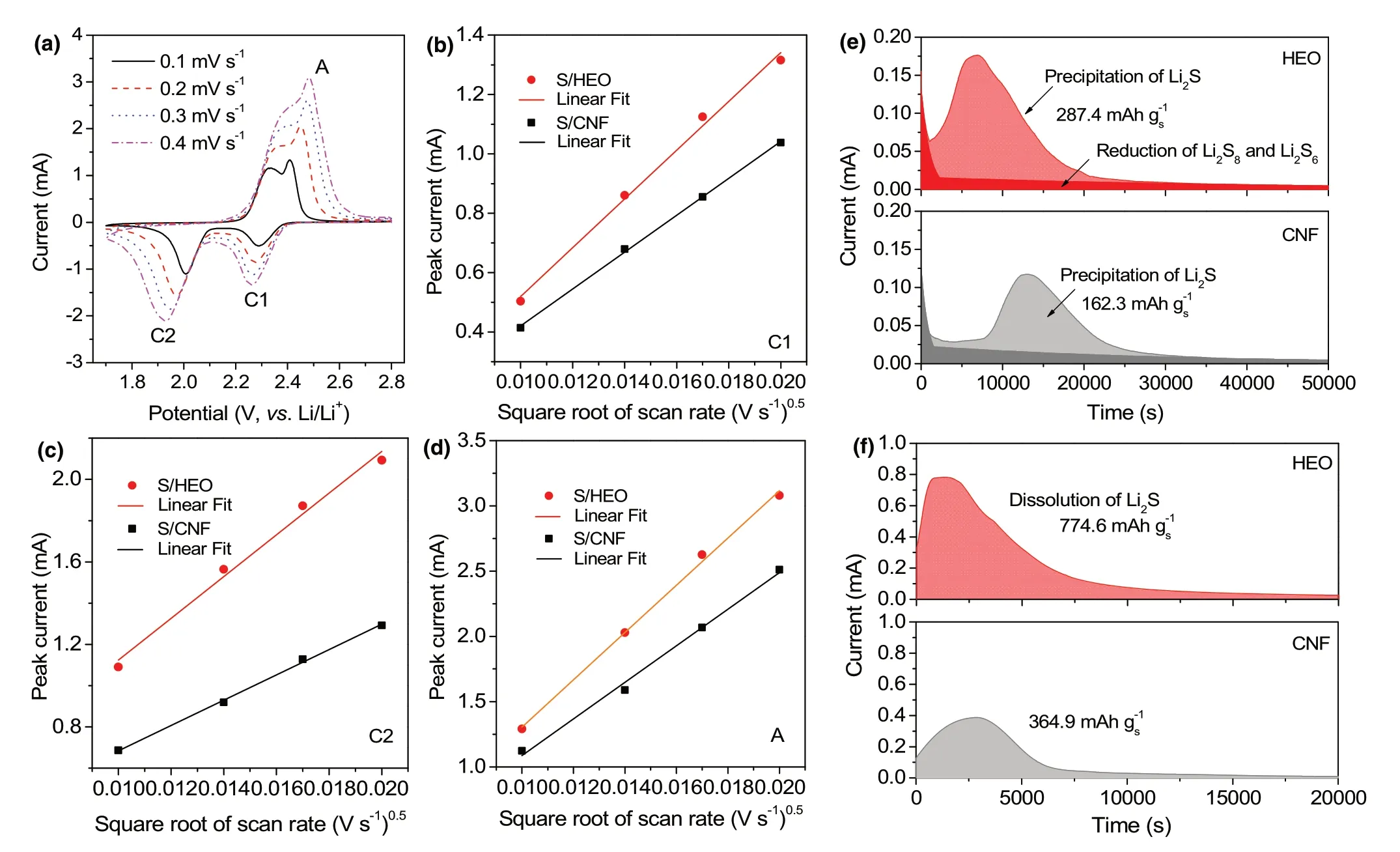
Figure 4. Diffusion catalysis of LiPS on HEO. a) CV profiles at different scan rates of S/HEO electrode. b–d) Linear correlation between CV peak current values and the square root of scan rates. e) Potentiostatic discharge profile at 2.05 V and f) potentiostatic charge profile at 2.4 V of different electrodes with Li2S8/tetraglyme catholyte for evaluating the nucleation and dissolution kinetics of Li2S.
Furthermore, the redox kinetics of sulfur cathode are explored based on CV evaluation of both S/HEO (Figure 4a) and S/CNF (Figure S8, Supporting Information) under various scan rates. The peak currents present a linear correlation with the square root of scan rate as shown in Figure 4b–d, and the fitted slope reflects the kinetics of Li-ion diffusion in the electrode according to the Randles–Sevcik equation.[52]Clearly, the S/HEO electrode shows larger slopes of both cathodic and anodic peaks, indicating the faster Li-ion diffusivity in both the reduction and oxidation processes. As mentioned above, the sulfur chemistry involves the two-step transformation from solid S8molecules into soluble LiPS and further solid Li2S2/Li2S. CV profiles and electrochemical impedance spectra (EIS) in symmetric cells with Li2S8electrolyte are conducted to explore the liquid–liquid conversion kinetics. The HEO electrode shows much higher peak current and lower charge transfer resistance (Figure S9, Supporting Information),suggesting fast electrochemical kinetics of the LiPS conversion on HEO electrode.

Figure 5. Impedance analysis. a) The initial discharge curve of the S/HEO electrode at 0.1 C rate. b, c)Nyquist plots of the S/HEO and S/CNF electrodes at different DOD. d–f) Plots of resistance values against DOD in the first discharge process.
Note that the vast majority of capacity contribution comes from the Li2S deposition from soluble LiPS species in the electrochemical process of sulfur.It is of great significance to evaluate the precipitation kinetics of Li2S reduction in the electrode, as well as the opposite process, that is,the oxidation of Li2S.Firstly,a potentiostatic discharge measurement at 2.05 V is conducted to reveal the nucleation of Li2S(Figure 3e).The HEO electrode exhibits higher peak current and reaches the current peak faster than CNF electrode.Meanwhile,the calculated capacity contribution of Li2S precipitation(light color region)is 287.4 mAh g?1in HEO electrode, near 1.77 times of CNF electrode (162.3 mAh g?1),demonstrating the faster kinetics of nucleation and deposition of Li2S on HEO nanofibers. Similarly, a potentiostatic charge measurement is conducted to investigate the dissolution kinetics of Li2S (Figure 3f).Usually, the deposition of non-conducting solid Li2S on the electrode surface leads to a significant increase in battery impedance and impedes both Li-ion diffusion and the subsequent oxidation of Li2S.[53,54]Therefore, the electrocatalysis on the oxidation of Li2S is urgently required for catalytic host materials to insure highly reversible processes of sulfur cathode. As calculated, HEO electrode exhibits much higher dissolution capacity(774.6 mAh g?1) than CNF electrode(364.9 mAh g?1). Overall, these results demonstrate the high electrocatalytic activity of HEO host in accelerating the kinetics of both the reduction and oxidation processes of sulfur cathode.
To deeply understand the electrochemical processes at the electrode/electrolyte interface,Nyquist plots at different depths of discharge(DOD)in the first cycle are presented in Figure 5a–c.Given that the plots are composed of two semicircles and a sloping line,the equivalent circuit (Figure S10, Supporting Information) involving charge transfer process, adsorption, and diffusion processes of soluble LiPS is established to investigate the interfacial properties of sulfur cathode.[47]The dissolution of LiPS into the electrolyte during discharging increases the viscosity of electrolyte,leading to an increase in electrolyte resistance (Re) value before point C (Figure 5d).[55]The subsequent decline in the discharge curve is related to the reduction of soluble LiPS to solid Li2S2/Li2S. The lower Revalue in the S/HEO electrode demonstrates the strong chemical entrapment of LiPS on HEO host. Similarly,the consumption of insulating sulfur in the initial process enables a highly active interface in the cathode,leading to smaller charge transfer resistance (Rct) values, while the deposition of insulating Li2S in the later process could increase the resistance values (Figure 5e). More importantly, the relatively low Rctsignifies the catalyzed conversion reaction on HEO surface. In addition, the fast kinetics of LiPS conversion in S/HEO electrode is also corresponding to smaller adsorption impedance(Ws, Figure 5f) and diffusion impedance (Wo, Table S2, Supporting Information). It is noted that at 90% DOD (point E) or in the end of discharge (point F), both the middle-frequency (MF) semicircle and low-frequency sloping line appear as an arc,which could be explained by the deposition of insulating Li2S on the electrode surface.[56]Furthermore,the measurements of the charge process with different depths are also presented (Figure S11 and Table S2, Supporting Information).Overall,the results from EIS spectra fully demonstrate the high catalytic activity of HEO host in promoting both the reduction and oxidation processes of sulfur cathodes.
It is worth noting that the concept of high-entropy configuration is the key to realizing high catalytic activity of polar hosts for sulfur redox reactions. Therefore, other counterparts including unary spinel oxide(NFO, NiFe2O4) and ternary spinel oxide (NCMFO, (Ni1/3Co1/3Mn1/3)Fe2O4)are also investigated to comprehensively understand the superiority of HEO host.Figure 6a shows the XRD patterns of the two oxides, and they share the same cubic spinel crystal structure. In the meantime,the nanofibrous morphology is well maintained for the two samples(Figure 6b,c).The Li2S precipitation on the surface of the two electrodes is further investigated. Compared with NiFe2O4, the higher capacity contribution of Li2S precipitation (Figure 6d) indicates that(Ni1/3Co1/3Mn1/3)Fe2O4is more effective in facilitating the Li2S nucleation, as well as the dissolution of Li2S (Figure 6e). This evidently demonstrates that multiple metallic compounds are more favorable for polysulfide regulator owing to the synergistic effect among different metallic cations compared with monometallic compounds. Moreover,the catalytic activity of oxide host can be further improved by mixing extra metal elements into a homogeneous and high-entropy spinel oxide. As a result, the S/HEO composite exhibits the best performance among the S/oxides composites including rate performance(Figure 7a) and cycle stability (Figure 7b). On the other hand,due to the strong chemical adsorption of LiPS (Figure S12, Supporting Information),better cycle stability and rate capability are achieved for all the S/oxide composites when compared with S/CNF composite. Notably,all the composites show high sulfur content of ~80 wt% (Figure S13 and Table S3, Supporting Information). Another shared feature is that the tap density of S/oxide composites exceeds much that of S/CNF(Table S3, Supporting Information), leading to much high volumetric capacities. As shown in Figure 7c, high volumetric capacities of 2052.9, 2233.5, and 2627.9 mAh cm?3are achieved for S/NFO,S/NCMFO,and S/HEO composites,respectively.Moreover,the S/HEO composite presents remarkable volumetric capacity as compared with previous sulfur-based composites with porous carbon,[46,50,55]dense graphene,[57,58]and heavy oxide[47,50,55,59,60]host materials, as summarized in Figure 7d. Therefore, the advantages of HEO materials including high density,desirable polysulfide adsorption, and enhanced catalytic activity are well established, which are critical for fabricating sulfur cathodes with both high gravimetric and volumetric capacity.

Figure 6. a) XRD pattern and SEM images of b) NCMFO and c) NFO nanofibers. d) Potentiostatic discharge profile at 2.05 V and e) potentiostatic charge profile at 2.4 V of NCMFO and NFO electrodes.
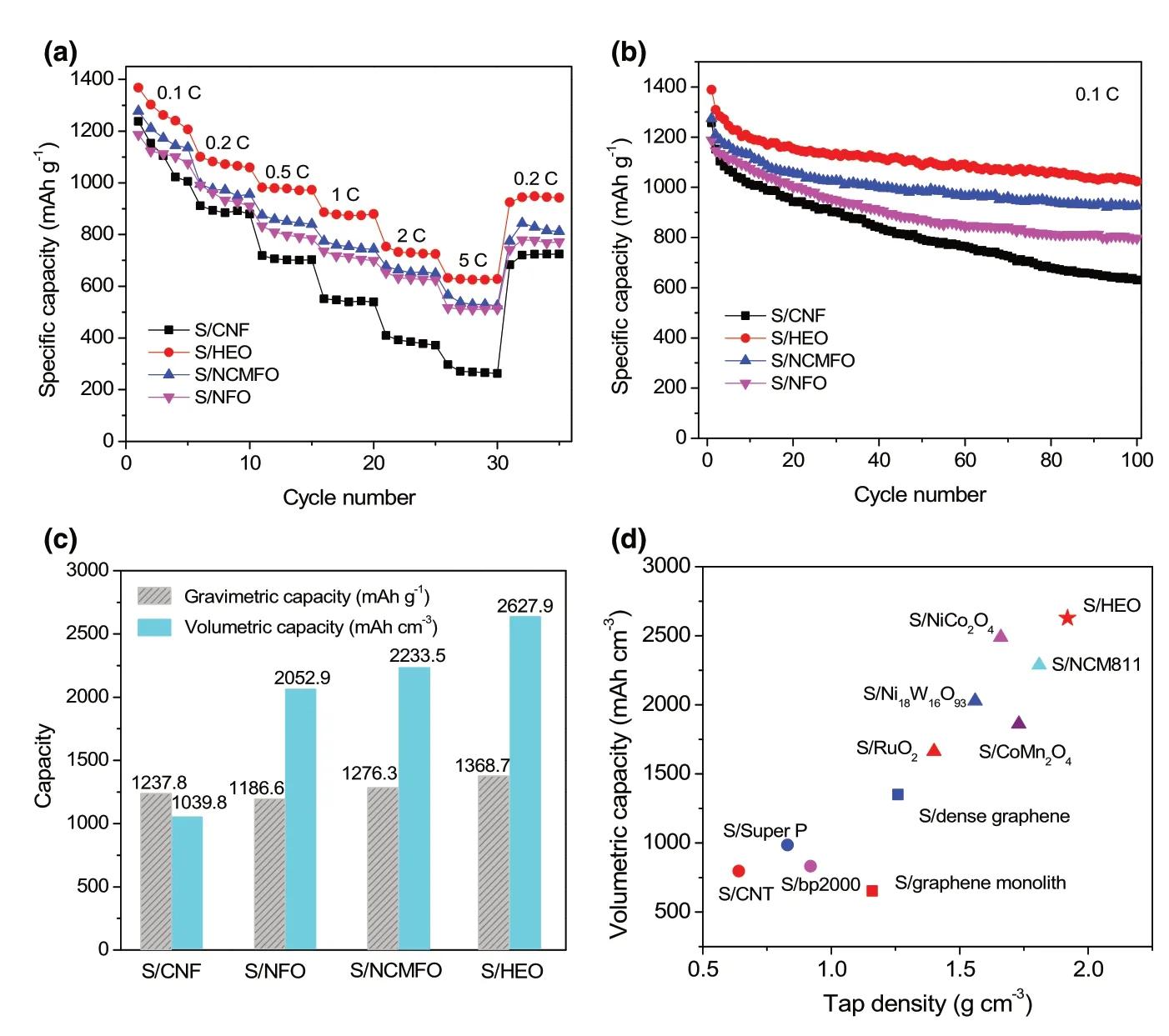
Figure 7. Electrochemical performance of various S/spinel oxide and S/CNF composites. a) Rate capability,b) cycle stability, and c) volumetric capacities. d) Comparison of volumetric capacity between S/HEO and various sulfur/host composites in the literatures.
3. Conclusion
In summary, we propose HEO ((Mg0.2Mn0.2Co0.2Ni0.2Zn0.2)Fe2O4)nanofibers as carbon-free sulfur immobilizer for highly efficient sulfur redox reactions. The inherent multiple meal cations offer sufficient binding sites for chemical entrapment of LiPS and exhibit an expected synergistic effect on boosting the diffusion and conversion of LiPS,and the deposition and dissolution of Li2S. The S/HEO composite presents high specific capacity of 1368.7 mAh g?1at 0.1 C rate, excellent rate capability up to 632.1 mAh g?1at 5 C rate, and good cycle stability over 500 cycles at 1 C rate.Satisfying cycle stabilities can be still realized with high sulfur loading of 4.6 mg cm?2or at low E/S ratio of 5 μL mg?1. Notably, the high-entropy feature of HEO benefits for its high catalytic activity on sulfur redox reactions as compared with the unary or ternary spinel oxides. In particular, the HEO host enables the high tap density of S/HEO composite that leads to a more than twofold volumetric capacity in comparison with S/CNF composite. Therefore,this work affords a strategy of developing highly efficient hosts in consideration of cathode density, LiPS entrapment, and electrocatalytic activity for practical Li-S batteries, and also inspires the future exploration of various high-entropy materials for energy storage and conversion.
4. Experimental Section
Synthesis of high-entropy (Mg0.2Mn0.2Co0.2-Ni0.2Zn0.2)Fe2O4nanofibers: Typically, a mixture of Mg(CH3COO)2?4H2O (0.02 mmol), Mn(CH3COO)2?4H2O (0.02 mmol), Ni(CH3COO)2?4H2O (0.02 mmol), Co(CH3COO)2?4H2O (0.02 mmol), Zn(CH3COO)2?2H2O (0.02 mmol), Fe(NO3)3?9H2O(0.2 mmol), and polyacrylonitrile (PAN, 1 g) was dissolved in N,N-dimethylformamide (DMF,10 mL)to obtain a homogenous solution with the solid content of 0.11 g mL?1under continuous stirring. The pristine nanofibers were prepared via the electrospinning method with a flow rate of 2 mL h?1with a voltage condition of 20 kV. The high-entropy(Mg0.2Mn0.2Co0.2Ni0.2Zn0.2)Fe2O4nanofibers could be obtained after a calcination at 500 °C for 3 h in air. Besides, unary spinel oxide(NiFe2O4) and ternary spinel oxide ((Ni1/3Co1/3Mn1/3)Fe2O4) were also obtained via the same procedure. As control, carbon nanofibers (CNFs)were prepared by electrospinning a DMF solution(10 mL) containing PAN (1 g) and polystyrene(PS, 0.5 g) with the solid content of 0.15 g mL-1,followed by a calcination at 850 °C for 3 h in Ar.
Preparation of sulfur cathodes: Sulfur composite was firstly prepared by combining sulfur with the host (HEO or CNF) via a heat treatment at 155 °C for 12 h in an Ar-filled sealed autoclave. Then, the as-obtained sulfur/host composites(70 wt%)were mixed with aligned carbon nanotubes(20 wt%,XFM61, XFNANO) and polyvinylidene fluoride (PVDF, 10 wt%) in N-methyl pyrrolidone.The uniform slurry was casted onto the Al foil using doctor blade.Finally, sulfur electrodes with a diameter of 12 mm were obtained after drying overnight and punching.
Materials characterization: Scanning electron microscope (JEOL, JSM-7800F)and transmission electron microscope(JEOL,JSM-2800)were used to characterize the microstructure and morphology. Phase purity of the samples was examined by X-ray diffraction (Rigaku MiniFlexII). X-ray photoelectron spectroscopy (XPS)was carried out on a Thermo Scientific ESCALAB 250Xi instrument. N2adsorption/desorption isotherms were recorded on an instrument (JW-BK112). Sulfur content was confirmed by thermogravimetry (METTLER TOLEDO, TG/DSC1).Tap density(g cm?3)of S/HEO and S/CNF composites was measured in a graduated glass cylinder with continuous shake until the measured volume change was below 2%.
Battery assembly and electrochemical evaluation: CR2032 coin cells were assembled using the above sulfur cathode, lithium metal anode, and Celgard 2300 membrane as the separator.The electrolyte consisted of 1 M lithium bis(trifluoromethanesulfonyl)imide(LiTFSI)and 0.2 M LiNO3in a mixture solvent of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) (1:1, v/v). For regular Li-S batteries, sulfur loading of 1.4 mg cm?2and the electrolyte/sulfur (E/S) ratio of 20 μL mg?1were applied.For high-loading batteries of 2.8 and 4.6 mg cm?2,E/S ratios of 15,10,and 5 μL mg?1were applied.Galvanostatic discharge/charge tests were performed in the potential range of 1.7–2.8 V (vs. Li/Li+) on LANDCT2001A instruments. Cyclic voltammogram (CV) profiles were collected using an electrochemical station(CHI 600e)at different scan rates,and electrochemical impedance spectra (EIS) were tested on Zahner IM6ex in the 10 mHz–100 kHz frequency region with an amplitude of 5 mV.
Symmetric cell assembly: HEO or CNF electrode was prepared by dropping HEO/ethanol or CNF/ethanol solution onto carbon paper (P75, 12 mm in diameter) following with drying at 60 °C. The mass loading of HEO or CNF was about 1 mg cm?2. CR2032 cells were assembled with two identical HEO or CNF electrodes and Celgard 2300 separator. 20 μL of 0.2 M Li2S6and 1 M LiTFSI in DOL/DME solution were added to each electrode. The Nyquist plots were measured in the 100 mHz–100 kHz frequency region, and CV curves were recorded in a voltage window of ?0.8 to 0.8 V at a scan rate of 5 mV s?1.
Nucleation and dissolution of Li2S on HEO and CNF: CR2032 cells were assembled with the above HEO or CNF electrode,lithium metal anode,and Celgard 2300 separator.20 μL of 0.25 M Li2S8/tetraglyme solution was added in the cathode side,and 20 μL of 1 M LiTFSI/tetraglyme solution was added in the anode side.To study the liquid–solid conversion kinetics,the assembled cells were galvanostatically discharged at 0.112 mA to 2.06 V and subsequently potentiostatically discharged at 2.05 V until the current was below 0.01 mA to ensure the complete nucleation of Li2S.For evaluating the dissolution of Li2S,the cells were firstly galvanostatically discharged at 0.112 mA to 1.8 V,then at 0.01 mA to 1.7 V,and finally potentiostatically charged at 2.4 V until the current was below 0.01 mA to ensure the complete dissolution of Li2S.
Acknowledgments
Financial supports from the National Natural Science Foundation of China(21935006 and 22008102)are gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Informationis available from the Wiley Online Library or from the author.
Keywords
catalytic host, high-entropy oxide, lithium–sulfur battery, polysulfide conversion, spinel oxide nanofibers
Received: April 10, 2021
Revised: May 4, 2021
Published online: May 11, 2021
[1] X. P. Gao, H. X. Yang, Energy Environ. Sci. 2010, 3, 174.
[2] P. G. Bruce, S. A. Freunberger, L. J. Hardwick, J. M. Tarascon, Nat. Mater.2012, 11, 19.
[3] L. Peng, Z. Y. Wei, C. Z. Wan, J. Li, Z. Chen, D. Zhu, D. Baumann, H. T.Liu, C. S. Allen, X. Xu, A. I. Kirkland, I. Shakir, Z. Almutairi, S. Tolbert, B.Dunn, Y. Huang, P. Sautet, X. F. Duan, Nat. Catal. 2020, 3, 762.
[4] Y. T. Liu, S. Liu, G. R. Li, X. P. Gao, Adv. Mater. 2021, 33, 2003955.
[5] Q. Pang, X. Liang, C. Y. Kwok, L. F. Nazar, Nat. Energy 2016, 1, 11.
[6] A. Bhargav, J. R. He, A. Gupta, A. Manthiram, Joule 2020, 4, 1.
[7] J. Lei, T. Liu, J. J. Chen, M. S. Zheng, Q. Zhang, B. W. Mao, Q. F. Dong,Chem. 2020, 6, 2533.
[8] H. J. Peng, J. Q. Huang, X. B. Cheng, Q. Zhang, Adv. Energy Mater. 2017,7, 1700260.
[9] B. H. Zhang, J. F. Wu, J. K. Gu, S. Li, T. Y. Yan, X. P. Gao, ACS Energy Lett. 2021, 6, 537.
[10] P. Chen, G. R. Li, T. T. Li, X. P. Gao, Adv. Sci. 2019, 6, 1900620.
[11] S. Q. Li, Z. Y. Fan, Energy Storage Mater. 2021, 34, 107.
[12] C. Deng, Z. W. Wang, L. L. Feng, S. P. Wang, J. X. Yu, J. Mater. Chem. A 2020, 8, 19704.
[13] C. Zhao, G. L. Xu, Z. Yu, L. C. Zhang, I. H. Hwang, Y. X. Mo, Y. Ren, L.Cheng, C. J. Sun, Y. Ren, X. B. Zuo, J. T. Li, S. G. Sun, K. Amine, T. S.Zhao, Nat. Nanotechnol. 2020, 16, 166.
[14] P. D. Xu, H. D. Liu, Q. W. Zeng, X. Li, Q. Li, K. Pei, Y. H. Zhang, X. F.Yu, J. Zhang, X. Qian, R. C. Che, Small 2021, 17, 2005227.
[15] D. H. Liu, C. Zhang, G. M. Zhou, W. Lv, G. W. Ling, L. J. Zhi, Q. H. Yang,Adv. Sci. 2018, 5, 1700270.
[16] Z. X. Sun, S. Vijay, H. H. Heenen, A. Y. S. Eng, W. G. Tu, Y. X. Zhao, S.W. Koh, P. Q. Gao, Z. W. Seh, K. Chan, H. Li, Adv. Energy Mater. 2020,10, 1904010.
[17] Y. Z. Song, W. L. Cai, L. Kong, J. S. Cai, Q. Zhang, J. Y. Sun, Adv. Energy Mater. 2019, 9, 1901075.
[18] R. Razaq, D. Sun, Y. Xin, Q. Li, T. Huang, L. Zheng, Z. Zhang, Y. Huang,Nanotechnology 2018, 29, 295401.
[19] H. Yuan, H. J. Peng, B. Q. Li, J. Xie, L. Kong, M. Zhao, X. Chen, J. Q.Huang, Q. Zhang, Adv. Energy Mater. 2019, 9, 1802768.
[20] Z. Zhang, A. H. Shao, D. G. Xiong, J. Yu, N. Koratkar, Z. Y. Yang, A. C.S. Appl, Mater. Interfaces 2020, 12, 19572.
[21] M. Zhao, H. J. Peng, B. Q. Li, X. Chen, J. Xie, X. Y. Liu, Q. Zhang, J. Q.Huang, Angew. Chem. Int. Ed. 2020, 132, 9096.
[22] Y. X. Yang, Y. R. Zhong, Q. W. Shi, Z. H. Wang, K. N. Sun, H. L. Wang,Angew. Chem. Int. Ed. 2018, 57, 15549.
[23] S. D. Seo, S. Yu, S. Park, D. W. Kim, Small 2020, 16, 2004806.
[24] X. Q. Zhang, W. Yuan, Y. Yang, Y. Chen, Z. H. Tang, C. Wang, Y. H.Yuan, Y. T. Ye, Y. P. Wu, Y. Tang, Small 2020, 16, 2005998.
[25] J. R. He, A. Bhargav, A. Manthiram, Adv. Mater. 2020, 32, 2004741.
[26] L. Wang, G. R. Li, S. Liu, X. P. Gao, Adv. Funct. Mater. 2021, 2010693.
[27] D. He, J. T. Meng, X. Y. Chen, Y. Q. Liao, Z. X. Cheng, L. X. Yuan, Z. Li,Y. H. Huang, Adv. Funct. Mater. 2021, 31, 2001201.
[28] L. L. Xu, H. Y. Zhao, M. Z. Sun, B. L. Huang, J. W. Wang, J. Xia, N. Li, D.D. Yin, M. Luo, F. Luo, Y. P. Du, C. H. Yan, Angew. Chem. Int. Ed. 2019,58, 2.
[29] Y. Yao, H. Y. Wang, H. Yang, S. F. Zeng, R. Xu, F. F. Liu, P. C. Shi, Y. Z.Feng, K. Wang, W. J. Yang, X. J. Wu, W. Luo, Y. Yu, Adv. Mater. 2019,31, 1905658.
[30] Z. Z. Du, X. J. Chen, W. Hu, C. H. Chuang, S. Xie, A. J. Hu, W. S. Yan, X.H. Kong, X. J. Wu, H. X. Ji, L. J. Wan, J. Am. Chem. Soc. 2019, 141, 3977.
[31] Y. W. Chen, H. Y. Fu, Y. Y. Huang, L. Q. Huang, X. Y. Zheng, Y. M. Dai,Y. H. Huang, W. Luo, ACS Mater. Lett. 2021, 3, 160.
[32] A. Sarkar, L. Velasco, D. Wang, Q. S. Wang, G. Talasila, L. Biasi, C. K¨ubel,T. Brezesinski, S. S. Bhattacharya, H. Hahn, B. Breitung, Nat. Commun.2018, 9, 3400.
[33] M. S. Song, S. C. Han, H. S. Kim, J. H. Kim, K. T. Kim, Y. M. Kang, H. J.Ahn, S. X. Dou, J. Y. Lee, J. Electrochem. Soc. 2004, 151, A791.
[34] Y. Zhang, X. B. Wu, H. Feng, L. Wang, A. Zhang, T. C. Xia, H. C. Dong,Int. J. Hydrogen Energy 2009, 34, 1556.
[35] Q. Fan, W. Liu, Z. Weng, Y. M. Sun, H. L. Wang, J. Am. Chem. Soc.2015, 137, 12946.
[36] A. Iqbal, Z. A. Ghazi, A. M. Khattak, A. Ahmad, J. Solid State Chem.2017, 256, 189.
[37] S. Evers, T. Yim, L. F. Nazar, J. Phys. Chem. C 2012, 116, 19653.
[38] Y. C. Jiang, H. M. U. Arshad, H. J. Li, S. Liu, G. R. Li, X. P. Gao, Small 2021, 17, 2005332.
[39] L. Kong, X. Chen, B. Q. Li, H. J. Peng, J. Q. Huang, J. Xie, Q. Zhang, Adv.Mater. 2018, 30, 1705219.
[40] B. Cantor, Entropy 2014, 16, 4749.
[41] C. M. Rost, E. Sachet, T. Borman, A. Moballegh, E. Dickey, D. Hou, J. L.Jones, S. Curtarolo, J. P. Maria, Nat. Commun. 2015, 6, 8485.
[42] M. J. Cui, C. P. Yang, B. Y. Li, Q. Dong, M. L. Wu, S. Hwang, H. Xie, X.Z. Wang, G. F. Wang, L. B. Hu, Adv. Energy Mater. 2020, 10, 2002887.
[43] Q. S. Wang, A. Sarkar, D. Wang, L. Velasco, R. Azmi, S. S. Bhattacharya,T. Bergfeldt, A. D¨uvel, P. Heitjans, T. Brezesinski, H. Hahn, B. Breitung,Energy Environ. Sci. 2019, 12, 2433.
[44] T.Wang,H.Chen,Z.Yang,J.Liang,S.Dai,J.Am.Chem.Soc.2020,142,4550.
[45] Y. N. Zheng, Y. K. Yi, M. H. Fan, H. Y. Liu, X. Li, R. Zhang, M. T. Li, Z.A. Qiao, Energy Storage Mater. 2019, 23, 678.
[46] Z. Zhang, D. H. Wu, Z. Zhou, G. R. Li, S. Liu, X. P. Gao, Sci. China Mater.2019, 62, 74.
[47] Y. T. Liu, D. D. Han, L. Wang, G. R. Li, S. Liu, X. P. Gao, Adv. Energy Mater. 2019, 9, 1803477.
[48] M. Zhao, B. Q. Li, H. J. Peng, H. Yuan, J. Y. Wei, J. Q. Huang, Angew.Chem. Int. Ed. 2020, 59, 12636.
[49] Z. Zhang, L. L. Kong, S. Liu, G. R. Li, X. P. Gao, Adv. Energy Mater. 2017,7, 1602543.
[50] L. Wang, Y. H. Song, B. H. Zhang, Y. T. Liu, Z. Y. Wang, G. R. Li, S. Liu,X. P. Gao, A. C. S. Appl, Mater. Interfaces 2020, 12, 5909.
[51] Z. Zhang, J. N. Wang, A. H. Shao, D. G. Xiong, J. W. Liu, C. Y. Lao, K. Xi,S. Y. Lu, Q. Jiang, J. Yu, H. L. Li, Z. Y. Yang, R. V. Kumar, Sci. China Mater. 2020, 63, 2443.
[52] X. Zhu, W. Zhao, Y. Song, Q. Li, F. Ding, J. Sun, L. Zhang, Z. Liu, Adv.Energy Mater. 2018, 8, 1800201.
[53] Z. Q. Ye, Y. Jiang, L. Li, F. Wu, R. J. Chen, Adv. Mater. 2020, 32,2002168.
[54] C. Q. Zhang, J. J. Biendicho, T. Zhang, R. F. Du, J. S. Li, X. H. Yang, J.Arbiol, Y. T. Zhou, J. R. Morante, A. Cabot, Adv. Funct. Mater. 2019, 29,1903842.
[55] L. Wang, Z. Y. Wang, J. F. Wu, G. R. Li, S. Liu, X. P. Gao, Nano Energy 2020, 77, 105173.
[56] Z. F. Deng, Z. A. Zhang, Y. Q. Lai, J. Liu, J. Li, Y. X. Liu, J. Electrochem.Soc. 2013, 160, A553.
[57] H. F. Li, X. W. Yang, X. M. Wang, M. N. Liu, F. M. Ye, J. Wang, Y. C.Qiu, W. F. Li, Y. G. Zhang, Nano Energy 2015, 12, 468.
[58] H. Li, Y. Tao, C. Zhang, D. H. Liu, J. Y. Luo, W. C. Fan, Y. Xu, Y. Z. Li, C.H. You, Z. Z. Pan, M. C. Ye, Z. Y. Chen, Z. Dong, D. W. Wang, F. Y.Kang, J. Lu, Q. H. Yang, Adv. Energy Mater. 2018, 8, 1703438.
[59] Z. Y. Wang, D. D. Han, S. Liu, G. R. Li, T. Y. Yan, X. P. Gao, Electrochim.Acta 2020, 337, 135772.
[60] S. J. Chen, Y. Ming, B. C. Tan, S. Y. Chen, Electrochim. Acta 2020, 329,135128.
 Energy & Environmental Materials2022年2期
Energy & Environmental Materials2022年2期
- Energy & Environmental Materials的其它文章
- Progress of Pb-Sn Mixed Perovskites for Photovoltaics:A Review
- Development Strategies in Transition Metal Borides for Electrochemical Water Splitting
- Polymer-/Ceramic-based Dielectric Composites for Energy Storage and Conversion
- Controllable Construction of Bifunctional CoxP@N,P-Doped Carbon Electrocatalysts for Rechargeable Zinc–Air Batteries
- Unveiling the Underlying Mechanism of Transition Metal Atoms Anchored Square Tetracyanoquinodimethane Monolayers as Electrocatalysts for N2 Fixation
- Rational Design of High-Performance Bilayer Solar Evaporator by Using Waste Polyester-Derived Porous Carbon-Coated Wood
