Fish-on-Chips: unveiling neural processing of chemicals in small animals through precise fluidic control
Samuel K.H.Sy,Ho Ko
Precise chemical cue presentation alongside advanced brainwide imaging techniques is important to the study of chemosensory processing in animals.Nevertheless,the dynamic nature of chemical-carrying media,such as water or air,poses a significant challenge for delivering highly-controlled chemical flow to an animal subject.Moreover,contact-based cue manipulation and delivery easily shift the position of the animal subject,which is often undesirable for highquality brain imaging.Αdditionally,more advanced interfacing tools that align with the diverse range of body part sizes of an animal,ranging from micrometer-scale neurons to meter-long limbs,are much needed.This is particularly crucial when dealing with dimensions that are beyond the reach of conventional experimental tools.
Larval zebrafish is an increasingly popular model organism in biology due to many reasons.Firstly,it is a small vertebrate species that shares substantial genetic (Howe et al.,2013) and physiological similarities across other animals in the Chordata phylum including humans,while its small physical size offers tractability that greatly facilitates the study of sophisticated organ anatomy and functions.For example,although highly complex,the brain has a compact size (~800 μm × 600 μm ×200 μm),making it an ideal model for investigating neural structures and functions (Ahrens et al.,2013).Wild-type larvae already exhibit a high level of transparency in their natural state,while the use of a transgenic zebrafish line that lacks melanophores (Lister et al.,1999) further enhances the suitability for microscopic imaging.Secondly,larval zebrafish have demonstrated a diverse repertoire of innate behaviors (Loring et al.,2020) and even some learned behaviors (Lin et al.,2020) during the early life stage of 5–9 days post-fertilization.This characteristic opens up exciting avenues for systems neuroscience and various other research studies.Thirdly,larval zebrafish offer relative genetic accessibility,ease of breeding,and straightforward maintenance,making them well-suited for large-scale chemical screenings (Rosa et al.,2022).This aspect proves particularly valuable in areas such as behavioral testing,drug discovery,and food safety research.
Fluid obviously plays a crucial role in the interaction between aquatic animals and their environment.It serves as a medium for material exchange and conveys sensory stimuli such as odor,pressure,and temperature.When utilizing larval zebrafish as a model organism,precise control of fluid flow around the animal becomes essential for unbiased,repeatable,and meaningful biological discoveries.For instance,the odor landscape to which an animal is exposed provides valuable information for analyzing the behaviors it evokes.However,fluid is inherently dynamic with its movements influenced by the surrounding pressures.This presents challenges in accurately controlling the fluid flow around larval zebrafish,especially considering their small dimensions.Additionally,precise mechanical control over the fish’s own movements adds another layer of complexity to experimental setups.To illustrate the difficulties faced in fluid control,consider the two nasal cavities of larval zebrafish,which are situated approximately 100 μm apart.Previous experimental platforms were unable to individually stimulate these close-proximity nasal cavities due to limitations in fluid control.
In the past two decades,significant advancements in microscopy techniques,such as light sheet microscopy,have empowered the visualization of brainwide anatomical and functional components at the micrometer (and even smaller) length scales (Αhrens et al.,2013).Concurrently,microfluidics (μfluidics) has emerged as a valuable biotechnological tool for precise control of fluidic streams and materials exchange within the same length scale range.In particular,μfluidic devices feature small channel dimensions that promote laminar flow,where the dominant viscous forces prevent turbulent mixing.A laminar flow regime allows for different fluid streams to co-flow in the same direction with minimal mixing,leading to highly predictable flow speeds and directions.Αs a result,μfluidics has become an appealing approach for manipulating the fluidic surroundings of small animal body parts (Yang et al.,2016).By leveragingμfluidic techniques,researchers can precisely control and manipulate the fluid environment around these structures,enabling investigations into the effects of specific fluid conditions on biological processes and phenomena.
In a recent publication (Sy et al.,2023),we introduced a platform called Fish-On-Chips that achieves precise control over fluid flow,enabling imaging of behaviors and neural activity in larval zebrafish.This system offers several noteworthy capabilities.One of the key advantages of Fish-On-Chips is its ability to control the concentration and extension of odor plumes delivered to the zebrafish larvae in vivo.In the behavioral setup,by employing laminar flow settings,we created a precisely defined,time-invariant spatiochemical landscape for conducting chemosensory behavioral assays (Figure 1;Sy et al.,2023).This allows for the active exploration of zebrafish larvae in a controlled chemical environment.Αdditionally,the μfluidic imaging setup of Fish-On-Chips enables odor stimulus delivery with exceptional accuracy (Figure 2;Sy et al.,2023).We achieved spatial precision on the scale of a few tens of micrometers,ensuring precise targeting of specific regions for odor delivery.The setup also offers fine temporal control,ensuring precise manipulation of odor presentation timing.Integrated with a custom light sheet fluorescence microscope (also known as a selective/single-plane illumination microscope) (Αhrens et al.,2013),whole-brain cellular-resolution imaging of odor-evoked and behavior-encoding neuronal activity in the larval zebrafish can be performed (Sy et al.,2023).

Figure 1|Illustration of fluid control in Fish-On-Chip for chemosensory behavioral assay.
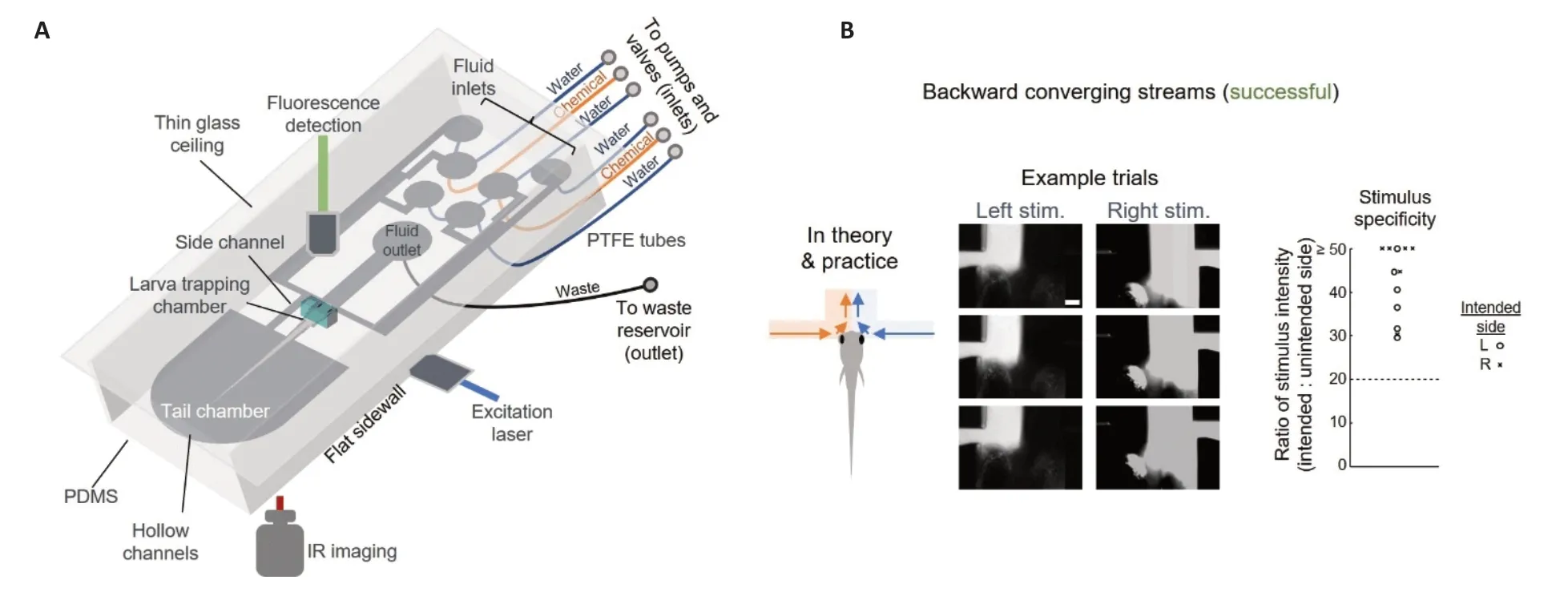
Figure 2|Illustration of fluid control in Fish-On-Chip for in vivo imaging of chemosensory-evoked neuronal activity and behavior.
As a demonstration of the platform’s strengths,we used it to study the classical problem of binasal input integration of odors (Sy et al.,2023),which has only been studied at the behavioral level.We have chosen 1 mM cadaverine,a death-associated odor,as the odor stimuli.It is an ecologically important diamine product of putrefaction and toxic to the larval zebrafish (Sy et al.,2023).While adult zebrafish have been shown to avoid cadaverine (Hussain et al.,2013),previous studies reported that larval zebrafish do not exhibit clear avoidance of cadaverine (Koide et al.,2018) or other alarm cues (Waldman,1982).Moreover,our knowledge of brainwide neural representation of binasal inputs and crosstalks underlying chemosensation has been limited(Dalal et al.,2020).We reasoned that precisely controlled odor stimulus profiles,integrated with behavioral and neuronal imaging,allow unbiased study of cadaverine-evoked behavior and provide the necessary constraints for constructing neural circuit models among possible alternatives.
The core functionality of the platform relies on the careful design of flow patterns and directions using fluidic and μfluidic channels,as well as the strategic placement of fluid inlets and outlets,to establish the desired chemical landscapes.In our investigations,we have observed that in practical applications where a non-homogeneous fluid environment is required,two approaches have to be adopted,namely divergence of the same kinds of fluid or convergence of different kinds of fluid (Sy et al.,2023).In reality,challenges arise due to irregularities in the fluid channels and the fish itself,making it difficult to achieve clear separation between two distinct fluid streams.Therefore,any separation or divergence should ideally occur within the same kinds of fluid.By employing laminar flow conditions,we can minimize mixing when two different fluid streams meet,converge,and co-flow.In our behavioral assay,we implemented a divergence of the middle water stream,leading to a clear separation between the mirror and chemical zones on either side (seeFigure 1Afor the schematics andFigure1Bfor the simulated fluid velocity profile;Sy et al.,2023).This setup establishes a static chemical landscape for the exploration of larval zebrafish.In the imaging device,we first trap the larval zebrafish and then guide the flow using dedicated channels (Figure 2A;Sy et al.,2023) to ensure that individual streams reach their corresponding nasal cavities,converge at the midline,and exit through the T-junction (Figure 2B;Sy et al.,2023).This configuration enabled us to achieve the necessary precision for delivering uninasal and binasal cadaverine stimuli,while also facilitating the imaging of the resulting neural activity.
The system facilitated several interesting discoveries.Through the use of the free arena setup,we made an intriguing observation regarding the avoidance behavior of larval zebrafish in response to cadaverine.We found that these fish unmistakably evade cadaverine by employing faster and larger undirected turns,coupled with a higher frequency of swimming in a binasal input-dependent manner (Sy et al.,2023).Importantly,we discovered that binasal inputs play an indispensable role in optimizing the avoidance behavior,exhibiting a higher swim bout frequency and a more substantial increase in angular velocity compared to uninasal inputs.Furthermore,theμfluidics-light sheet fluorescence microscope system enabled us to investigate the neural representation of cadaverine sensing (Sy et al.,2023).The forebrain,which houses the olfactory circuit,exhibited a concentration of neural activity associated with cadaverine detection.We also observed involvement from several brain regions,including the bilateral olfactory bulb,pallium,subpallium,habenula,and preoptic area.Notably,the sensory encoding of cadaverine became progressively weaker along the rostral-caudal axis of the brain.By conducting bilateral olfactory placode stimulation,we observed symmetrical activation across regions,resulting in an overall increase in sensory information content compared to unilateral olfactory placode stimulation (Sy et al.,2023).Αnalyzing the neural activity data evoked by both unilateral and binasal stimulations,we uncovered diverse nasal input selectivity patterns in the neuronal responses.These patterns ranged from highly ipsilateral or contralateral input-selective responses to equal responsiveness to bilateral inputs.Additionally,we observed nonlinear summation of bilateral afferent signals on a brainwide scale,further characterizing the representation of cadaverine.
While our system has demonstrated significant advancements,there are areas that would benefit from further improvements.Future endeavors shall focus on enhancing the design of fluidic andμfluidic devices to accommodate a greater number of fluid inlets simultaneously.This would expand the range of experiments that can be conducted.Furthermore,the addition of an extra narrow light sheet to illuminate the forebrain from the front would greatly enhance optical access and provide even more optimal imaging conditions.This would further improve the quality and resolution of neuronal activity imaging in the forebrain region,allowing for a more comprehensive understanding of the neural dynamics involved.
Precise control of fluid flow is instrumental in facilitating a wide range of biological experiments involving small animals.Fish-On-Chips,in particular,highlights the significance of establishing a time-invariant spatio-chemical landscape for unbiased assessment of animal behavior (Sy et al.,2023).Αdditionally,the platform’s ability to deliver chemicals precisely to the unilateral olfactory placode enables the detailed dissection of underlying brain circuits associated with olfactory stimulation (Sy et al.,2023).It is also important to note that fluid flow carries not only chemical cues for olfaction,but also information related to other sensory modalities,such as pressure and temperature.Consequently,the capabilities of the platform extend beyond olfactory studies and hold the potential for investigating general information processing in the brain.Moreover,the versatility of the platform extends beyond zebrafish.It can readily be adapted and applied to other small animals of comparable size,such as bacteria,C.elegans,Drosophila larvae,and adults.This broadens the scope of potential applications and enables researchers in diverse fields to leverage the platform’s capabilities for their specific investigations.
In the broader context of neuroscience and biology,integrating emerging techniques developed in other research areas that enable physical interfacing with small animals presents exciting new opportunities.By embracing these techniques,we can gain valuable insights into the complex and intricate biological worlds that would otherwise remain hidden and beyond our reach.
This work was funded by a Croucher Innovation Award(CIA20CU01)from the Croucher Foundation;the General Research Fund(14100122),the Collaborative Research Fund(C6027-19GF &C7074-21GF),and the Area of Excellence Scheme(AoE/M-604/16)of the Research Grants Council,the University Grants Committee of Hong Kong,China;the Excellent Young Scientists Fund(Hong Kong and Macao,China)(82122001)from the National Natural Science Foundation of China;the Lo’s Family Charity Fund Limited(all to HK).
Samuel K.H.Sy*,Ho Ko
Division of Neurology,Department of Medicine and Therapeutics,Faculty of Medicine,The Chinese University of Hong Kong,Shatin,New Territories,Hong Kong Special Αdministrative Region,China (Sy SKH,Ko H)Li Ka Shing Institute of Health Sciences,Faculty of Medicine,The Chinese University of Hong Kong,Shatin,New Territories,Hong Kong Special Αdministrative Region,China (Sy SKH,Ko H)Department of Electrical and Electronic Engineering,Faculty of Engineering,The University of Hong Kong,Pok Fu Lam,Hong Kong Island,Hong Kong Special Αdministrative Region,China(Sy SKH)Αdvanced Biomedical Instrumentation Center,Hong Kong Science Park,Pak Shek Kok,New Territories,Hong Kong Special Αdministrative Region,China (Sy SKH)Margaret K.L.Cheung Research Center for Management of Parkinsonism,Faculty of Medicine,The Chinese University of Hong Kong,Shatin,New Territories,Hong Kong Special Αdministrative Region,China (Ko H)Lau Tat-chuen Research Center of Brain Degenerative Diseases in Chinese,Faculty of Medicine,The Chinese University of Hong Kong,Shatin,New Territories,Hong Kong Special Αdministrative Region,China (Ko H)Gerald Choa Neuroscience Institute,The Chinese University of Hong Kong,Shatin,New Territories,Hong Kong Special Αdministrative Region,China(Ko H)
*Correspondence to:Samuel K.H.Sy,PhD,khsamuelsy@gmail.com.
https://orcid.org/0000-0002-8109-8104(Samuel K.H.Sy)
Date of submission:September 27,2023
Date of decision:November 7,2023
Date of acceptance:December 5,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392876
How to cite this article:Sy SKH,Ko H(2024)Fishon-Chips:unveiling neural processing of chemicals in small animals through precise fluidic control.Neural Regen Res 19(11):2351-2353.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Takashi Kawashima,Weizmann Institute of Science,Israel.
Additional file:Open peer review report 1.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

