Right versus left fully robotic live donor nephrectomy and open kidney transplantation: Does the laterality of the donor kidney really matter?
Brinn Ruch ,Deki Tsering ,Chndr Bhti ,Dhiren Kumr ,Muhmmd Seed , Seung Duk Lee , Amir Khn ,Disuke Imi ,Dvid Bruno ,Mrlon Levy ,Adrin Cotterell ,Amit Shrm ,*
a Hume-Lee Transplant Center, Virginia Commonwealth University, Richmond, VA, USA
b Division of Transplant Surgery, University of Maryland, Baltimore, MD, USA
KEYWORDS Robotics;Laparoscopy;Donor nephrectomy;Complication;Renal transplant;Outcome
Abstract Objective: Robotic-assisted live donor nephrectomy (LDN) is being gradually adopted across transplant centers.The left donor kidney is preferred over right due to anatomical factors and ease of procurement.We aimed to study donor and recipient outcomes after robotic procurement and subsequent open implantation of right and left kidneys.Methods: All fully robotic LDNs and their corresponding open kidney transplants performed at our center between February 2016 and December 2021 were retrospectively analyzed.Results: Out of 196 robotic LDN(49[right]vs.147[left]),10(5.1%)donors had intra-operative events(6.1%[right]vs.4.8%[left],p=0.71).None of the LDN required conversion to open surgery.The operative times were comparable for the two groups.Nausea (13.3%) was the most common post-operative complication.There was no mortality in either LDN group.Herein,we report our outcomes on 156 recipients (39 right and 117 left allografts) excluding robotic implants, exports, and pediatric recipients.There were no significant differences between right and left kidney recipients with respect to 1-year post-transplant patient survival (100.0% vs.98.1%, p=0.45) or graft survival (93.9% vs.97.1%, p=0.11), or delayed graft function (7.7%vs.5.1%, p=0.55).Conclusion: Non-hand-assisted robotic live donor nephrectomies can be safely performed with excellent outcomes.Right LDN was not associated with higher incidence of complications compared to left LDN.Open implantation of robotically procured right renal allografts was not associated with higher risk of recipient complications.
1.Introduction
Laparoscopic live donor nephrectomy (LDN) was first introduced in 1995[1]and quickly became the standard of care for living donation[2,3].In general practice,the use of robotics for native nephrectomies has begun to replace the laparoscopic approach secondary to improved ergonomics, visualization, and instrument articulation [4].Despite the utility shown in non-transplant nephrectomies, robotic LDN continues to be infrequently performed.A large study involving 97 centers in the United States reported that between 2008 and 2012, the majority (94%) of LDNs were performed laparoscopically,with only 2%performed robotically[5].
For LDN, the left kidney is preferred secondary to the right renal vein, being short and thin, making the subsequent recipient implantation technically difficult.There has also been some suggestions of the association between right live donor kidneys and venous thrombosis [6,7], but this has not been replicated uniformly [8,9].A recent meta-analysis comparing left to right live donor kidneys observed a borderline increase in the incidence of venous thrombosis for right donor kidneys [10].
Post-transplant outcomes of robotically procured live donor kidneys have not been widely reported [11,12].In particular, right-sided robotic donor nephrectomies and their recipient outcomes are lacking in the literature.A recent systematic review of eighteen studies found only 56 of the 910 donors (6.2%) underwent a robotic right donor nephrectomy [7].It is our aim to share our single center outcomes of live kidney donors who underwent robotic-assisted right and left nephrectomy and their respective recipient outcomes.
2.Materials and methods
We retrospectively compared our center’s outcomes for robotically procured right and left live donor kidneys between February 2016 and December 2021.The outcomes of adult recipients of these right and left kidneys that were transplanted using the conventional“open”technique were also analyzed.We excluded pediatric recipients, robotically implanted transplant recipients, and recipients of the allografts that were exported to other centers from this analysis.Our Institutional Review Board at Virginia Commonwealth University approved the study(HM20018309).This study was retrospectively performed with data obtained for clinical purposes.Individual consent was waived after discussion with our Institutional Review Board.
2.1.Pre-operative evaluation
All live donors were cleared by a multi-disciplinary renal transplant committee consisting of surgery, nephrology,psychology, social work, and an independent donor advocate.A measured glomerular filtration rate by iothalamate clearance greater than the 5th age-adjusted percentile was required to be considered for donation.All donors underwent a computed tomography angiogram with three-dimensional reconstruction to evaluate the renal vasculature.The right kidney was used when: (a) there were more than two left renal arteries and fewer renal arteries on right side; (b) the right kidney had some pathology (e.g., stones or cysts); or (c) the right kidney was significantly smaller than the left.
2.2.Robotic LDN technique
The da Vinci surgical system (either Si or Xi patient cart,Intuitive Surgical Inc.,Sunnyvale,CA,USA)was used for the LDN.All LDNs were performed using the trans-peritoneal approach.For the left LDN, one camera port(three-dimensional vision system endoscope 12 mm, 30-degree; Intuitive Surgical, Sunnydale, CA, USA), two working ports for a fenestrated bipolar forceps (EndoWrist bipolar cautery instrument;Intuitive Surgical,Sunnydale,CA,USA) and a vessel sealer (EndoWrist vessel sealer instrument; Intuitive Surgical, Sunnydale, CA, USA), and an assistant port (12-mm Air Seal SurgiQuest; ConMed Corporation, Milford, CT, USA) placed through a Pfannenstiel incision were used.On the right side, an additional 5-mm assistant port was placed in epigastrium to retract the right liver lobe.For left nephrectomy, the descending colon and splenic flexure were mobilized using the vessel sealer.Gerota’s fascia was divided lateral to psoas muscle to free the retroperitoneal space.The gonadal vein and the ureter were identified at the medial edge of the psoas muscle.The gonadal vein was traced to its confluence with the renal vein on the left side.The periureteric sheath was preserved to avoid damage to the nutritive vessels of the ureter.The renal vessels were gently freed from the surrounding lymphatic tissue.Complete mobilization of the kidney was then performed.
For right donor nephrectomy, an additional 5-mm port was placed in the epigastrium to assist in retracting the right lobe of the liver.The ascending colon and hepatic flexure were mobilized, and the duodenum was reflected medially.The ureter was identified and followed proximally.The gonadal vein was preserved in most cases.The confluence of the right renal vein and inferior vena was identified.The renal vein was circumferentially dissected.The right renal artery was identified posterior to the vein and partially cleared.The kidney was completely mobilized and reflected medially.The right renal arterial dissection was then completed.
For both sides,the ureter and gonadal vein were clipped and divided at the pelvic brim.A co-surgeon switched the 12-mm air seal port in the Pfannenstiel incision to a 15-mm port through which an endocatch bag (Endocatch; US surgical, Norwalk, Los Angeles, CA, USA) was introduced into the abdomen.The iliac fossa robotic arm was undocked, and the robotic 8-mm port was upsized to a 10-mm port to allow for stapler introduction.After heparinization, the kidney was positioned into the endocatch bag; the renal artery and vein were divided using laparoscopic, double-row, vascular 35-mm GIA stapler loads(Ethicon US, LLC, Cincinnati, OH, USA).The kidney was retrieved and flushed on the back-table.The remaining robotic arms were undocked;the fascia at the Pfannenstiel incision was closed; pneumoperitoneum was re-insufflated to inspect the renal fossa and vascular stumps for hemostasis using a 5-mm laparoscope by the primary surgeon.All port sites and the incisions were then closed.
2.3.Renal transplantation
The retrieved renal allograft was then prepped and implanted to the external iliac vessels using the conventional, extra-peritoneal approach.The ureteroneocystostomy was performed using the Lich-Gregoir technique over an indwelling double-J stent, which was removed at 4 weeks.
2.4.Post-operative care
Early ambulation and nutrition, as tolerated, was encouraged.Patient-controlled analgesia was switched to oral analgesics on the first post-operative day.Donors were discharged home and followed up in clinic at regular intervals.
In the recipients, transplant renal allograft vascular anatomy was evaluated on post-operative Day 0 by color duplex Doppler sonography.All transplant recipients received induction using rabbit antithymocyte globulin(1.5 mg/kg, thymoglobulin; Genzyme Corp., Cambridge,MA, USA) from Day 0 to Day 3.Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil,and a steroid dose tapered to 5 mg daily.
2.5.Statistical methods
Graft loss (GL) was defined as patient requiring removal of the renal allograft, permanent return to dialysis, retransplantation, or recipient death.Early GL was defined as GL within 30 days of kidney transplantation.Delayed graft function was defined as need for dialysis in the first week after transplant.The outcomes of LDN (right vs.left)and the renal allograft recipients were compared using the Mann-Whitney U test,Chi-square test of independence,and the log-rank test.A p-value of <0.05 was considered significant.
3.Results
Between February 2016 and December 2021, 196 fully robotic (non-hand-assisted) LDNs were performed at our center.Data on all 196 robotic donors and 156 recipients are reported.To minimize recipient variability, pediatric recipients, robotic implantations, and exports to other facilities were excluded from the final recipient analysis.We performed 49 (25.0%) right LDNs and 147 (75.0%) left LDNs.There was no statistically significant difference between the mean (standard deviation [SD]) ages (43.8 [SD 13.3]years vs.41.5 [SD 11.8] years, p=0.23) and body mass indexes (BMIs) (27.0 [SD 4.2] kg/m2vs.27.3 [SD 4.5] kg/m2,p=0.69) of right and left live kidney donors (Table 1).The majority of donors in both groups were Caucasian (right:73.5%, left: 61.9%) and female (right: 67.3%, left: 60.5%).
Most of the right and left LDNs were performed using the da Vinci Xi robotic patient cart (81.6% vs.76.2%, p=0.43)(Table 2).Multiple renal arteries were present in 16.3% of right and 19.0% of left kidney donors (p=0.67).A significantly higher proportion of right kidney donors had multiple renal veins (20.4% vs.4.8%, p=0.001).There was no statistically significant difference between the mean donor operative times (186.8 [SD 32.5] min vs.195.0 [SD 40.9]min),blood loss(41.4[SD 63.8]mL vs.35.0[SD 29.4]mL),or hospital stay (3.4 [SD 0.8] days vs.3.3 [SD 0.8] days)between the right and left LDNs.The pre- and postdonation (2-week and 6-month) serum creatinine and estimated glomerular filtration rate were comparable between the two groups.
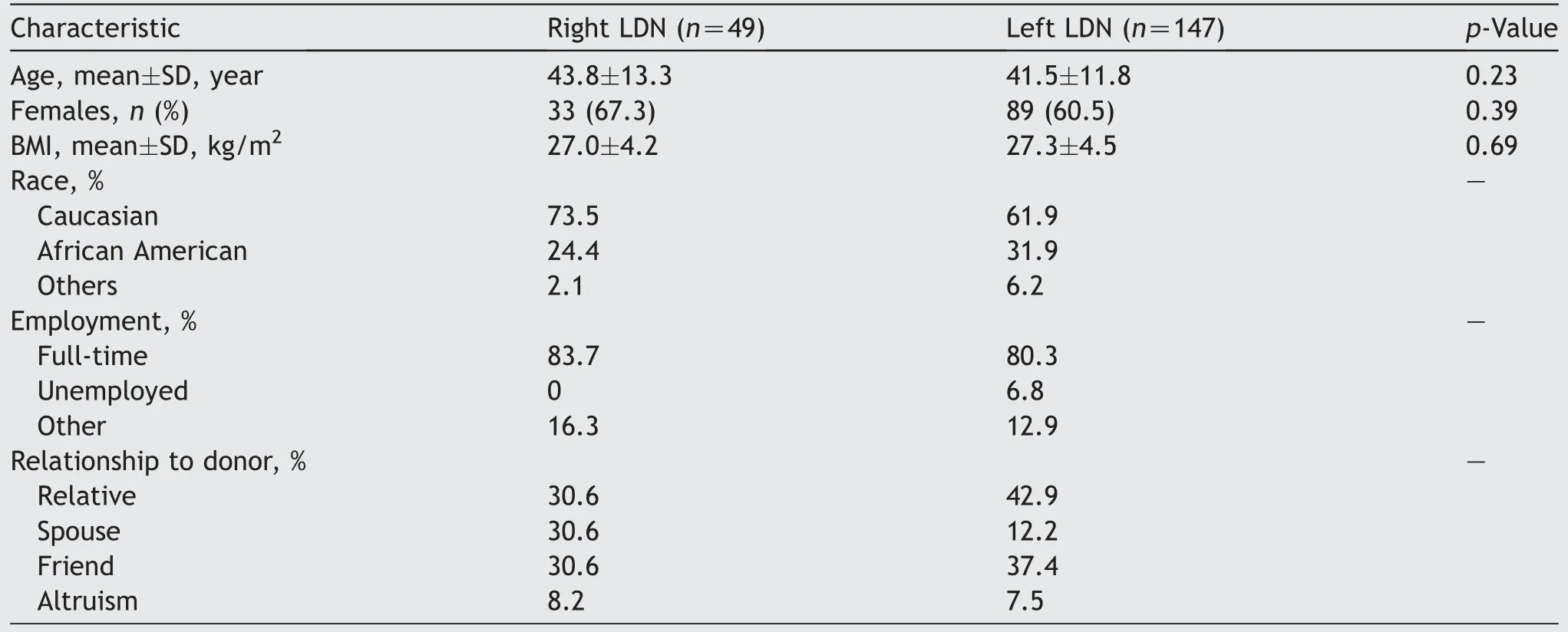
Table 1 Demographics of live kidney donors.
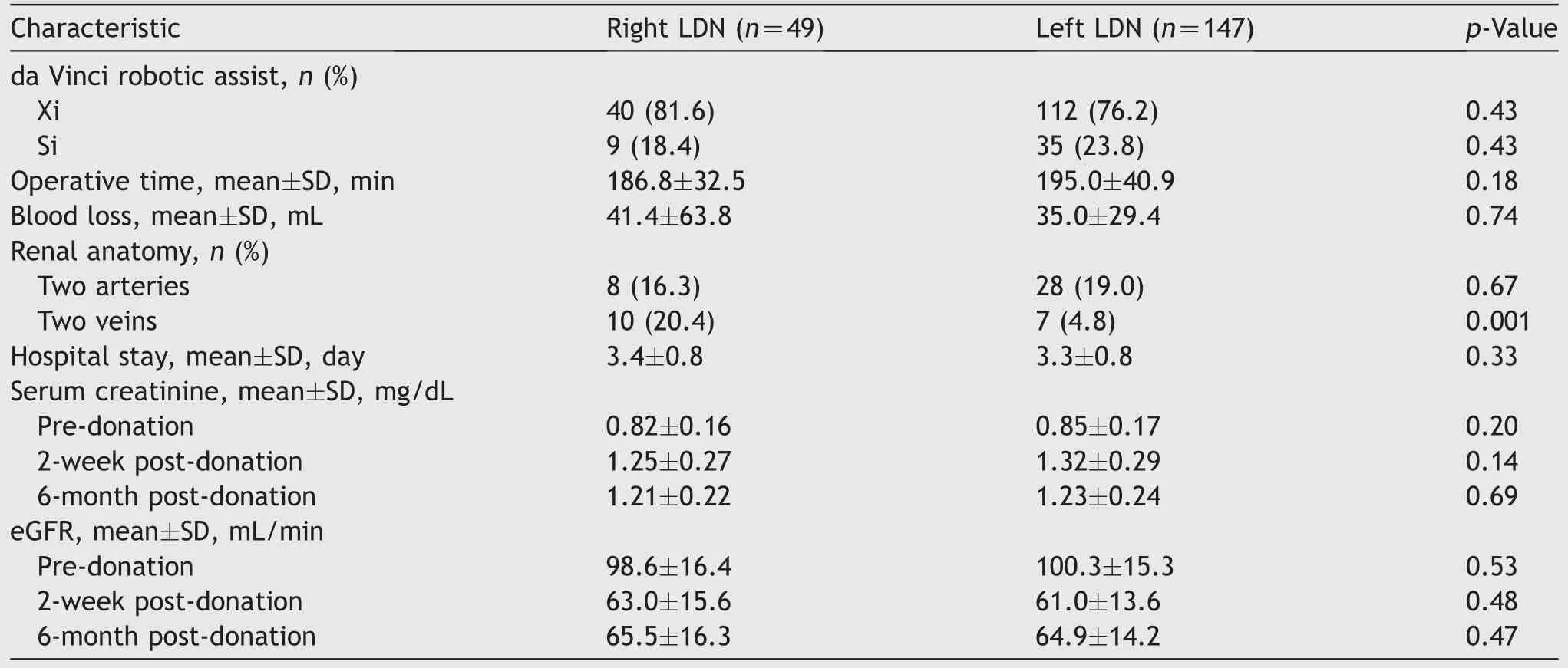
Table 2 Peri-operative characteristics of robotic live kidney donors.
Overall, 10/196 (5.1%) donors had intra-operative events(6.1% [right] vs.4.8% [left], p=0.71) that were managed without conversion to open surgery(Table 3).There were 49 post-operative complications (Table 3).There was nodifference in the incidence of post-operative complications between right and left kidney donors (28.6% vs.23.8%,p=0.51).The majorityofcomplications were relativelyminor and required no procedural interventions (Clavien-Dindo grades I and II).Early post-operative nausea was the most common overall complication occurring in 13.3%of all donors.Four patients had Clavien-Dindo Grade III complications that included meralgia paresthetica,spontaneous pneumothorax,chylous ascites,and internal hernia through descending colon mesentery.There were no deaths in either group.
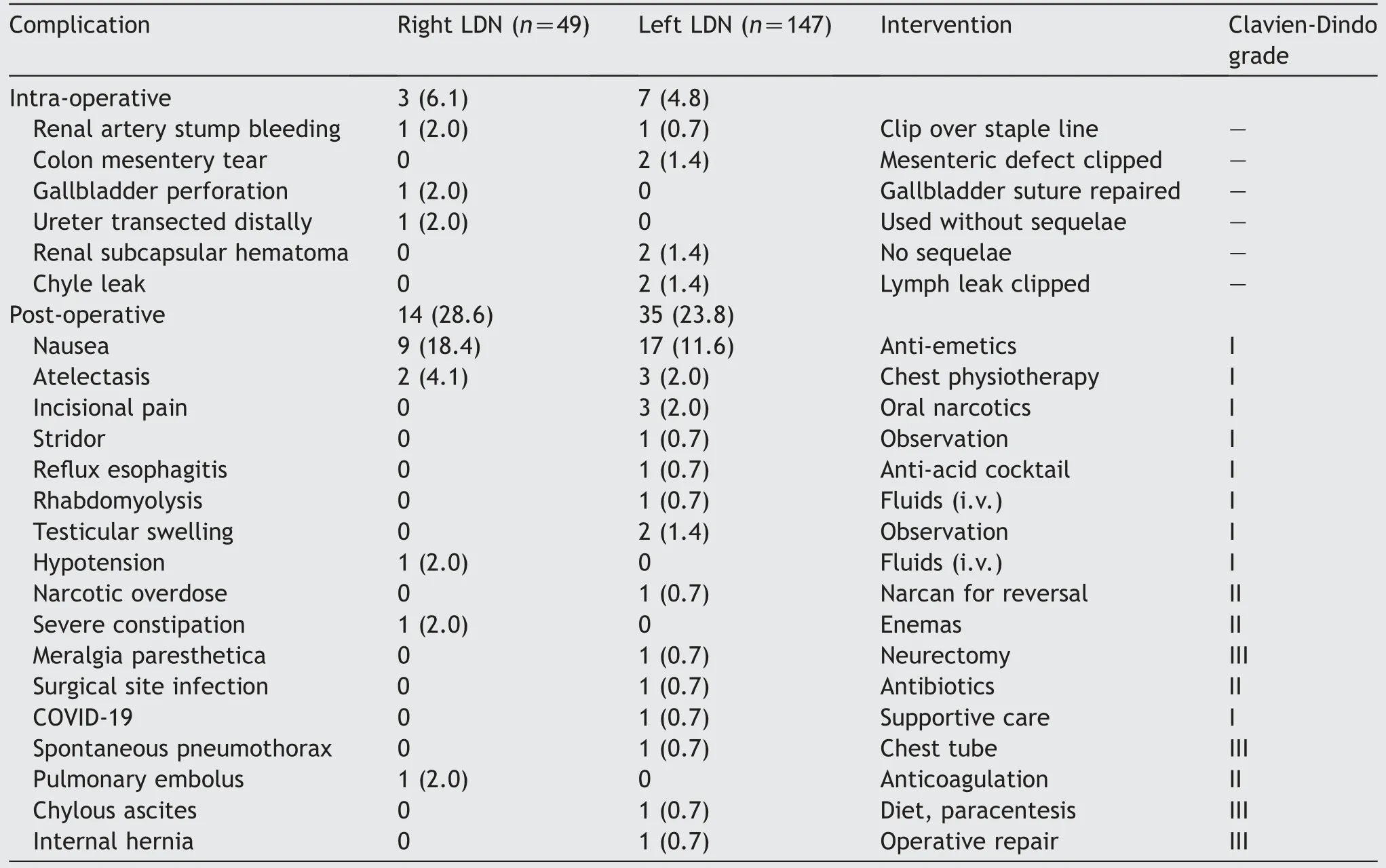
Table 3 Peri-operative complications of robotic LDN.
The recipients of right and left renal allografts were comparable in terms of age, BMI, gender, the duration of pre-transplant dialysis, and history of prior kidney transplantation (Table 4).There was no significant difference between the cold or warm ischemia times for the recipients of right or left allografts.The mean estimated blood loss was comparable between the two groups.
The mean length of hospital stay in recipients of right and left kidneys was not statistically different(5.1[SD 1.8]days vs.5.2[SD 3.0]days,p=0.85)(Table 5).Delayed graft function rates were comparable between the two groups(7.7% vs.5.1%, p=0.55).Although right kidney recipients did obtain a lower mean serum creatinine at 1-year (1.29[SD 0.48] vs.1.42 [SD 0.38] mg/dL, p=0.12), there was no significant difference in graft survival at 1-year (93.9% vs.97.1%, p=0.11).
Patient survival at 1-year was 100.0%and 98.1%for right and left kidney recipients, respectively.One recipient of left kidney had sudden death with a functioning graft while another patient succumbed to sepsis after developing aparotid tumor.Two right renal allografts were lost early:one due to renal vein thrombosis and another due to diffuse intra-vascular thrombosis.A left allograft was lost due to renal artery thrombosis.One recipient of right allograft developed renal artery stenosis that responded to angioplasty by interventional radiology.There was no statistically significant difference in the rates of acute cellular rejections, deep venous thrombosis, pulmonary embolism,or coronary events between the two groups.Wound seroma and lymphocele were observed only in left donor kidney recipients (3.4% each).
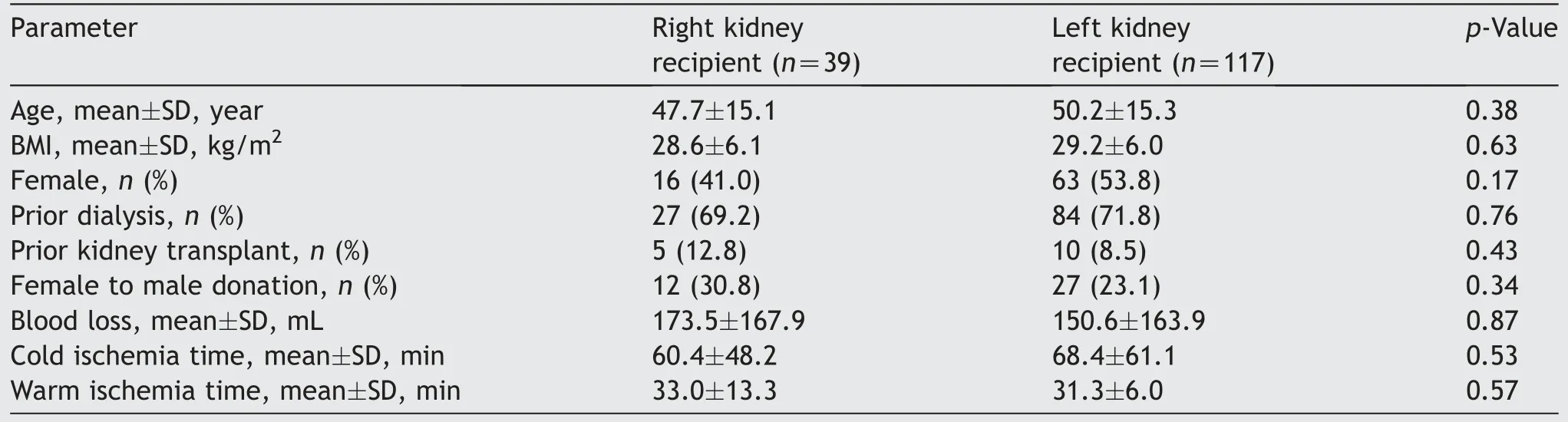
Table 4 Demographics and peri-operative parameters of renal transplant recipients.
4.Discussion
We present our experience of robotic live LDNs using the“non-hand-assisted”technique.We have compared the live donor outcomes after right and left nephrectomies as well as the recipient outcomes after implantation of those kidneys.To the best of our knowledge, this is one of the largest published single center studies on outcomes of robotic right LDN inclusive of recipient outcomes [7].
The first robotic-assisted LDN was performed by Horgan and colleagues in 2002 [13].Currently, about 2% of donor nephrectomies are performed robotically in the United States [5].The left kidney is preferred for donor nephrectomy due to operative difficulty on the right[14].However,not many studies have compared the donor and recipient outcomes after robotic right and left LDN[15].In our series,the right kidney donors frequently had more than a single renal vein, but we did not see any statistically significant difference in operative times, venous thrombosis, blood loss, hospital stays, or post-operative donor renal function between the two groups.
There were ten intra-operative events(5.1%)during 196 robotic donor nephrectomies.In our opinion,none of them can be directly ascribed to the use of the robot for procurement.These intra-operative complications were not related to the laterality of kidney donation (6.1% [right]vs.4.8% [left]; p=0.71).No intra-operative events required conversion to open surgery nor did they impact donor or recipient outcomes.Similar instances reported in literature often required conversion to open procedure and the reported conversion rates decreased, to an average of about 1.5%,as centers gained more experience[16].It has been pointed out that during the hemostatic action in the abdominal cavity, the robot, far from being the cause of acute bleedings,may be helpful in facilitating the surgical team in the suture repairing of the vascular lesions [17].
There were 49 post-operative complications in the 196 donors (25.0%) that were not related to the laterality of kidney donation(28.6%[right]vs.23.8%[left];p=0.51).Of these,83.7%(41/49)were classified as Clavien-Dindo Grade I,8.2%(4/49)as Grade II,8.2%(4/49)as Grade III,and none as Grade IV.Early post-operative nausea was the most common complication (53.1%) and appeared to be related to narcotic use.We have now switched to use of nonnarcotic analgesics in most of our donors.At other centers in the United States, the most common peri-operative complications include gastrointestinal (4.4%), bleeding(3%), respiratory (2.5%), and surgical/anesthesia-related injuries (2.4%) [5].
In a meta-analysis of published work on 32 038 kidney procurements using different surgical techniques, Kortram et al.[18] reported a worldwide complication rate of only 9.3%,but pointed out potential biases in registration of the complications.Horgan et al.[11] reported a complication rate of 24% in their first 74 cases of hand-assisted robotic donor nephrectomies and this decreased to 7% in the following 140 procedures.Lentine and colleagues [5] reported that in the United States, for the subset of nephrectomies performed with robotic assistance (359 corresponding to 2.4% of the total), the frequency of serious complications (Clavien-Dindo Grade IV) was twice than for traditional laparoscopic procurements.The frequency of complications appeared to be directly correlated with the number of procurements performed in different centers, as well as with the BMI of the donors [5].None of our donors had Clavien-Dindo Grade IV complications and only 2.0% (4/196) had Grade III complications that were managed successfully.We attribute these outcomes to the presence of skilled surgical teams for donor and recipient’s surgeries.Robotic nephrectomies were exclusively performed by two surgeons (Sharma A and Bhati C) while implantation was performed by Cotterell A and Levy M in this series.In addition, we have a dedicated operating room transplant team that is well versed in robotic technology as well as conventional “open” transplant procedures.
We have analyzed the outcomes of robotically procured kidneys that were implanted using the traditional “open”technique.In live donor kidney recipients, some factors related to poor outcomes include early vascular complications requiring intervention, human leukocyte antigen mismatches, acute rejection, and female recipients [19].Analysis of 4372 live donor kidney transplants in the Dutch registry showed that implantation of right kidneys from live donors is associated with a higher incidence of technique-related graft failure as compared to left kidneys[20].The recipients of right kidneys had a significantly higher creatinine level at 3-month when compared to their left counter parts (145 μmol/L vs.134 μmol/L, p=0.01).However,the 1-year serum creatinine levels post-transplant were comparable.It was suggested that this could be related to the prolonged suturing time for right kidneys(30.1 min vs.27.6 min, p=0.01), thus implying a greater technical challenge.Another proposed explanation for this difference in early allograft function is that the relatively short right renal vein and long renal artery could lead to compression of the renal vein in case of intrinsic or extrinsic swelling, e.g., urinary obstruction or peri-nephric fluid collection [21].
The mean serum creatinine levels in our recipients at 1-year post-transplant were slightly lower in the right kidney recipients (1.29 [SD 0.48] mg/dL vs.1.42 [SD 0.38] mg/dL,p=0.02) even though the mean warm ischemia times for implanting the right kidneys were numerically, but not significantly,longer than the left kidney(33.0[SD 13.3]min vs.31.3[SD 6.0]min,p=0.57).
Our 1-year post-transplant GL of 2% (3/156) is lower than the 9% in open LDN, comparable to the 2% in hand-assisted laparoscopic LDN, 2% in pure laparoscopic LDN, but slightly higher than the 1% loss reported for robotic-assisted LDN in one large series of 4286 donor nephrectomies [12].Although hyperacute or accelerated acute rejections are occasionally responsible for early GL,the most common causes are non-immunological; vascular thrombosis accounts for up to one-third of early kidney transplant loss.It has also been reported that when compared to ABO blood groups compatible matched controls, long-term patient survival of ABO blood groups incompatible kidney recipients is not significantly different,but the GL is significantly higher,particularly in the first 2 weeks after transplantation [22].
In our series, one right renal allograft was lost due to renal vein thrombosis and another due to diffuse intravascular thrombosis in a planned ABO-incompatible graft.One left kidney was lost due to renal arterial thrombosis.A single-center study of technical GL in 714 consecutive renal transplant recipients has shown that the incidence of technical GL (2%) was significantly higher in diabetic recipients, none of whom received kidneys with multiple renal arteries.In this study, the rate of arterial thrombosis(0.8%) was related to older donor age (46.7 years vs.38.1 years).The authors attribute this to lower sensitivity of computed tomography scans to detect subtle atherosclerotic changes during donor screening.In addition, venous thrombosis(1%)was noted to be more common in recipients with positive hypercoagulable workup [23].
Our report carries the inherent limitations of a single center study with retrospective analysis.Although providing one of the larger analysis of right robotic donor nephrectomy,our workforce may still be too small given the relative infrequency of certain live donor and recipient complications.In addition, since we switched from performing nephrectomies through an open “mini-incision” to robotic nephrectomies,we are unable to provide a comparison with laparoscopic donor nephrectomies.As one-fourth of donor nephrectomies and implants included right kidney,our data provide a robust comparison of donor as well as recipient outcomes for a single-center with standardized protocols.
5.Conclusion
Robotic LDN can be safely performed with excellent outcomes.The incidences of donor complications after right and left robotic nephrectomy were not significantly different.Implantation of right renal allografts was not associated with any higher risk of recipient complications in comparison to the left renal allografts.Skilled surgical teams, standardization of surgical techniques, and preoperative planning played a vital role in achieving those results.The application of robotic technique to donor nephrectomy provides three-dimensional views, greater control, and maneuverability, thus allowing it to be a safe and attractive option for the donors as well as the surgeons.
Author contributions
Study concept and design: Amit Sharma, Chandra Bhati,Dhiren Kumar, Marlon Levy, Adrian Cotterell.
Data acquisition:Brianna Ruch,Deki Tsering,Amit Sharma.Participated in the performance of the research: Brianna Ruch, Deki Tsering, David Bruno, Muhammed Saeed, Daisuke Imai, Aamir Khan, Seung Duk Lee.
Writing of the manuscript: Brianna Ruch, Amit Sharma.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2023年4期
Asian Journal of Urology2023年4期
- Asian Journal of Urology的其它文章
- Robot-assisted adrenalectomy: Step-by-step technique and surgical outcomes at a high-volume robotic center
- The application of internal suspension technique in retroperitoneal robot-assisted laparoscopic partial nephrectomy with a new robotic system KangDuo Surgical Robot-01: Initial experience
- A systematic review of robot-assisted partial nephrectomy outcomes for advanced indications: Large tumors (cT2-T3), solitary kidney, completely endophytic, hilar,recurrent, and multiple renal tumors
- Three-dimensional automatic artificial intelligence driven augmented-reality selective biopsy during nerve-sparing robot-assisted radical prostatectomy:A feasibility and accuracy study
- First 100 cases of transvesical single-port robotic radical prostatectomy
- Robot-assisted oncologic pelvic surgery with Hugo?robot-assisted surgery system: A single-center experience
