Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
Felix Kohle,Michael Schroeter
Immune-mediated neuropathies are rare diseases of the peripheral nervous system (PNS),substantially affecting patients’ functionality and quality of life.They are amenable to immunomodulatory treatments,which in many cases stabilize disease progression,but long-term deficits persist in many patients.Such long-term deficits are particularly observed in monophasic autoimmune neuropathies like Guillain-Barré syndrome and also in chronic variants,e.g.,chronic inflammatory demyelinating neuropathy (Fadia et al.,2019).In chronic immune neuropathies,we increasingly recognize a slowly progressing degeneration of neurons despite highly active immune therapies– a fact that has been rediscovered in multiple sclerosis,the prototypic central nervous system (CNS) immune disease.
Even though the PNS,compared to the CNS,offers a significant neuro-regenerative potential and constitutes,from a pharmacological pointof-view,an easier target than the CNS with its blood-brain barrier,this is most often neglected in pre-clinical and clinical studies of immunemediated neuropathies.They concentrate on the acute effector phases of the diseases to find new immunomodulatory pathways,treatments,or biomarkers for disease activity or treatment response (Kohle et al.,2023a).Further,most studies regarding neuro-regeneration in the PNS are conducted in models like sciatic nerve transection,which show substantial differences in the pathophysiology of immune-mediated neuropathies and display great limitations regarding their direct translation (Griffin et al.,2010).E.g.,Guillain-Barré syndrome and chronic inflammatory demyelinating neuropathy exhibit pathological hallmarks of heterogeneous demyelination and axonal damage due to infiltration of inflammatory cells,which are also seen in experimental autoimmune neuritis(EΑN) (Kohle et al.,2023a).In difference to nerve transection,completely interrupted nerve fibers are rarely observed,as well as secondary denervation of the muscles due to Wallerian degeneration,resulting in a higher probability for regenerating nerve fibers to follow the original paths along an intact basal lamina for correct innervations of muscle agonists and antagonists(Griffin et al.,2010).The overall duration and success of this regenerative process highly depend on motor proteins as neurons are large post-mitotic cells with an overall length of up to one meter (Hirokawa et al.,2010).Without cell division,only axonal and neurite outgrowth remain a cellular correlate for neuronal regeneration,implying that cell organelles like mitochondria must move these distances along microtubules to cover the needed energy demand (Prokop,2013).Proteins of the superfamilies kinesin,myosin,and dynein convert ATP into force and movement along the microtubules or in the case of myosin along filament.They are not only the key mediator of neuronal plasticity but also of neuronal survival and for cargo of synaptic vesicle precursors (Hirokawa et al.,2010).The motor protein superfamilies are constituted of several protein families,e.g.,kinesin-1,each involved in a different motor function.Knock-out and genetic studies found that mutations in the genes of the protein families are also involved in disease pathogenesis.Motoneuron dysfunction,the neuron type most commonly affected in immunemediated neuropathies,was described for mutations of the kinesin-1 family and correlated to spastic paraplegia,amyotrophic lateral sclerosis,and Huntington’s disease.Kinesin-3 mutations are associated with Charcot-Marie-Tooth disease and Multiple sclerosis,while cytoplasmic dynein mutations were also described in amyotrophic lateral sclerosis (Hirokawa et al.,2010).However,motor proteins are not only involved in neurodegeneration but also in neuro-regeneration.Kinesin-5 was characterized as one of the most interesting targets,as its inhibition surprisingly was described to accelerate neuronal outgrowth bothin vivoandin vitroin the CNS and PNS.The mechanism of kinesin-5,also known as Eg5,was deciphered by Myers and Baas (2007),showing that it acts as a brake,effectively limiting the rate of other motor proteins to move along the microtubules as it is mainly involved in antiparallel microtubule crosslinking.Because motor proteins are also needed for cell division,a small molecule inhibitor,monastrol,was initially developed as a chemotherapeutic and was hoped to significantly decrease tumor growth without any neurotoxic side effects,which are often observed and therapy-limiting in other chemotherapeutics.
Overall,this led us to the hypothesis that (a) the recovery phase alone in autoimmune neuropathies might be a critical determinator of the functional outcome,which could be potentially exploited,and (b) the crucial regulators of this phase are motor proteins.Of note,the interplay of motor proteins is complex,and an unselective interaction can result in neuro-degeneration,as displayed by the agent paclitaxel.
Notably,monastrol alleviated bortezomibinduced neuropathy by reducing morphological features of axonal neuropathy and improving sensory neuropathic symptoms (Bobylev et al.,2017).We therefore investigated the neuroregenerative potential of kinesin-5 inhibition in autoimmune-neuritis (Kohle et al.,2023b).EΑN,induced with injections of a neuritogenic P253-78peptide dissolved in complete Freund’s adjuvant in Lewis rats,is a monophasic disease course similar to Guillain-Barré syndrome,mimicking its pathophysiological changes of secondary axonal damage,demyelination,and nerve infiltration of immune cells.After an induction period,the first motor and sensory symptoms are observed around day 10 or 11 post-immunization (p.i.),marking the beginning of the effector phase until the disease peaks around day 18 p.i.,the inflammatory chain reactions ceases,and the recovery phase begins (Kohle et al.,2023c).This allows a good distinction between antiinflammatory and/or neuroprotective effects(applied during the induction and effector phase)and neuro-regenerative effects (recovery phase).Therefore,we treated the animals thrice with monastrol at the beginning of the recovery phase,starting on day 18 p.i.,every fourth day.Remarkably,an improved functional outcome was observed by the end of the observation period at day 30 p.i.,accompanied by a significantly enhanced recovery of the motor nerve conduction velocity,indicating an impact on remyelination.Our histological data supported this,showing a significantly thicker myelin sheath in our ultrastructural analysis and a higher myelination rate as assessed via fluoromyelin staining (Kohle et al.,2023b).To evaluate if these findings were due to an accelerated neuronal outgrowth,we treated human induced pluripotent stem cellderived secondary motor neurons with different concentrations of monastrol.In the outgrowth assay,a dose-dependent increase of the overall neurites was observed,mainly attributed to an increase in the primary neurite length,without an overt increase in neurite branching.This observation highlights the pivotal mechanistic impact of kinesin-5 inhibition on axonal trafficking and neurite outgrowth on secondary motor neurons.We next investigated the impact on the neuromuscular junction (NMJ).Our data indicated that the neuromuscular junction is targeted in EAN,as we observed denervated and partially denervated junctions on day 30 in both groups.However,the treatment group displayed more intact or partially (re-)innervated neuromuscular junctions,indicative of an induced re-innervation under kinesin-5 inhibition (Kohle et al.,2023b).
To conclude,we provided evidence for a crucial role of neuronal trafficking,specifically the motor protein kinesin-5,in the regeneration phase in autoimmune neuritis.In our study,we focused on motor deficits and functional recovery.However,sensory deficits like hypesthesia or chronic positive symptoms like pain commonly occur and persist in EAN and human immune-neuropathies.Kinesin-5 inhibition might reverse pathological pain conditions,which warrants further studies focusing on sensory behavioral assays (Wei et al.,2022;Figure 1).
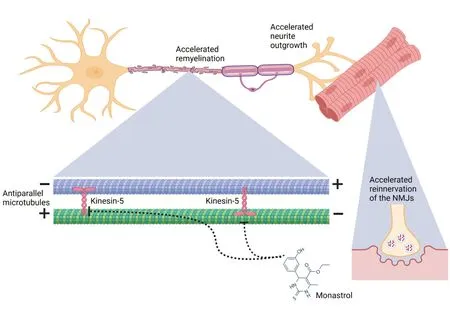
Figure 1|Impact of kinesin-5 inhibition on motor nerve regeneration in autoimmune neuritis.
The advantage of using the small molecule inhibitor monastrol is the direct pharmacological exploitation,with a potentially higher translational value.Our study observed no behavioral abnormalities in the rats,and treatment was well tolerated.Regarding potential side effects,monastrol affects neurons and mitotic spindle formation of fast-dividing cells.Therefore,we assessed the red/white blood cell ratio and found no differences between the control and treatment groups (Kohle et al.,2023b).Notably,a more specific assessment of myelosuppressive side effects is warranted in future studies.
Even though some kinesin-5 inhibitors entered clinical trials to investigate its potential as a chemotherapeutic,nearly all showed limited therapeutic potential,which was attributed to high mutation rates at the binding site of kinesin-5.Preliminary safety profiles of kinesin-5 inhibitors seem favorable,but reliable safety data from extensive clinical trials are missing (Garcia-Saez and Skoufias,2021).On the other hand,further indications for kinesin-5 inhibition,apart from PNS disease,are worth considering.In the CNS,kinesin-5 might contribute to Αlzheimer’s disease progression and impaired dendritic morphology(Yoon et al.,2005).
Our study highlights the need for further neuroregenerative and therapeutic approaches in peripheral neuropathies.Repurposing of known drugs like monastrol could be a valuable tool to address this void,especially as immune-mediated neuropathies constitute rare and heterogeneous diseases with debilitating consequences.Further,large and well-characterized cohorts and biomarkers or image-based established disease activity markers are missing (Kohle et al.,2023a).
To conclude,addressing neuronal trafficking appears to be a promising approach to enhance neuro-regeneration.This might also translate to other motor proteins as inhibition of kinesin-12in vivowas shown to accelerate neuronal outgrowths in developing neurons (Liu et al.,2010).They further constitute a promising therapeutic target for neurodegenerative disorders,e.g.,kinesin-1 or cytoplasmic dynein in amyotrophic lateral sclerosis.To minimize potential side effects and to fully elucidate their function in diseases,the use of cell-type and motor protein-specific inhibition,knock-out,or overexpression should be explored.
This work was supported by the Deutsche Forschungsgemeinschaft(DFG,German Research Foundation):project ID 431549029–SFB 1451(to MS).
Felix Kohle*,Michael Schroeter
Department of Neurology,University of Cologne and University Hospital Cologne,Cologne,Germany
*Correspondence to:Felix Kohle,MD,felix.kohle@uk-koeln.de.
https://orcid.org/0000-0002-4429-0367(Felix Kohle)
Date of submission:October 8,2023
Date of decision:December 15,2023
Date of acceptance:December 29,2023
Date of web publication:January 31,2024
https://doi.org/10.4103/NRR.NRR-D-23-01676
How to cite this article:Kohle F,Schroeter M(2024)Neuronal trafficking as a key to functional recovery in immune-mediated neuropathies.Neural Regen Res 19(11):2331-2332.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments
- Harnessing endothelial cells and vascularization strategies for nerve regeneration

