Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
Surajit Hansda ,Prateeksha Prateeksha ,Hiranmoy Das
Neurological disorders and injuries lead to the impairment or depletion of neurons in terms of quantity,structure,or function,resulting in the loss of cognitive,emotional,and physical abilities in human beings.This,in turn,accompanies the shortfall of valuable human resources and economic potential,causing a detriment to society.Addressing these challenges,therefore,becomes imperative not only from a humanitarian perspective but also as a means of preserving human capital and bolstering economic prosperity.Extensive scientific efforts have been directed toward developing effective,precise,and safe therapeutics for neuroregeneration,encompassing neurogenesis,neurorepair,and neuroprotection to enhance neurological recovery and health.Comprehending the molecular complexity of neuron damage and recovery is imperative to unravel the mysteries of the brain’s resilience.This understanding opens a new avenue for developing innovative treatments by targeting the specific pathways and molecules involved in several neurological illnesses.These neurological diseases include stroke,epilepsy,Alzheimer’s disease,learning impairments,neuromuscular disorders,autism,attention deficit disorder,brain tumors,cerebral palsy,and acute neural injuries.
Research in this area has shifted its focus to unveiling the connection of transcription factors in the restoration of neuron structural and functional integrity.Transcription factors are central players in the orchestration of gene expression and molecular processes critical for neurological restoration (Moore et al.,2011;Yin et al.,2015).Their regulation of various aspects of neural repair,plasticity,and protection makes them promising targets for future therapies aimed at promoting recovery and enhancing the quality of life for individuals with neurological disorders or injuries.One notable family of transcription factors is the Krüppel-like factors (KLFs),characterized by their diverse roles in governing gene expression across various cellular processes,particularly in development (Yin et al.,2015).KLF family includes seventeen members (KLF1-KLF17),each with its distinct characteristics,tissue-specific expression patterns,and functions.However,all the KLF members comprise highly conserved Cys-2/His-2 zinc-finger domains that bind to the GC-rich area and CACCC-elements of DNA sequences in the promoter (Das et al.,2006,2019).Almost all 17 KLFs are moderately and highly expressed in the retinal ganglion cells (RGCs) and brain (Moore et al.,2009),and are critical regulators of neuronal development and regeneration to maintain brain homeostasis.Dysregulation of KLFs has been associated with various neurological disorders(Yin et al.,2015).It was reported that the neurons in the central nervous system (CNS) lose their ability to regenerate early in development,but it is still not known the actual mechanisms.By screening genes developmentally regulated in RGCs,it was identified that one of the members of the KLF family,KLF4 represses the growth of axons in RGCs and other CNS neurons.Deficiency in the level of KLF4 in RGCs showed the increased level of axon growth bothin vitro,and after optic nerve injuryin vivo.It was reported that the coordinated activities of different KLFs regulate the regenerative capacity of CNS neurons (Moore et al.,2009).KLF2,a prominent member of the KLFs family,plays a significant role in a wide range of biological processes,including neovascularization,endothelial and bone cell proliferation and function,preservation of B cell homeostasis and plasma cell homing,induction of the granulocyte inflammatory response,immune cell activity,and stimulation of inflammatory T cells,according to some earlier studies (Yin et al.,2015).
Recent scientific studies have been undertaken to comprehend the expression of KLF2 during neurological illness and its interaction with other key molecules involved in neuroregeneration (Wu and Li,2022;Prateeksha et al.,2023).Notably,KLF2 mitigates hypoxic-ischemic brain damage by stimulating neuron survivability.KLF2’s interaction with the promoter region of interferon regulatory factor 4 (IRF4) facilitated the subsequent binding of IRF4 to the promoter region of histone deacetylase 7 (HDAC7),thereby enhancing the expression of HDAC7 and,as a result,mitigating neuronal apoptosis and brain damage.KLF2 overexpression leads to augmented average neurite length and the average number of primary neurites,indicating its role in neuron repair (Wu and Li,2022).The interplay among KLF2,IRF4,and HDAC7 could potentially serve as promising therapeutic focal points for addressing neonatal hypoxic-ischemic brain injury.This warrants a deeper investigation in research endeavors aimed at developing more effective treatments for hypoxic-ischemic brain damage.
In the CNS,postnatal or adult neurons fail to regenerate their axons after injury.Previous research demonstrated that KLF members govern the intrinsic axon regeneration ability;Krüppel-like factor-4 (KLF4) is recognized as a transcriptional repressor of axon growth in RGCs and other CNS neurons.However,related KLF family members suppressed or enhanced axon growth to differing extents,and several growth-enhancing KLFs were down-regulated postnatally,whereas growthsuppressive KLFs were up-regulated (Moore et al.,2011).This evidence proposed that diverse members of KLFs exhibit a specific role during axon regeneration.Notably,neurons in the peripheral nervous system exhibit the ability to regenerate their axons after injury.Therefore,a scientific study was conducted to establish the functionality of KLF2 in axon growth and demonstrated that elevated KLF2 expression at early and regenerative stages after sciatic nerve lesion enters the nucleus to further interrupt the signaling cascade involved neuron damage.As an early injury response,peripheral nerve injury leads to calcium influx that is back propagated from injured axons to neuronal soma of dorsal root ganglion,thereby,inducing KLF2 promotes Vav1 transcription.Αt the regenerative stage,injury-induced Vav1 triggers the activation of Rac1 GTPase.Collectively,these biological processes facilitate axon regeneration and improve the functional recovery of dorsal root ganglion neurons after peripheral nerve injury (Wang et al.,2021).In essence,KLF2 aids in axon growth in peripheral nervous system after injury.Moreover,the binding of brain-derived neurotrophic factor (BNDF) to the tropomyosinrelated kinase receptor type B (TrkB) is implicated in regulating axon growth in the CNS.KLF2 has also been found to protect BV2 microglial cells from OGD damage by activating the BDNF/TrkB pathway(Zhou et al.,2020).This evidence discloses the possibility of KLF2’s ability to regenerate the axon in CNS.It is worth noting that both mature oligodendrocytes and mature astrocytes contribute to an inhibitory environment for axon regeneration in the injured adult mammalian CNS.Further studies in this dimension warrant to get insights into the role of KLF2 in axon regeneration.
Brain endothelial cells interact with astrocytes,pericytes,and neurons to form a dynamic interface between the peripheral circulation and the CNS.They play a crucial role in establishing the blood-brain barrier,characterized by tight interendothelial junctions formed by various factors such as occludin.During pathological conditions,oxygen and nutrient deprivation trigger proteases,causing the breakdown of endothelial cell tight junctions,and increasing blood-brain barrier permeability.This allows inflammatory cells from the bloodstream to infiltrate the brain,initiating an inflammatory cascade that harms neurons.KLF2 is well known for its antiinflammatory action in endothelial cells and monocytes.Interestingly,KLF2 restores the bloodbrain barrier via upregulating the expression of a factor,occludin in a rat model of stroke and ameliorates the pathogenesis of stroke (Shi et al.,2013).
Similarly,a murine model of Alzheimer’s disease,Tg2576 showed a higher accumulation of amyloid-β (Αβ) in the brain,which activates the P53 molecule to suppress the expression of KLF2 (Wu et al.,2013).Lentiviral-mediated overexpression of KLF2 reversed the expression of tight junction protein,occludin induced by Αβ peptide fragment 1 to 42 (Αβ1–42) in primary human brain endothelial cells.
Numerous microRNAs (miRNAs) have been reported to mediate the molecular mechanisms of neuronal processes (Duan and Si,2019;Wang et al.,2021).The effect of miR-25 on hippocampal neuron injury in Alzheimer’s disease induced by Αβ1–42was evaluated and found that miR-25 inhibits neuron proliferation via KLF2 through the nuclear factor-E2-related factor 2 signaling pathway (Duan and Si,2019).Similarly,miR-125b overexpression restore the neurological function in spinal cord injured rat model by upregulating the KLF2 expression and downregulating the Smurf1 in neural stem cells,which provokes KLF2 degradation through its E3 ubiquitin ligase function.This,in turn,repressed the expression of ATF2 in neural stem cells (Zhao et al.,2021).The neuroprotective functions of KLF2 extend beyond its primary role.KLF2 can downregulate the expression of long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) in P12 cells.Notably,increased expression of lncRNA SNHG12 has been implicated in the development of neuropathic pain by regulating the expression of RΑD23B,a nucleotide excision repair protein,in the dorsal root ganglion of rats with spared nerve injury(Wang et al.,2021).These findings collectively highlight the significant role of KLF2 in controlling neurological damage (Figure 1).
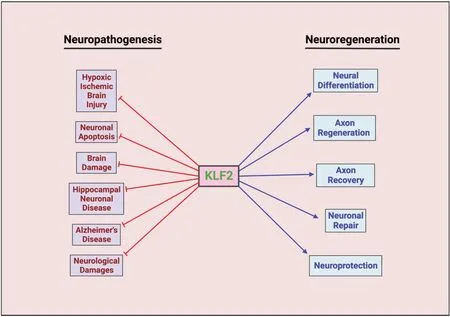
Figure 1|Effect of KLF2 on neuropathogenesis and neuroregeneration pathways.
Stem cells in the CNS have the potential to differentiate into various neural cell types,including neurons,through a process called neurogenesis.Autophagy and mitophagy play a critical role during the differentiation of stem cells to meet the desire for a high amount of energy and nutrition.Autophagy and mitophagy govern various signaling pathways necessary for effective neuron proliferation.In our recent research,our team investigated the role and expression of KLF2 in dental-pulp stem cells throughout the neural differentiation process.We observed a significant upregulation of KLF2 in dental-pulp stem cells during neural differentiation,demonstrating its influence on maintaining autophagy and mitochondrial functions (Prateeksha et al.,2023).These findings hold promise for the development of strategies involving the manipulation of KLF2 levels during neurogenesis.Such approaches have the potential to complement existing methods in the field of regenerative therapies for neurodegenerative diseases.Administration of pre-differentiated stem cells with or without KLF2 inducer,the safe and appropriate pharmacological agent in diverse models of neurological disorders may improve neuron proliferation and functionality and should be proven in the future.
In conclusion,the above scientific evidence strongly corroborates the potential of KLF2 to ameliorate neuron damage and restore neuronal health,however,the understanding of its functional significance and molecular control in the etiology of nervous system diseases is still in its infancy.Specifically,all the aforementioned studies should be validated across the diverse range of experimental models of neurological disorders.Emphasizing the need to elucidate the crosstalk of KLF2 with other signaling molecules involved in neuroregeneration is imperative to comprehend the pathogenesis of neurological disorders and to further develop KLF2-based therapies for neurodegenerative diseases.
This work was supported in part by National Institutes of Health grants,R01AR068279(NIAMS),STTR R42EY031196(NEI),and STTR 1R41AG057242(NIA)(to HD).
Surajit Hansda#,Prateeksha Prateeksha#,Hiranmoy Das*
Department of Pharmaceutical Sciences,Jerry H.Hodge School of Pharmacy,Texas Tech University Health Sciences Center,Amarillo,TX,USA
*Correspondence to:Hiranmoy Das,PhD,hiranmoy.das@ttuhsc.edu.
https://orcid.org/0000-0002-3343-0096(Hiranmoy Das)
#Both authors contributed equally to this article.
Date of submission:October 25,2023
Date of decision:December 1,2023
Date of acceptance:December 22,2023
Date of web publication:January 31,2024
https://doi.org/10.4103/NRR.NRR-D-23-01758
How to cite this article:Hansda S,Prateeksha P,Das H(2024)Krüppel-like factor 2(KLF2),apotential target for neuroregeneration.Neural Regen Res 19(11):2327-2328.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments
- Harnessing endothelial cells and vascularization strategies for nerve regeneration

