Effects of electroacupuncture on gut microbiota and related inflammatory factors in rats with Crohn disease
LIU Qiong (劉瓊), HE Haolong (何灝龍), YANG Jingjing (陽晶晶), CAO Sihui (曹思慧), CHEN Lin (陳琳),ZHOU Jingying (周競穎), LIU Xia (劉霞), YANG Zongbao (楊宗保), LIU Mi (劉密)
1 College of Acupuncture & Tuina and Rehabilitation, Hunan University of Chinese Medicine, Changsha 410208, China
2 Chongqing Three Gorges Medical College, Chongqing 404120, China
3 Xiamen University, Xiamen 361102, China
Abstract Objective: To observe the effects of electroacupuncture (EA) on gut microbiota and serum inflammatory factors interleukin (IL)-1β and tumor necrosis factor (TNF)-α in Crohn disease (CD) model rats.
Keywords: Acupuncture Therapy; Electroacupuncture; Crohn Disease; Gut Microbiome; Interleukin-1beta; Tumor Necrosis Factor-alpha; Rats
Crohn disease (CD) is an inflammatory bowel disease(IBD) characterized by abdominal pain, diarrhea, and weight loss[1-3].In recent years, the global incidence rate of CD has been increasing[4].At present, the clinical treatment of CD mainly includes salicylic acid drugs,corticosteroids, immunomodulators, combination therapy, and surgical treatment[5-6].Drug therapy for CD is mainly based on immunosuppression.However,long-term drug use may lead to an increased risk of infection, bone marrow suppression, liver injury,osteoporosis, and other adverse reactions[7].Therefore,searching for effective and safer treatment is needed.
Genetic factors, environmental factors, changes in gut microbiota, and immune system disorders are some of the factors affecting CD[8].Abnormal intestinal immuno-inflammatory reactions mediated by gut microbiota disorders have been receiving more and more attention lately[9].Recent studies have reported lower stability of gut microbiota in active CD patients than in healthy people, manifested by changes in gut microbiota diversity and structure[10].The imbalance of gut microbiota further leads to the expression of intestinal inflammatory factors, triggering inflammatory reactions[11], e.g., inflammatory factor interleukin (IL)-1β can promote inflammatory reactions and aggravate tissue damage, while tumor necrosis factor (TNF)-α can increase IL-1β.
Acupuncture and moxibustion have been widely used as alternative therapies for the treatment of IBD[12].Studies have shown that acupuncture can reduce the disease activity index (DAI) score and improve the quality of life in patients with mild and moderate active CD[13-14].However, the mechanism is still not fully understood.In this study, we assessed the effect of electroacupuncture (EA) on gut microbiota and inflammatory factors in rats with CD induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS).
1 Materials and Methods
1.1 Experimental animals
Thirty-six specific-pathogen-free male Sprague-Dawley rats [body mass (160±10) g, License No.SYXK(Min) 2013-0006] were raised in the Animal Laboratory of Xiamen University in an environment with a temperature of (25±1) ℃, relative humidity of(60%±10%), and a light/dark cycle of 12 h/12 h.
After one week of adaptive feeding, animals were randomly divided into a normal control (NC) group(n=10) and a modeling group (n=26).The experiment was conducted in compliance with theLaboratory Animal:Guideline for Ethical Review of Animal Welfare(GB/T35892-2018)[15]and passed the ethical approval of Xiamen University (Approval No.LL2017020803).
1.2 Model preparation
According to the literature[16], TNBS combined with 50% ethanol solution was used for modeling.The modified catheter was connected to the syringe, and the enema was administered with TNBS enema solution at 3 mL/(kg·bw).After the enema was injected, the rats were kept upside down for 1 min to promote the full absorption of the solution.Rats in the NC group were given 0.9% normal saline enema.The enema was repeated once every 7 d for a total of 4 times.
Model identification methods: After the modeling, 2 rats in each group were randomly selected to observe the pathological colon injury by hematoxylin-eosin (HE)staining.Under the light microscope, the absence and discontinuity of the epithelial tissue of the colon mucosa, disordered glandular arrangement, and glandular hyperplasia or disappearance were observed.Ulcers appeared in the mucosa and submucosa.Submucosa showed obvious congestion, edema and thickening, significant inflammatory cell infiltration,lymphocyte aggregation, and granulomatous lesions.After the success of model preparation was confirmed,rats in the modeling group were divided into a model(CD) group, a Western medicine (WM) group, and an EA group according to the random number table method,and then the follow-up experiment was carried out.
1.3 Intervention mode
The NC group was not given any intervention or treatment.The CD group was fixed on the self-made fixator, and no treatment was given.Rats in EA group were fixed in the self-made fixator.Bilateral Shangjuxu(ST37) and Tianshu (ST25) were taken[17-18].Shangjuxu(ST37) is located below the external knee joint of the rat, about 10 mm below the small head of the fibula.Tianshu (ST25) is located 40 mm below the xiphosternal synchondrosis of the rat, 5 mm beside the anterior median line.Disposable sterile acupuncture needles were inserted into Shangjuxu (ST37) for 7 mm and Tianshu (ST25) for 5 mm.Then, the electrical apparatus was connected, and Shangjuxu (ST37) and Tianshu(ST25) on the same side were connected to the same group of wires.The continuous wave was selected.The frequency was 10 Hz, and the current was 1.5 mA.The time was 20 min.The intervention was performed once a day for 7 consecutive days.
Rats in the WM group received intragastric treatment.The ground powder of mesalazine enteric-coated tablets and double distilled water were prepared into gavage fluid, and the daily dosage was calculated according to the ratio of adults (body mass 70 kg, 4 g/d)to rats (body mass 200 g), which was 56:1[19], once a day,for a total of 7 times of gavage.

Figure 1 Schematic diagram of point location and electroacupuncture operation in rats
1.4 Sample collection
After the last intervention, the stool of rats was collected in an EP tube, snap-frozen in liquid nitrogen,and then stored in a -80 ℃ refrigerator for gut microbiota detection.After fasting without water for 12 h, rats in each group were anesthetized with 1%pentobarbital sodium [50 mg/(kg·bw)] injected intraperitoneally, and blood samples were collected from the abdominal aorta.After resting, the serum was centrifuged at 3 000 r/min at 4 ℃ for 15 min, and the separated serum was stored in the refrigerator at -20℃for later detection of serum TNF-α and IL-1β.In addition,5 cm of colon was taken and fixed in 4%paraformaldehyde fixative solution for pathological examination.
1.5 Observation indicators and methods
1.5.1 General condition and DAI score of rats in each group
The changes in rat body mass and fecal characteristics were recorded daily.DAI was calculated according to the literature[20]using the following formula: DAI = (Body mass decline fraction + Stool trait fraction + Stool blood fraction) ÷ 3.
1.5.2 Pathological examination of colon
After treatment, the diseased colon tissues of rats were taken, fixed, dehydrated, and embedded in paraffin, after which HE staining was performed.The pathological changes in colon tissues were observed under a light microscope using the following scoring criteria: structural change only, 0 points; chronic inflammation, 1 point; neutrophil infiltration was observed in the lamina propria of the intestinal mucosa,2 points; neutrophil infiltration was observed in the upper cortex, 3 points; recess structure failure, 4 points;erosions or ulcers, 5 points.
1.5.3 Serum TNF-α and IL-1β concentrations
After treatment, serum samples were taken from rats,and the contents of TNF-α and IL-1β were detected according to enzyme-linked immunosorbent assay kit instructions.
1.5.4 Detection of gut microbiota
The previously frozen stool samples of rats were taken, and 16S rDNA high-throughput sequencing was used for gut microbiota detection, including the following experimental procedures.
DNA extraction and inspection: The total genomic DNA of fecal bacteria was extracted in strict accordance with the operating instructions of the DNA kit.The DNA concentration and purity were determined by Multiskan TM GO, and the DNA completeness was detected by agarose gel electrophoresis.
Amplification and library construction of polymerase chain reaction (PCR): Primers 341F and 806R were used to amplify the 16SV3-V4 region.Also, agar gel electrophoresis was detected, fluorescence quantification was performed, and the 16S library was constructed by Illumina.
Library quality testing: Library quality testing includes accuracy testing, length testing, and contaminant testing.In this study, Qubit3.0 was used for quantitative analysis and dilution, after which Agilent2100 was used to detect the effective concentration in the library.Finally, real-time quantitative PCR (RT-qPCR) was used to quantify the effective concentration in the library.
MiniSeq high-throughput sequencing: After the library was qualified, the Illumina high-throughput sequencing platform (HiSeq/MiniSeq) was used for sequencing.After the sequencing platform obtained the offline dual-end data, the Flash software was used for splice and quality control to obtain high-quality clean reads, and then the chimera filtering was performed.Finally, the QIIME software was used to cluster the OTU.According to the OTU abundance table of each sample,α diversity, β diversity, and LEfSe difference analyses were performed.
1.6 Statistical methods
Data analyses were conducted using the SPSS version 22.0 statistical software.The measurement data were tested for normality and homogeneity of variance.The measurement data conforming to normal distribution were presented as mean ± standard deviation (±s),while the data without normal distribution were presented as median (quartile) [M (Q)].Analysis of variance was used for multiple-group comparisons if the measurement data obeyed normal distribution.If there was a significant difference, a comparison between the two groups was carried out.When the variance was homogeneous, the least significant difference was used to analyze the variance; otherwise, the Dunnet T3 test was used.If the measurement data were not normally distributed, the rank-sum test was used for comparisons.P<0.05 indicated statistical significance.
2 Results
2.1 General behavior observation
In the NC group, the fur color of rats was glossy.The rats were active, and their food intake and stool were normal.In the CD group, the hair color of rats was haggard, the behavior was sluggish, the body mass was decreased, and the stool was mushy.Some rats had mucous, pus, bloody stools, and perianal stains.After EA intervention, the mental state of rats improved.The rats were more active than before and had smooth hair and soft stool without pus or blood.In the WM group,the mental state improved, the hair color did not significantly change, the nature of stool improved, and there was no pus and blood stool.
2.2 Body mass and DAI score
There was no significant difference in body mass among all groups before modeling (P>0.05).After modeling, compared with the NC group, the body mass of rats in the CD group was significantly decreased(P<0.01).After intervention, the body mass of the EA and WM group was higher than that of the CD group(P<0.05).Please see Table 1.After intervention, the DAI scores in the EA group and WM group were significantly decreased compared with the CD group (P<0.01).Please see Table 2.
Table 1 Change of body mass in each group (±s) Unit: g

Table 1 Change of body mass in each group (±s) Unit: g
Note: Compared with the normal control group, 1) P<0.01; compared with the model group, 2) P<0.01.
Group n Before modeling After modeling After intervention Normal control 8 203.45±12.13 256.53±26.51 267.25±27.21 Model 8 205.13±12.91 175.98±15.511) 171.32±15.741)Electroacupuncture 8 207.15±19.10 179.85±11.171) 218.55±10.012)Western medicine 8 201.61±13.28 181.63±12.651) 216.54±31.332)
Table 2 DAI score of rats in each group ( ±s) Unit: point

Table 2 DAI score of rats in each group ( ±s) Unit: point
Note: Compared with the model group, 1) P<0.01.
Group n After modeling After intervention Normal control 8 0 0 Model 8 2.13±1.01 2.09±0.98 Electroacupuncture 8 2.10±0.22 1.03±0.291)Western medicine 8 2.21±0.88 1.05±0.951)
2.3 Histopathological changes
In the NC group, the colonic tissue layers were clear,and the glands were intact.In the CD group, the colonic tissue layers were fuzzy, the structure was disordered,and the glands were bent and deformed; also, a large number of inflammatory cells were seen, the goblet cells were few, and the granulation tissue was proliferated.After EA or intervention with Western medicine, the colon mucosa structure was relatively complete, and the gland structure was restored, with little inflammatory cell infiltration (Figure 2).
Compared with the NC group, the histopathological score of the CD group was significantly higher (P<0.05).On the other hand, after intervention, compared with the CD group, the histopathological score of the EA group and WM group decreased significantly (P<0.01).Please see Table 3.
2.4 Comparison of serum concentrations of TNF-α and IL-1β
Compared with the NC group, the serum concentrations of TNF-α and IL-1β in the CD group increased (P<0.01).After intervention, the concentrations of TNF-α and IL-6 in the EA group and WM group decreased significantly (P<0.01).Please see Table 4.
2.5 Analysis of gut microbiota in each group
2.5.1 Venn diagram and α diversity analysis
Venn diagram was designed according to the OTU division of effective sequence (Figure 3).The total number of OTU in the NC group, CD group, EA group,and WM group was 1 003, 950, 980, and 999,respectively, and the number of unique OTU in the NC group, CD group, EA group, and WM group was 83, 56,51, and 60, respectively.The results indicated that the abundance of the CD group decreased, while the number of EA and WM groups increased after treatment.Compared with the NC group, the Shannon index of the CD group decreased (P<0.05), and that of the EA group increased significantly (P<0.05) compared with the CD group.There was no significant difference in the Simpson index among the groups (P>0.05).Please see Table 5.
2.5.2 β diversity analysis in each group
β diversity is the comparative analysis of the microbial community structure of different samples.Each point in the PCA analysis chart represents a sample, and different colors represent different groups.The closer the distance between the points, the more similar the samples are.The contribution rate of abscissa PC1 was 12.63%, and the contribution rate of ordinate PC2 was 10.04%, which showed that the composition of the gut microbiota of rats in each group was different (Figure 4).

Figure 2 Comparison of colonic histomorphology (hematoxylin-eosin staining, ×200)
Table 3 Comparison of pathological score of rats in each group( ±s)
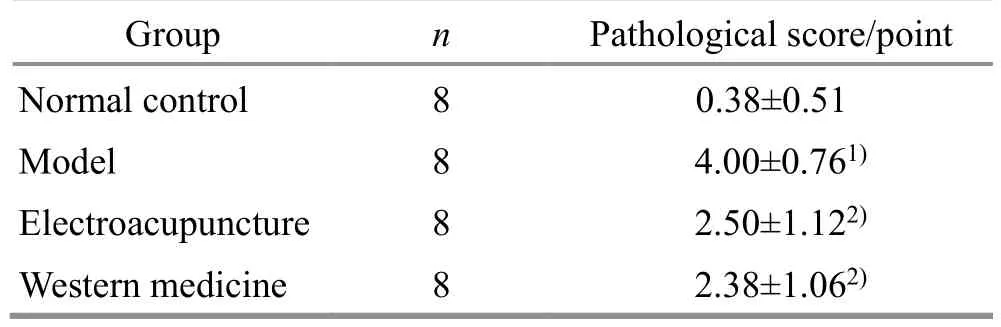
Table 3 Comparison of pathological score of rats in each group( ±s)
Note: Compared with the normal control group, 1) P<0.05;compared with the model group, 2) P<0.01.
Group n Pathological score/point Normal control 8 0.38±0.51 Model 8 4.00±0.761)Electroacupuncture 8 2.50±1.122)Western medicine 8 2.38±1.062)
Table 4 Comparison of the serum concentrations ofTNF-α and IL-1β of rats in each group ( ±s) Unit: pg/mL

Table 4 Comparison of the serum concentrations ofTNF-α and IL-1β of rats in each group ( ±s) Unit: pg/mL
Note: IL-1β=Interleukin-1β; TNF-α=Tumor necrosis factor-α;compared with the normal control group, 1) P<0.01; compared with the model group, 2) P<0.01.
Group n IL-1β TNF-α Normal control 8 165.61±51.45 20.54±7.34 Model 8 546.98±123.611) 60.63±19.521)Electroacupuncture 8 248.36±60.072) 32.25±7.672)Western medicine 8 270.27±99.532) 32.82±10.162)
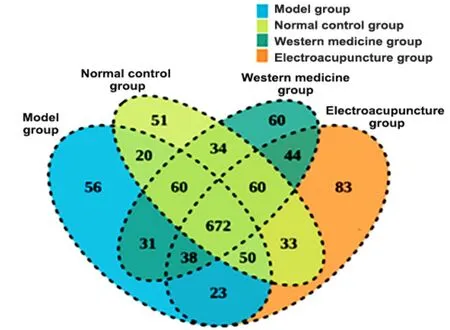
Figure 3 Venn diagram
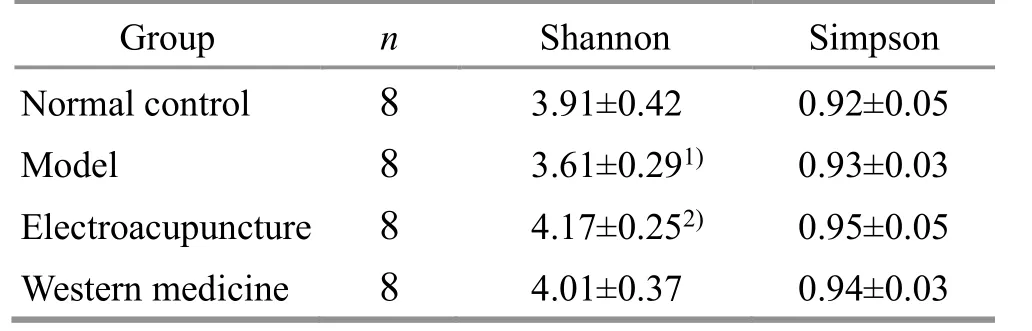
Table 5 β diversity analysis in each group

Figure 4 β diversity PCA analysis chart of gut microbiota in each group
2.5.3 Comparison of phylum level and genus level of gut microbiota in rats in each group
The relative abundance of gut microbiota at the phylum level of rats in each group was counted.The primary microbes includedBacteroidetes,Firmicutes,Proteobacteria, TM7,Tenericutes,Cyanobacteria,Elusimicrobia,Actinobacteria,Fusobacteria,Spirochaetes, etc.There was no statistically significant difference in the relative abundance of gut microbiota in the phylum level among the groups (Figure 5).
Figure 5 The relative abundance of gut microbiota at the phylum level
At the genus level, the relatively abundant species in the four groups of rats mainly included S24-7-unclassified,Lactobacillus,Prevotella,Clostridiumunclassified, CF231,Ruminococcaceae-unclassified,Bacteroidetes,Oscillospira, and YRC22.Compared with the NC group,LactobacillusandOscillospirain the CD group decreased, and the intervention of EA significantly increased the relative abundance.The relative abundance ofPrevotellaincreased in the CD group and decreased after EA intervention.Compared with the other groups,Rosariaincreased in the EA group (Figure 6).
2.5.4 Analysis of species differences of gut microbiota
LEfSe analysis can realize the comparison among multiple groups to find species with significant differences in abundance between groups.The species with an LDA threshold of 2 were selected in this analysis.The length of the histogram represents the impact of significantly different species.Different genera of bacteria were found in rats of each group.The different bacteria in the NC group wereBacillales,Sutterella, andStaphylococcaceae.The different bacteria in the CD group includedPhascolarctacterium,Firmicutes, andPhascolarctobacteriumofVeillonellaceae.The different bacteria in the EA group consisted ofProteobacteria,Lachnospiraceae,Roseburia,Paraprevotalla,ParabacteroidesofPorphyromonadaceae, andButyrisimonas.The different bacteria in the WM group includedAllobaculumofErysipelotrichaceae,Proteobacteria, andTuricibacter-unclassified (Figure 7).

Figure 6 The relative abundance of gut microbiota at the genus level
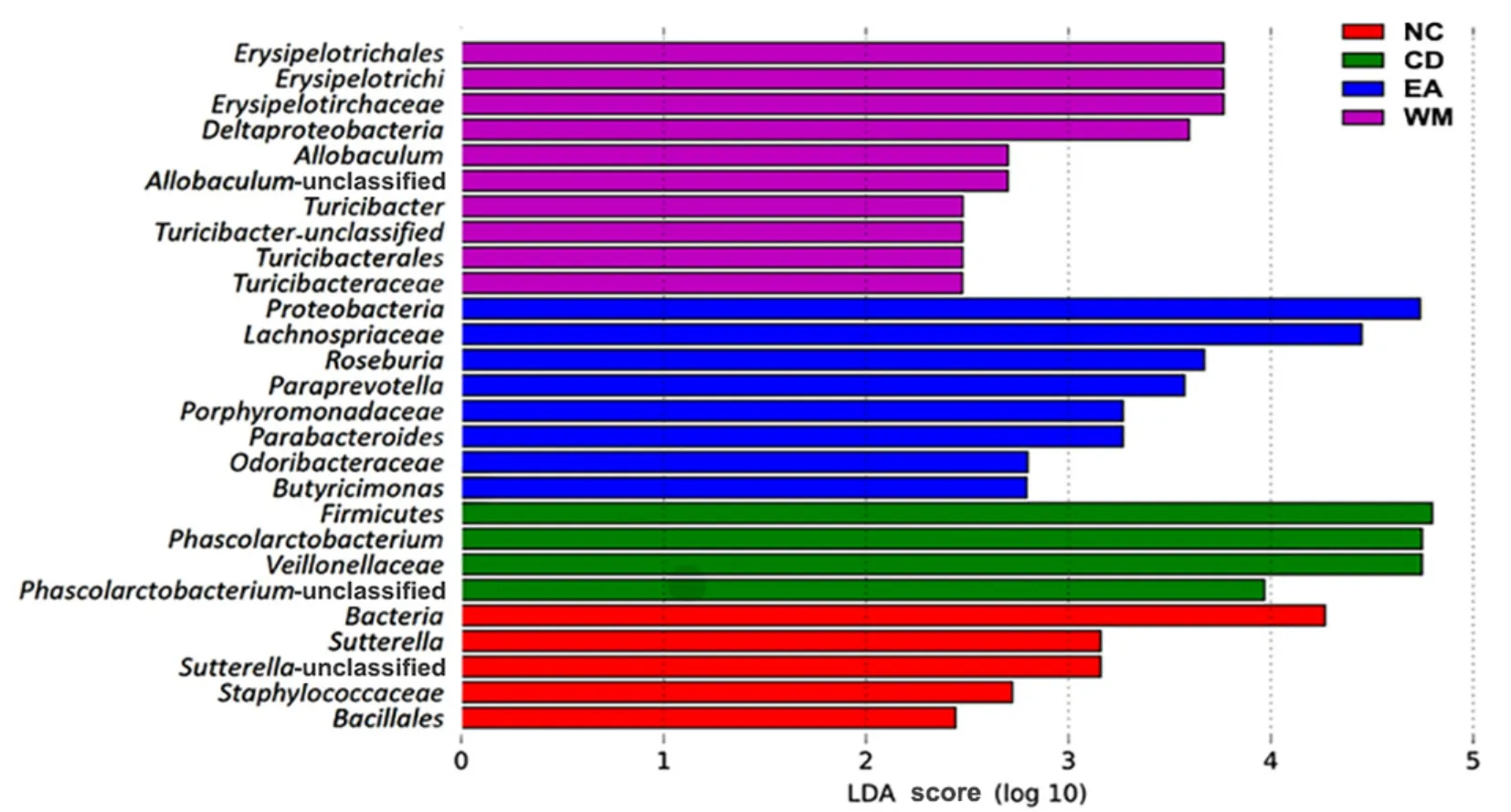
Figure 7 The LEfSe multi-level species difference discriminant analysis chart of gut microbiota in each group
3 Discussion
This study demonstrated the effect of EA in treating CD from three aspects, overall efficacy, related inflammatory factors, and gut microbiota structure.In the EA group, the stool traits of rats improved, the body mass increased (P<0.01), and the DAI score decreased compared with the CD group (P<0.01).In addition,compared with the CD group, the intestinal gland structure improved, and the inflammatory cell infiltration decreased, indicating that EA has a positive effect on CD rats.
As a type of recurrent IBD, the pathological mechanism of CD is closely related to the increased inflammation-related cytokines and protein molecules,including TNF-α and IL-1β[21-22].In this study, the serum expression of TNF-α and IL-1β in the CD group was significantly increased (P<0.01), while the expression of TNF-α and IL-1β decreased after the EA and Western medicine interventions (P<0.01), indicating that both EA and Western medicine have good effects in improving the inflammatory state in this disease.
The gut microbiota has an important role in CD’s pathogenesis, development, and prognosis, and its changes are closely related to the degree of inflammation.Therefore, after confirming the link between how EA acts in treating CD and inflammatory factors, this study sequenced and functionally annotated the microbiome in rats’ feces using 16S rDNA to analyze the diversity and composition.In the analysis of α diversity, the Shannon index in the CD group decreased compared with the NC group (P<0.05), which is consistent with the previous study[23].After the intervention, compared with the CD group, the Shannon index increased significantly in the EA group(P<0.05); the Simpson index in the EA group increased,but the difference between groups was not statistically significant (P>0.05), which may be partly related to the short intervention time in this study.Also, EA increased the α diversity of the gut microbiota of CD rats.For the PCA analysis chart of β diversity, the samples of the NC group, WM group, and EA group were concentrated,while the samples of the CD group were far from the other three groups, suggesting that EA and Western medicine can regulate the gut microbiota of CD rats.
The analysis of the results shows that EA can improve the gut microbiota of CD rats, which was mainly manifested by the reduced abundance of opportunistic pathogens (such asPrevotella) and increased abundance of beneficial bacteria (Lactobacillus,Oscillospira, andRoseburia).Lactobacillusis a natural probiotic that exists in the gut and suppresses the reproduction of pathogenic bacteria.Probiotics are increasingly used in clinical practice to treat IBD(including CD).DOU X N,et al[24]applied theLactobacillus caseiATCC 393 to treat dextran sodium sulfate-induced ulcerative colitis in C57BL/6 mice.LactobacilluscaseiATCC 393 and its metabolites reduced the inflammatory response in ulcerative colitis through the NLRP3-(Caspase-1)/IL-1β signaling pathway.In this study,Lactobacilluswas the dominant gut microbiota in the EA group.
The decreased abundance ofRoseburiain CD patients may lead to insufficient production of short-chain fatty acids (SCFAs)[25].In turn, SCFAs can regulate the gut microbiota, promote the proliferation and repair of intestinal cells, and regulate intestinal immunity to relieve the CD symptoms and maintain intestinal health and stability[26].BAO C H,et al[27]showed that acupuncture increased the relative abundance ofRoseburiain patients with mildly and moderately active CD, which was negatively correlated with the C-reactive protein level.Like the findings of the previous studies, in our study,Roseburiain the EA group was also significantly increased compared with the CD group, which confirmed the benign regulatory effect of EA onRoseburia.
Oscillospirais a Gram-positive bacterium.Studies have shown that the abundance ofOscillospirais closely related to health status.The abundance ofOscillospirain patients with ulcerative colitis and irritable bowel syndrome drops[28].A meta-analysis study on the composition of intestinal microflora in IBD patients showed thatOscillospirawas significantly reduced in CD patients[29].Oscillospirahas the function of producing SCFAs, which can protect the intestinal barrier.In this study,Oscillospirain the CD group was lower than that in the NC group, indicating that the risk of intestinal diseases was significantly increased.After EA intervention,Oscillospiraincreased significantly,indicating the benign regulatory effect of EA onOscillospira[30].
Prevotellais a common conditional pathogen.Mucosal inflammation mediated byPrevotellaleads to the systemic transmission of inflammatory mediators,bacteria, and bacterial products, which may affect the outcome of systemic diseases[31].In this study, the CD group had an increased abundance ofPrevotella, and EA reduced the relative abundance ofPrevotella.
In the present study, based on the classic TNBSinduced CD model, we observed the effect of EA on the inflammatory factors and gut microbiota diversity and composition in CD rats.According to the results, EA can improve the symptoms of CD, and its mechanism may be related to increasing the diversity of gut microbiota,reducing the abundance of conditionally pathogenic bacteria, increasing the abundance of beneficial bacteria, promoting the rebalance of gut microbiota,and down-regulating TNF-α and IL-1β expression.However, considering the significant differences between animal and human flora, we need to explore further the mechanism of EA treatment of CD in combination with clinical observation.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Projects of National Natural Science Foundation of China (國家自然科學基金項目, No.81774438, No.81603705, No.81904097); Fund Project of Hunan Province Education Office (湖南省教育廳資助科研項目, No.21A0235); Projects of Administration of Traditional Chinese Medicine of Hunan Province (湖南省中醫藥管理局科研項目, No.B2023109,No.C2022027); Natural Science Foundation of Changsha(長沙市自然科學基金, No.kq2208183); Science and Technology Talent-lifting Program of Hunan (湖南省科技人才托舉工程項目, No.2019TJ-Q04); “Furong Scholar Award Program” of Hunan Province [湖南省“芙蓉學者獎勵計劃”, No.湘教通(2020)58 號]; Science and Technology Research Program of Chongqing Municipal Education Commission (重慶市教育委員會科學技術研究項目, No.KJQN202002714, No.KJQN202102708);Graduate Student Research Innovation Project of Hunan Province (湖南省研究生科研創新項目,No.QL20220188); Research Fund of Hunan University of Chinese Medicine (湖南中醫藥大學校級科研基金,No.2021XJJJ013, No.2022CX108).
Statement of Human and Animal Rights
The treatment of animals in this experiment conformed to the ethical criteria.
Received: 14 November 2022/Accepted: 17 January 2023
——生態學
——馬克思主義學科
 Journal of Acupuncture and Tuina Science2024年1期
Journal of Acupuncture and Tuina Science2024年1期
- Journal of Acupuncture and Tuina Science的其它文章
- Study on the mechanism of herb cake-partitioned moxibustion inhibiting tumor growth in colitis-associated colorectal cancer based on KDM4D receptor
- Effects of Tuina static training on vascular endothelial cell dysfunction and adiponectin in obese rats
- Clinical study of electroacupuncture combined with exercise therapy in improving the balance function of patients with knee osteoarthritis
- Effects of warming triple needling plus Chinese medication on inflammatory responses and daily functioning ability in knee osteoarthritis patients
- Clinical observation of kidney-tonifying and mindcalming acupuncture therapy in the treatment of perimenopausal insomnia
- Clinical study of electroacupuncture improving sleep electroencephalogram and event-related potential in patients with somatoform disorders
