Robot-assisted adrenalectomy: Step-by-step technique and surgical outcomes at a high-volume robotic center
Federco Prmde *, Crlo Andre Brv ,Mrco Pcott ,d, Luc Srch ,e, Lug Nocer ,f,Adele Pro ,g, Mr Perre Lores ,Eleonor Blestrzz ,h, Angelo Mottrn ,h, Ru Frnh ,Huert Ncols , Peter De Bcker , Frederek D’hondt ,Peter Schttemn , Ruen De Groote , Geert De Neyer ,Alexndre Mottre
a Department of Urology, Onze-Lieve-Vrouwziekenhuis, Aalst, Belgium
b ORSI Academy, Ghent, Belgium
c Department of Oncology, Division of Urology, University of Turin, San Luigi Gonzaga Hospital, Turin,Italy
d Department of Urology, Humanitas Research Hospital- IRCCS, Rozzano, Italy
e Department of Urology, ASST Santi Paolo e Carlo, University of Milan, Milan, Italy
f Unit of Urology,Division of Experimental Oncology,Urological Research Institute,IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy
g Department of Urology, Ospedale Policlinico e Nuovo Ospedale Civile S.Agostino Estense Modena,University of Modena and Reggio Emilia, Modena, Italy
h Division of Urology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
i Urological Department, La Citadelle, Lie`ge, Belgium
KEYWORDS Robotics;Adrenalectomy;Pheochromocytoma;Malignant;Surgical technique
Abstract Objective: In the last years,robotic surgery was introduced in several different settings with good perioperative results.However, its role in the management of adrenal masses is still debated.In order to provide a contribution to this field, we described our step-by-step technique for robotic adrenalectomy (RA) and related modifications according to the type of adrenal mass treated.Methods: We retrospectively analyzed 27 consecutive patients who underwent RA at Onze-Lieve-Vrouw hospital (Aalst, Belgium) between January 2009 and October 2022.Demographic,intra- and post-operative, and pathological data were retrieved from our prospectively maintained institutional database.Continuous variables are summarized as median and interquartile range (IQR).Categorical variables are reported as frequencies (percentages).Results: Twenty-seven patients underwent RA were included in the study.Median age, body mass index, and Charlson’s comorbidity index were 61 (IQR: 49-71) years, 26 (IQR: 24-29)kg/m2,and 2(IQR:0-3),respectively,and 16(59.3%)patients were male.Median tumor size at computed tomography scan was 6.0 (IQR: 3.5-8.0) cm.Median operative time and blood loss were 105 (IQR: 82-120) min and 175 (IQR: 94-250) mL, respectively.No intraoperative complications were recorded.Overall postoperative complications rate was 11.1%, with a postoperative transfusion rate of 3.7%.A total of 10(37.0%)patients harbored malignant adrenal masses.Among them, 3 (11.1%) had adrenocortical carcinoma, 6 (22.2%) secondary metastasis, and 1 (3.7%) malignant pheochromocytoma on final pathological exam.Only 1(10.0%)patient had positive surgical margins.Conclusion: We described our step-by-step technique for RA,which can be safely performed even in case of high challenging settings as malignant tumors,pheochromocytoma,and large masses.The standardization of perioperative protocol should be encouraged to maximize the outcomes of this complex surgical procedure.
1.Introduction
Since the first report of Gagner et al.[1] in 1992, laparoscopy progressively gained popularity for the treatment of adrenal masses,and nowadays laparoscopic adrenalectomy(LA) is considered the gold standard treatment option for adrenal masses less than 6 cm without risk of malignancy[2].However, although LA allows for good perioperative results as compared to its open counterpart, it usually requires a high level of laparoscopic skills and sometimes may become a challenging procedure.For instance, a higher rate of complications has been described in large masses, with high risk of malignancy and/or pheochromocytoma, and in obese patients [3-5].
As it happened in other settings [6-8], also in this complex surgical scenario robotics-thanks to its many intrinsic advantages-may help surgeons improve the intraoperative management of adrenal masses.Therefore, some surgeons started to assess the potential role of robotic surgery in this setting.In this regard, current literature showed that, as compared to LA, robotic adrenalectomy (RA) may have comparable operative time and complication rates, but lower blood loss and shorter hospital stay[9,10].However,a detailed description of standardized surgical technique for RA is still lacking.For this reason, and in order to provide a contribution to this field, we described our step-by-step technique for RA and related modifications according to the type of adrenal mass treated.
2.Patients and methods
2.1.Patient population
This is a retrospective single center study including 27 consecutive patients who underwent robot-assisted adrenalectomy at Onze-Lieve-Vrouw hospital (Aalst,Belgium) between January 2009 and October 2022.The study was conducted in accordance with good clinical practice guidelines and approved by Onze-Lieve-Vrouw hospital ethical committee (approval number: 2021/042);informed consents were obtained from the patients regarding the procedure and the management of their clinical data.Demographic, intra- and post-operative, and pathological data were retrieved from our prospectively maintained institutional database.
2.2.Surgical indication and preoperative assessment
All patients with a finding of an adrenal mass underwent a multidisciplinary evaluation, including complete hormonal assessment (serum levels of aldosterone, cortisol and catecholamines,as well as urine levels of metanephrines).The adrenal mass was evaluated by non-enhanced computed tomography (CT) scan or magnetic resonance imaging.An enhanced CT scan was performed if needed (inconclusive diagnosis, suspicious of malignancy).
The indication to adrenal surgery was given according to the current guidelines [2,11] including: functioning adenoma (Conn’s syndrome, Cushing’s syndrome, and hyperproduction of sexual hormones), non-functioning adenoma of larger than 5 cm or growing on seriate imaging, and pheochromocytomas and lesion suspected for malignancy.Secondary adrenal masses (i.e., adrenal metastases) were considered eligible for surgical resection.Infiltrative growth pattern and/or involvement of surrounding structures were the major contraindications for robotics.
In case of suspected catecholamines production, patients received preoperative preparation with oral alphablockers.During surgery, all patients were carefully monitored (arterial catheterization) to prevent hemodynamic instability, especially during tumor manipulation prior to adrenal vein ligation.In case of pheochromocytoma, hydrocortisone and adequate fluid administration were given perioperatively to compensate the loss of adrenergic tone after removal of the mass.
2.3.Surgical technique
All procedures were performed transperitoneally by two high-experienced robotic surgeons (Mottrie A and De Naeyer G) using the da Vinci Si or Xi surgical system (Intuitive Surgical, Sunnyvale, CA, USA).Our surgical technique is described in detail in the enclosed video(Supplementary Video 1).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajur.2023.04.001.
2.3.1.Patient positioning, port placement, and docking of the robotic system
Under general anesthesia, the patient was positioned in a 60°flank position with the abdomen on the edge of the surgical bed.The pressure points were padded and checked before starting the operation.The robotic system was used in a four-arm configuration, starting with the da Vinci Si system and-in more recent years-with the Xi system,using the same port placement for both systems.As shown in Fig.1, robotic trocars were placed 6-8 cm apart in a linear fashion starting from the pararectal line-2 cm below the costal rim-with the lowest one(i.e.,the closest to the pelvis)approximately 4 cm more lateral than the first one.During left procedures, the fourth robotic instrument was utilized in the rightmost trocar, whereas for right procedures the fourth robotic instrument was used in the leftmost trocar.The AirSeal port was placed near the umbilicus.No additional assistant trocars for retracting the liver or the spleen are usually placed.
2.3.2.Identification of the adrenal lodge
2.3.2.1.Left side.The surgical procedure started with the correct exposure of adrenal lodge.Therefore,surgeons began with the medialization of the colon and rotation of the spleen and the pancreas.Spleen lateral attachments as well as spleno-renal ligaments were incised in order to obtain a proper mobilization of the spleen.During the dissection of the upper margin of the adrenal gland, it is possible to identify the splenic vessels and the pancreas tail, which is medialized as well.During this phase of the operation, it is of paramount importance to proceed carefully in order to avoid injuries to the pancreas tail as it can be easily mistaken for the adrenal gland.

Figure 1 Left side trocars placement.For right side procedure, the trocars were placed in a mirror fashion.
2.3.2.2.Right side.On the right side, the first step was the mobilization of the liver in order to obtain enough space to get to the adrenal lodge.After the incision of the triangular ligament and the posterior peritoneum, the colon and the duodenum were medialized in order to identify the inferior vena cava (Fig.2).
2.3.3.Identification and control of adrenal vein
On the left side,the identification of renal vein was needed in order to adequately control the adrenal vein.To identify the renal hilum, it is possible to either assess it directly or by identifying and following the gonadal vein.On the right side, the adrenal vein was identified following the lateral margin of inferior vena cava towards the diaphragm.Once properly dissected and isolated, the adrenal vein was clipped between Weck?Hem-o-lok?applied by the bedside assistant (Fig.3).
2.3.4.Dissection of the adrenal gland
The procedure was continued with the incision of the adreno-renal ligament, dissecting the adrenal gland from the upper pole of the kidney.Then, the adrenal gland was dissected from the psoas muscle moving upwards to the diaphragm.During this phase, the adrenal arterial pedicles were identified and clipped with Weck? Hem-o-lok?(Fig.4).
2.3.5.Hemostasis and removal of the specimen
After the section of the last adrenal pedicle, the adrenal gland was placed into a laparoscopic endobag.Then, the surgeon checked the hemostasis with particular attention to the clipped pedicle.If needed, hemostatic agents may be applied to control and secure the hemostasis.The robot was undocked and the specimen was removed by the AirSeal port incision after a minimum extension.No drain was left inside at the end of the procedure.

Figure 2 Identification of right renal vein and inferior vena cava during right adrenalectomy.

Figure 3 Clipping of right adrenal vein.
2.3.6.Special situations
2.3.6.1.Pheochromocytoma.Pheochromocytoma requires a careful preoperative endocrinologic and anesthesiologic assessment in order to adequately prepare the patient for the surgery.Similarly, some intraoperative technical details differ from standard adrenalectomy and are utilized to reduce the risk of hemodynamic instability.First, it should be kept in mind that elevated intraabdominal pressure of CO2may cause a release of catecholamines [12].Therefore, pneumoperitoneum should be induced gradually, and pressure should be maintained as low as possible.On this matter, medical systems able to maintain constant intraabdominal pressure should be preferred.Second, the early adrenal vein control is crucial to minimize the risk of intraoperative hemodynamic instability.Thus, efforts should be made to isolate and clip the adrenal vein as soon as possible during the operation, and to minimize gland manipulation until the adrenal vein is controlled.Third, all tractions should be done on perirenal fat and not on the capsule to avoid compression of the tumor.Finally, a meticulous surgical dissection is needed throughout the operation to avoid capsular rupture with subsequent bleeding (which may occur easily given the high vascularization of pheochromocytomas) and risk of local relapse.

Figure 4 Development of posterior plane.During this phase,the arterial pedicles were identified and clipped.
2.3.6.2.Risk of malignancy.In case of lesions suspicious for malignancy,the principles of oncologic surgery must be respected.First, the mass should be handled carefully in order to avoid tumor rupture and spillage.In this regard, it should be always kept in mind that the handling of these masses may be challenging as they are frequently larger than benign ones.Second, the lesion should be removed en-bloc together with surrounding tissue and perirenal fat to avoid positive surgical margins and risk of recurrence.Finally, especially in case of suspected adrenal primary tumors, a locoregional lymphadenectomy might be necessary (pericaval nodes on the right, and periaortic and renal hilar nodes on the left).
2.4.Postoperative care
Postoperative management followed our institutional protocol, including intravenous fluid, analgesics, and thromboprophylaxis.Corticoid supplementary therapy was given according to endocrinological indications.Hospital discharge usually takes place on the 3rd postoperative day.Subsequently, patients underwent endocrinological follow-up at our institution by our team of dedicated endocrinologists.
2.5.Statistical analysis
Continuous variables are summarized as median and interquartile range (IQR).Categorical variables are reported as frequencies(percentages).Data analysis is performed using the Jamovi software v.2.3 (Sidney, Australia).
3.Results
Demographic characteristics of our cohort are shown in Table 1.Median age, body mass index, and Charlson’s comorbidity index were 61(IQR:49-71)years,26(IQR:24-29)kg/m2, and 2 (IQR: 0-3), respectively, and 16 (59.3%)patients were male.Median American Society of Anesthesiologists score was 2 (IQR: 2-3) and previous abdominal surgery was recorded in 9(33.3%)patients.The lesions were on the right side in 16 (59.3%) patients, and median tumor size was 6.0 (IQR: 3.5-8.0) cm.The adrenal mass was incidentally detected in the 55.6% of the cases.The two most common clinical indications for adrenalectomy were uncontrolled hypertension(11.1%)and suspected metastasis finding during oncological follow-up for other malignancies(22.2%).
Surgical variables are reported in Table 2.Median operative time and blood loss were 105 (IQR: 82-120) min and 175 (IQR: 94-250) mL, respectively.No intraoperativecomplications were recorded.Overall postoperative complications rate was 11.1% with a postoperative transfusion rate of 3.7%.Length of stay was 3 (IQR: 3-4) days.

Table 1 Demographic variables.
Pathological data are shown in Table 3.A total of 10 (37.0%) patients harbored malignant adrenal masses.In detail, 3 (11.1%) adrenocortical carcinoma, 6 (22.2%) secondary metastasis, and 1 (3.7%) malignant pheochromocytoma were identified on final pathological exam.The most common origin of metastasis was clear cell renal carcinoma(5 out of 6 patients).Only 1(10.0%)positive surgical margin was recorded.This patient experienced local recurrence of the tumor (renal cell carcinoma), underwent systemic oncological therapy, and died of tumor related causes 5 years later.
4.Discussion
In this study, we described our step-by-step standardized technique for transperitoneal robot-assisted adrenalectomy, including some technical tips to improve the management of challenging adrenal masses.

Table 2 Surgical variables.
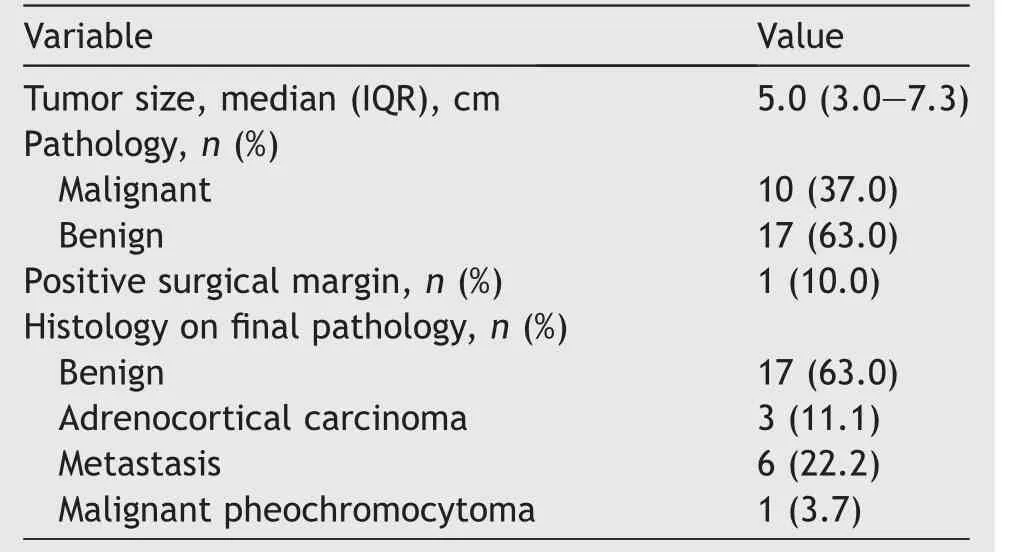
Table 3 Pathological variables.
The standardization of surgical pathway (from patient selection to surgical technique and postoperative management) is a helpful tool that, especially in some challenging scenarios (as adrenal surgery), could lead surgeons to maximize perioperative patients’ care.
As in other fields, the correct patient selection plays a very important role in adrenal surgery scenario.This is particularly true in the selection of candidates to RA since adrenal masses may present with extremely challenging features.For instance, malignant masses with infiltrative growth pattern and/or local nodal enlargement should be treated with an open surgical approach in order to decrease the risk of positive surgical margins and tumor capsule rupture.That said,besides these features,radiologic risk of malignancy per se should not be considered a contraindication for minimally invasive surgery.As reported by Donatini et al.[13] and Porpiglia et al.[14], if principles of oncologic surgery are respected (careful handling of the gland, avoiding tumor capsule rupture or spillage, and no positive surgical margins), the laparoscopic approach had oncological outcomes comparable to those of open surgery.Therefore, it is reasonable to assume that robotics could achieve at least the same results of laparoscopy, and our results suggest that this might be the case.However, it should be kept in mind that, while performing a RA, the threshold for conversion to open surgery must remain very low.If there are signs of infiltration of surrounding structures and/or capsule violation,or a resection with negative margins cannot be assumed,the conversion to open surgery should not be delayed [15].
Another important aspect for RA is a standardized approach,which involves trocar placement and step-by-step surgical technique.We described our trocar placement,which is very similar to commonly used settings for kidney surgery, unlike other settings described by other authors[16,17], and as such, it is easily reproducible.At the same time, it can be adjusted as per specific circumstances such as large masses or particular anatomies.Furthermore, our trocar placement has been easily used with both da Vinci systems, with no modifications needed, similar to what reported by Feng et al.[18],who also reported no differences in terms of perioperative outcomes.
Of course, with respect to our surgical technique, it shares some steps with other previously reported experiences[16,19]but also some differences as the type of instruments used for the dissection, the direction of the dissection of the gland[16], or the absence of an additional assistant instrument used as liver retractor [19].Furthermore, in this paper, we underline some important tips that may help during this procedure.The preliminary dissection plays a pivotal role, allowing a correct exposure of every anatomical structure, and should be performed carefully in order to avoid accidental injuries of surrounding organs and structures.Here, robotics may help surgeons thanks to the tractions made by the fourth arm, facilitating the exposure of adrenal lodge.Another important point of attention should be the management of the vascular pedicle, which requires a precise dissection and sometimes may be problematic.For example, in case of large malignant masses or pheochromocytoma, the frequent presence of neoangiogenesis may increase the intraoperative bleeding.Moreover,especially in case of right pheochromocytoma, surgeons should check for additional adrenal veins before handling the mass in order to minimize the risk of unexpected intraoperative release of catecholamines [5].
With respect to surgical outcomes, our findings are comparable with other large robotic series in terms of operative time, diameter of treated lesions, and perioperative complications [20-23].Considering possible advantages of robotics over laparoscopy,a recent study from the EUROCRINE surgery registry on more than 1000 patients showed a lower complication rate and shorter duration of stay [24].Two large metanalysis demonstrated reduced blood loss and length of stay of robotics, whereas robotic surgery was found to have higher costs per procedure as compared to laparoscopy [9,25].Although this is usually described as the main disadvantage of robotic surgery,there are aspects of surgery that might indirectly affect the overall cost of a procedure,and that might be improved by robotic surgery.For instance, recent literature suggests that robotics may reduce the rate of perioperative complications, especially in high complex settings [26-29].Moreover,robotics showed lower rate of conversion to open surgery in case of large adrenal masses [26,30], less hemodynamic instability in case of pheochromocytoma [27],and shorter operative time and less blood loss for obese patients [31].Taken together, it seems reasonable to believe that improvements of perioperative outcomes might translate to shorter length of stay and thus balance the higher technical costs of robotics.Obviously, this also depends on intrinsic aspects of different health care systems.For example, the length of stay after surgical procedures is generally shorter in North America than in Europe or in Asia as a result of different post-discharge facilities and organizations [32].Finally, the introduction of new robotic platforms has the potential to increase competition in the field and thus lower costs of robotic surgery [33,34].
Our study is not devoid of limitations.Given the small sample size, the lack of a comparative group, and the absence of a long follow-up, the results reported in this study should be handled with caution since their generalizability could be limited.Nevertheless, our series showed that a robust robotic experience, together with a rigorous standardized perioperative protocol, may achieve adequate perioperative outcomes after RA.
5.Conclusion
In this study, we described our step-by-step technique for RA, which can be safely performed even in case of high challenging settings as malignant tumors, pheochromocytoma, and large masses.The standardization of perioperative protocol should be encouraged to maximize the outcomes of this complex surgical procedure.
Author contributions
Study concept and design: Alexandre Mottrie, Ruben De Groote, Carlo Andrea Bravi, Federico Piramide.
Data acquisition: Federico Piramide, Carlo Andrea Bravi,Marco Paciotti,Luca Sarchi,Luigi Nocera,Adele Piro,Maria Peraire Lores, Eleonora Balestrazzi, Angelo Mottaran, Rui Farinha, Hubert Nicolas, Pieter De Backer, Frederiek D’hondt, Peter Schatteman, Ruben De Groote, Geert De Naeyer, Alexandre Mottrie.
Data analysis: Federico Piramide.
Drafting of manuscript: Federico Piramide, Carlo Andrea Bravi.
Critical revision of the manuscript: Federico Piramide,Carlo Andrea Bravi, Marco Paciotti, Luca Sarchi, Luigi Nocera, Adele Piro, Maria Peraire Lores, Eleonora Balestrazzi,Angelo Mottaran,Rui Farinha,Hubert Nicolas,Pieter De Backer,Frederiek D’hondt,Peter Schatteman,Ruben De Groote, Geert De Naeyer, Alexandre Mottrie.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2023年4期
Asian Journal of Urology2023年4期
- Asian Journal of Urology的其它文章
- The application of internal suspension technique in retroperitoneal robot-assisted laparoscopic partial nephrectomy with a new robotic system KangDuo Surgical Robot-01: Initial experience
- A systematic review of robot-assisted partial nephrectomy outcomes for advanced indications: Large tumors (cT2-T3), solitary kidney, completely endophytic, hilar,recurrent, and multiple renal tumors
- Three-dimensional automatic artificial intelligence driven augmented-reality selective biopsy during nerve-sparing robot-assisted radical prostatectomy:A feasibility and accuracy study
- First 100 cases of transvesical single-port robotic radical prostatectomy
- Robot-assisted oncologic pelvic surgery with Hugo?robot-assisted surgery system: A single-center experience
- Prospective observational study on the prognosis of ureteral lesions caused by impacted stones via dual-energy spectral computed tomography
