Advances in spinal cord injury: insights from non-human primates
Gaetan Poulen ,Florence E.Perrin
Abstract Spinal cord injury results in significant sensorimotor deficits,currently,there is no curative treatment for the symptoms induced by spinal cord injury.Basic and pre-clinical research on spinal cord injury relies on the development and characterization of appropriate animal models.These models should replicate the symptoms observed in human,allowing for the exploration of functional deficits and investigation into various aspects of physiopathology of spinal cord injury.Non-human primates,due to their close phylogenetic association with humans,share more neuroanatomical,genetic,and physiological similarities with humans than rodents.Therefore,the responses to spinal cord injury in nonhuman primates most likely resemble the responses to traumatism in humans.In this review,we will discuss nonhuman primate models of spinal cord injury,focusing on in vivo assessments,including behavioral tests,magnetic resonance imaging,and electrical activity recordings,as well as ex vivo histological analyses.Additionally,we will present therapeutic strategies developed in non-human primates and discuss the unique specificities of non-human primate models of spinal cord injury.
Key Words: behavior;cellular analysis;non-human primates;spinal cord injury;tissue analysis
Introduction
The mean worldwide incidence of spinal cord injury (SCI),a debilitating neuropathological condition,is approximately 909,000,but significant regional differences exist (Ding et al.,2022).The most common causes of SCI in humans are traumatisms resulting from falls and motor vehicle accidents,with the majority of SCIs occurring at cervical levels.Motor,sensory,and autonomic deficits are correlated with the severity of the lesion and the rostro-caudal location of the injury.SCIs generally carry a poor prognosis for the recovery of all symptoms,resulting in a substantial economic burden(Gooch et al.,2017).
One major challenge in neuroscience is the development of clinically relevant animal models.Ideally,animal models of SCI should allow us to investigate functional sensorimotor and autonomic deficits,as well as decipher cellular and molecular pathways modulated by the lesion.These prerequisites are essential for translational purposes,enabling us to accurately assess the efficacy of therapeutic strategies.Furthermore,the “ideal”SCI model should be reproducible and easily available.While such a model may not exist,the availability of several models permits to investigate various aspects of SCI physiopathology and facilitates the development and evaluation of new therapeutic strategies.
Many SCI models have been developed,including various severities of section,contusion,distraction,and dislocation(for review see Cheriyan et al.,2014).However,given the complexity and heterogeneity of human SCI,none of the existing animal models can encompass all aspects.The first animal model of SCI,developed in 1911 by Allen,consisted of weight-drop contusion in dogs (Allen,1911).Subsequently,numerous other models were developed in different species,including but not restricted to rodents,rabbits,cats,dogs,pigs,and non-human primates (NHPs).However,rodent models are the most commonly used in the SCI field;specifically,mice provide abundant transgenic animals that permit genetic studies,and rats display some similar morphological,functional,and electrophysiological outcomes to humans following SCI.
Undeniably,NHPs,given their close phylogenetic association with humans,share more genetic,neurodevelopmental,neuroanatomical,and physiological similarities with humans than rodents.Therefore,responses to SCI of NHPs most likely resemble responses in human (Perez et al.,2021).Moreover,some NHPs share homologies with humans in functions such as bipedal walking,having a hand with an opposable thumb,emotion,cognition,and social behavior,all of which may also be modulated by SCI.Thus,for the purpose of clinical translation,experiments involving NHPs are often necessary.
Several NHP models of SCI have been developed in bothProsimiiandSimii.Macaque monkeys,due to their phylogeny,are the closest to human neurophysiology,neuroanatomical organizations,and human metabolism (Figure 1).However,macaque models come with limitations,such as their large body size,which makes housing and handling challenging.Breeding is also an issue,as macaque models have delayed sexual maturity,low reproductive output,and a long interbirth interval (for review see Fischer and Austad,2011).As an alternative to larger NHPs (Figure 1),other NHP models of SCI have been developed in marmosets (Callithrix jacchus)(Iwanami et al.,2005) andMicrocebus murinus(Le Corre et al.,2018).
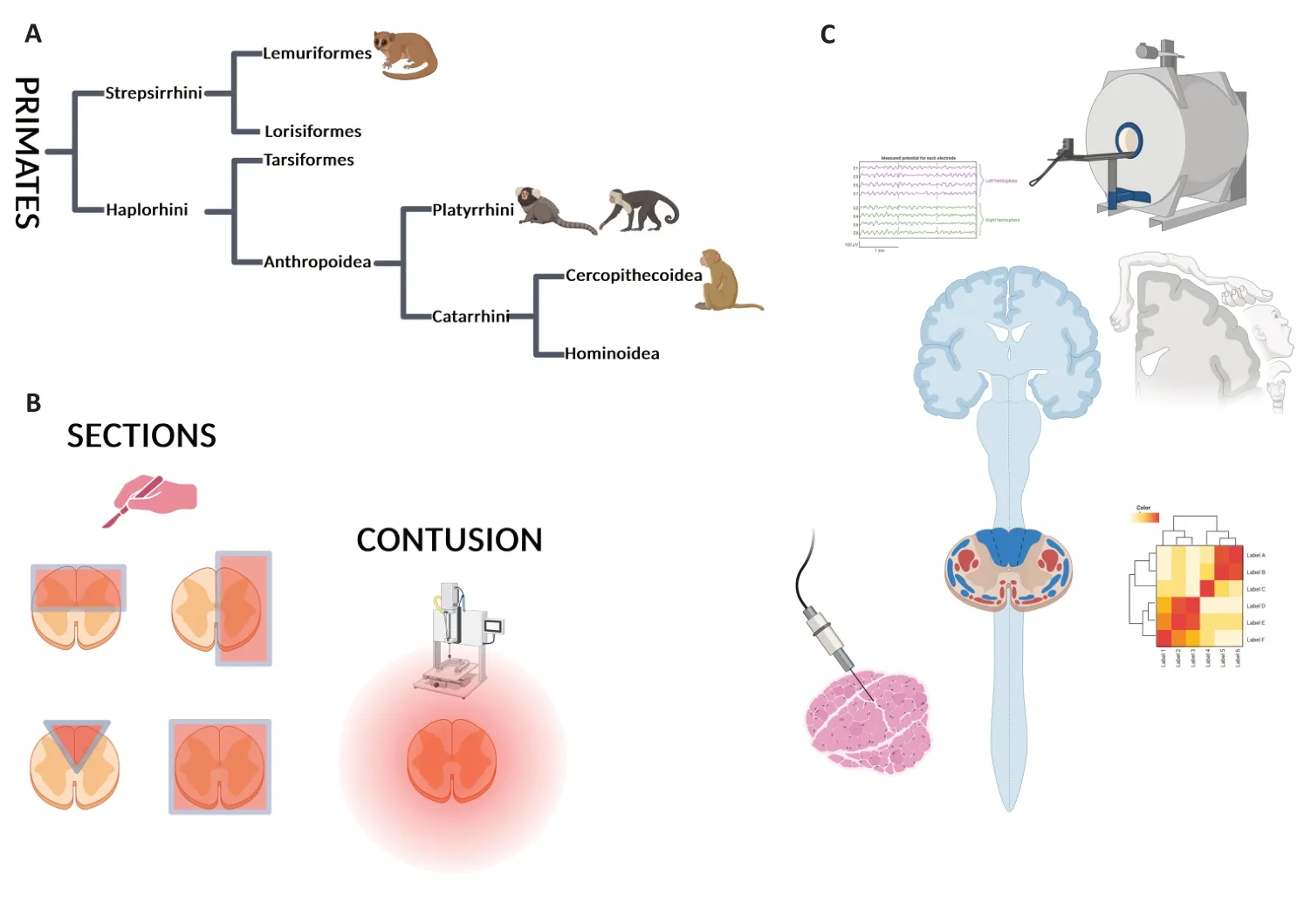
Figure 1|Non-human primate models of spinal cord injury and methodologies to assess specific outcomes.
In this review,after a brief introduction of the simplified taxonomy of non-human primates,we first introduce traumatic models of SCI in NHPs.Second,we reportin vivoassessments,such as behavioral tests and magnetic resonance imaging (MRI),andex vivohistological analyses.Third,we present therapeutic strategies developed in NHPs,excluding cell transplantation.Lastly,we highlight the unique specificities of non-human primate models of SCI.Throughout our review,we will present experiments from NHPs,ranging from the more phylogenetically distant to the closest to human.
Search Strategy
Studies cited in this review published from 1980 to 2023 were searched on the PubMed database using the following keywords: “spinal cord injury AND non-human primate”.We exclude cell transplantation studies.
Taxonomy of Non-Human Primates
As shown inFigure 1A,primates are divided into 2 semiorders,StrepsirrhiniandHaplorrhini,which further divide into two infraorders.Strepsirrhinidivides into Lemuriformes and Lorisiformes,whereasHaplorrhinidivides into Anthropoidea and Tarsiformes.Anthropoidea further branches intoPlatyrrhiniandCatarrhini,which further divide intoCercopithecoideaandHominoidea(Figure 1).Most common models of SCI have been developed in Lemuriformes (M Murinus),Platyrrhini(Marmoset),andCatarrhini(Macaque)(Figure 1andAdditional Table 1;Fleagle and Seiffert,2020).
Several SCI models have been developed in different species of NHPs to better understand the molecular,cellular,and functional mechanisms underlying SCI and to evaluate the outcomes of therapeutic strategies.Each model/species displays advantages and disadvantages.For example,asMacacasand human share similarities in hand control,this large NHP is favored for cervical SCI and post-injury investigation of hand dexterity (Figure 1C).Indeed,the evolutionary emergence of direct cortical projections to spinal motoneurons correlates with the appearance of a precise grip between the thumb and the index finger,which exists in only some primates (for review see Lemon et al.,2004;Lemon,2008).Humans,macaques,and other Old World monkeys share manual prehensile tasks (for review see Courtine et al.,2007),and both macaques and humans are characterized by a well-developed monosynaptic cortico-motoneuronal system(Figure 1C).
Spinal Cord Injury Models Developed in Non-Human Primates
Spinal cord sections
Various severities of surgical spinal cord sections,including both complete and incomplete injuries,have been developed at different rostro-caudal levels in various NHP species.
Incomplete spinal cord lesions
Lateral hemisection:Xiong et al.(2018) developed a model of hemisection at thoracic level 10 in tree shrews,a small group of Asian mammals recently classified as NHP due to certain brain similarities to primates.Following SCI,they observed spastic and flaccid paralysis in the hindlimbs on both sides of the lesion.However,motor function spontaneously but partially recovered starting from 7 days post-injury (dpi).Le Corre et al.(2018) characterized a model of lateral spinal cord hemisection (Figure 1B) at lumbar level 1 (L1) in adultMicrocebus murinus,a small lemur.The L1 lesion induced deficits in the ipsilateral hindlimb and allowed for high-quality of MRI acquisitions.Cervical lateral hemisection (Figure 1) was also performed on common marmosets (Callithrix jacchus)(Fujiyoshi et al.,2007;Takemi et al.,2014).Αfter laminectomy at either C3,C4,C5,or C6 levels,the entire dorsoventral extent of one side of the spinal cord was cut.This level of lesion induced deficits in the ipsilateral fore-and hindlimbs.Several research groups have established models of spinal cord hemisection at different rostrocaudal levels in larger primates.In macaques,injuries were conducted at various thoracic levels,including T12/L1 (Suresh Babu et al.,2000;Babu and Namasivayam,2008),T11 (Levi et al.,2002;Li et al.,2008;Xiyang et al.,2009;Jia et al.,2011),T9/T10 (Slotkin et al.,2017;Zhao et al.,2017;Zheng et al.,2023) and T7/T9 (Wu et al.,2013;Rao et al.,2015,2018;Wang et al.,2020).Initially developed at T10,a lateral hemisection model onMacaca mulattawas further extended to the C7 level since the most common level of human SCI is cervical (for review see Nout et al.,2012a).Numerous studies were conducted at cervical levels,including C5/C6 lesion in green monkeys (Wang et al.,2020),C6/C7 in macaques (Freund et al.,2006,2009;Yang et al.,2006;Nout et al.,2012a,b;Fregosi et al.,2018;Nakagawa et al.,2018;Beaud et al.,2020),C7 inMacaca mulatta(Rosenzweig et al.,2010) and C7/C8 injury in macaques(Schmidlin et al.,2004;Freund et al.,2006).
Unilateral section of the corticospinal tract (CST):In human,the CST is divided into lateral and anterior CST.The lateral CST crosses the midline and controls limbs and digits,while the anterior CST remains on the same side without crossing the midline and controls trunk muscles.In NHPs,the CST contains axons originating from both the left and right motor cortex and primarily projects through the dorsolateral column(Friedli et al.,2015).The CST serves as the major descending pathway for voluntary hand movements [for a comprehensive review see (Lemon,2008)].However,following a lateral CST lesion,it can be challenging to determine whether the entire pyramidal tract is completely injured.In this context,Brinkman et al.(1978),conducted bilateral lesions of the spinal dorsal columns at C1/C2 and C5 levels in macaques.Freund et al.(2009) and Schmidlin et al.(2004) performed unilateral section at the C7/C8 level (thus including the CST)and assessed the recovery of manual dexterity.In another study,Tohyama et al.(2017) carried out a unilateral lesion of the dorsolateral funiculus at the C4–C5 segment inMacaca fuscatato interrupt direct cortico-motoneuronal connections.
Unilateral dorsal column lesion:SCI typically results in sensorimotor deficits.Lesion of the dorsal column of the spinal cord of NHPs provides a valuable approach to investigating the somatosensory system with precision.Dorsal column lesions allow researchers to disrupt ascending tactile inputs while preserving descending pathways [for an extensive review see(Reed et al.,2016)].In alignment with this approach,Wang et al.(2016) developed a unilateral dorsal column section at the C4–C5 levels in squirrel monkeys (Saimiri sciureus)and conducted a longitudinal assessment using quantitative magnetization transfer.Similarly,unilateral section of the C4–C6 dorsal column on squirrel monkeys (Saimiri boliviensis)was established (Qi et al.,2011;Chen et al.,2012;Yang et al.,2014).In larger NHPs,Darian-Smith et al.(2014) compared corticospinal sprouting inMacaca fascicularisfollowing either a dorsal column lesion,a dorsal root lesion,or a combination of both lesions.
Complete section of the spinal cord
Only a few research groups established models of complete spinal cord section in NHPs.Han et al.(2019) conducted a complete T9 section in rhesus monkeys;while a T8 section in macaque monkeys had been utilized to assess motor function and evaluate implications for humans (Hernández-Laín et al.,2011;Piedras et al.,2011).The development and characterization of complete spinal cord section in NHPs are important,considering the significant prevalence of complete paraplegia and tetraplegia in human.Moreover,one advantage of this model is its reproducibility.However,a complete spinal cord lesion raises ethical concerns,and very strict ethical guidelines are necessary.It is crucial to monitor the bladder function of the animals to prevent urinary retention and infections,and to strictly monitor the general health status of the animals;making post-operative cares more challenging.Similar to other species,including rodents,the existence of numerous post-operative complications reported after complete spinal cord section likely explains the preference for incomplete SCI in NHPs.
Spinal cord contusion
To replicate the variabilities observed in human SCI,models of spinal cord contusion injuries have been developed in NHPs.Contusive SCI models are often considered more suitable for preclinical studies than section injuries due to their similarities with human SCI pathophysiology.Iwanami et al.(2005) established graded contusion injury models using three different weight drops on marmosets (Callithrix jacchus).They dropped weights of either 15,17,or 20 g onto the exposed C5 spinal cord from a height of 50 mm,resulting in graded severities of SCI (mild,moderate,or severe).Nishimura et al.used a 17-g weight with a diameter of 3.5 mm,which is dropped from a height of 50 mm onto the exposed dura matter at the C5 level (Nishimura et al.,2014) In larger NHPs,Ye et al.conducted thoracic spinal cord contusions at T8–T9 (Ye et al.,2016) and T10 (Ye et al.,2018) levels incynomolgusmonkeys,and Ma et al.(2016) used the same approach in rhesus monkeys.Finally,Bresnahan et al.(1976,1978) employed a model of contusion (500 g·cm) at upper thoracic levels on rhesus monkeys and conducted histological assessments.
Like in other species,each NHP injury has its advantages and disadvantages.Spinal cord sections are relatively easy to reproduce,allowing for good homogeneity in responses to injury.However,section injuries may not be the most representative of clinical situations.Partial sections of the spinal cord,unlike complete sections,not only allow for selective alterations of functions but also preserve some functions.Partial sections therefore require less complex postoperative care,particularly concerning bladder expression.Contusion injuries are more representative of clinical situations,but the limitation may be the heterogeneity of the lesions induced,even when using the same weight and height drop for the injury.
Behavioral Assessments Following Spinal Cord Injury in Non-Human Primates
As exhibited inFigure 1C,many different functional tests have been developed to assess motor function after SCI in NHPs.Often,locomotion is analyzed using behavioral tests adapted from rodents.However,the specificities of NHPs include assessments of manual prehensile tasks,such as grip and grasp,and bipedal walking patterns,at least for some species.
Motor function assessments
Spontaneous activity:Αfter SCI,spontaneous motor activity in NHPs can be assessed to evaluate the overall deficits and general health status of the animals.In this context,motor function evaluation after various types of injuries in several NHP species has been developed.Parameters,including,but not restricted to weight bearing,motion (walking,climbing,jumping,overground locomotion,perch use,sitting),trunk stability griping,and smooth movements are quantified by experimenter either by direct and/or video-recorded observation.
Functional motor activity scoring has been developed to evaluate motor impairments and recovery inMicrocebus murinusafter L1 hemisection (Le Corre et al.,2018;Poulen et al.,2021) and of common marmosets in three severities of C5 contusive injuries (Iwanami et al.,2005).To quantify spontaneous motor activity,cages with infrared sensors were developed to continuously record the 3D motion of animals,assessing the coordination of the fore-and hindlimbs.Moreover,a seven-point scale allowed evaluating the ability of the marmosets to hold on to their cage,reverse their position,and escape upwards when placed upside down on the side of the cage (Iwanami et al.,2005).Takemi et al.(2014) and Kitamura et al.(2011) adapted the rating scale originally developed in rats (Tarlov and Klinger,1954) to assess motor recovery of common marmosets after C3,C4,or C5 hemisection and C5 contusion,respectively.Likewise,voluntary movements were scored in African green monkeys after T9–T10 hemisection (Pritchard et al.,2010) and T9–T10 hemicontusion (Jacobson et al.,2021),incynomolgusmonkeys after T8–T9 contusion (Ye et al.,2016) and in rhesus macaques after C7 hemisection (Nout et al.,2012a,b).Ko et al.(2019) developed a video recording system to analyze the motion of knee and hip joints after T10 hemisection in rhesus macaques.
Walking pattern analysis:static and dynamic parameters including but not restricted to print length,print width,stride length,base of support,regularity index,stand,and swing have been assessed inMicrocebusmurinus following an L1 lateral hemisection of the spinal cord using CatWalk (Le Corre et al.,2018;Poulen et al.,2021).The CatWalk? system,originally developed for rodents,can be used for lemurs due to their ability to walk quadrupedally and their small size.
A significant advantage of NHPs compared to rodents is the investigation of bipedal walking.Scholars (Suresh Babu et al.,2000,2012;Babu and Namasivayam,2008) designed footprint analysis using ink and paper to analyze distances to the opposite foot,print length,toe-spread and distance between the intermediary toes inMacaca radiataafter T12–L1 spinal cord hemisection.The treadmill is also used to evaluate bipedal locomotion in NHPs after SCI (Suresh Babu et al.,2000;Babu and Namasivayam,2008;Nout et al.,2012b;Krucoff et al.,2017) (for review see Nout et al.,2012a;Rangasamy,2013).In particular,Rosenzweig et al.(2010) recorded rhesus monkeys walking on a treadmill and performed 3D-video reconstruction using four cameras and reflective markers attached to the skin overlying body landmarks (knee joint,greater trochanter,malleolus,fifth metatarsal,outside tip of the fifth digit,head of the humerus,elbow joint,distal head of the ulna,metacarpo-phalageal joint,and outside tip of the third digit).3D coordinates of markers were obtained with motion capture,allowing the quantification of gait timing,joint kinematics,interlimb coordination,and limb endpoint trajectory after a C7 spinal cord hemisection.
Grip and grasp tests:manual prehensile tasks are often used to assess fine motor function in NHPs.The ability to grip a bar has been scored in hemisectedMicrocebus murinus(Le Corre et al.,2018;Poulen et al.,2021).The gripping reflex in marmosets after contusion of the spinal cord was quantified(Iwanami et al.,2005).Αfter hemisection in marmosets,a motion capture system (infrared cameras with millimeter and millisecond accuracy) permitted the reconstruction of 3D hand positions and the evaluation of hand kinematics during food retrieval (Takemi et al.,2014).Several tasks to evaluate impaired forelimb function were developed particularly through the assessment of the ability to retrieve and grasp food (Sasaki et al.,2004;for review see Nout et al.,2012a).The Kluver board is a commonly used test to assess hand function and performance in monkeys.It consists of multiple slots of differing depths and widths from which the NHP extracts food using a precision grip and has been used after SCI (for review see Darian-Smith,2007).Abilities to reach,grasp and analyze finger dexterity were quantified following corticospinal tract lesions in macaques (Sasaki et al.,2004;Nishimura et al.,2007,2011;Alstermark et al.,2011;Sawada et al.,2015;Tohyama et al.,2017).Α reach-and-grasp task has been developed where monkeys were trained to reach for a small piece of food through a narrow vertical slit and to grasp it between the pads of the index finger and thumb (Sasaki et al.,2004;Nishimura et al.,2007,2011;Alstermark et al.,2011;Kinoshita et al.,2012;Sawada et al.,2015;Tohyama et al.,2017).Schmidlin et al.(2004) used the Brinkman board that contains slots allowing single-digit access to food pellets in rhesus monkeys with a cervical spinal cord hemisection to assess manual dexterity.Similarly,Freund et al.(2009)employed the modified Brinkman board task to assess manual dexterity in rhesus orcynomolgusmonkeys with a C7/C8 CST lesion.The modified board consists of 50 slots,with 25 oriented vertically and 25 horizontally,containing food pellets.This design allows for the evaluation of grip precision,as retrieving pellets requires fractionated finger movements with an opposition of the index finger and the thumb.Correspondingly,Rosenzweig et al.(2010) examined the manual dexterity of rhesus monkeys with a motor task that involved retrieving a piece of food after spinal cord section.Finally,to assess functional recovery in rhesus monkeys after SCI,the Monkey Hindlimb Score consisting of a digital function score and a hindlimb locomotor score was also been developed (Ma et al.,2016).
Sensory function assessments
Signs of discomfort or pain are always evaluated during the regular follow-up of NHPs after injury.However,very few studies have specifically investigated pain in NHPs following injury.The ability to discriminate between smooth and rough textures in contact with the glabrous skin of the foot following thoracic section of the dorsal spinal column inMacaca arctoideshas been evaluated.Responses were impaired for at least 2 months after injury,then heterogenous responses ranging from no recovery to full recovery,were reported(Vierck,1998;Vierck and Cooper,1998).Αfter T6/T7 complete spinal cord section inMacaca mulatta,somatosensory function was rated using light,deep and painful stimuli.When these stimuli were applied to the skin of lower limbs,abdomen,and thorax,animals never exhibited any reliable response (Hernández-Laín et al.,2011).No evidence of persistent pain was observed after unilateral cervical contusion inMacaca mulattausing von Frey testing (Salegio et al.,2016).Similarly,following T10-hemisection in rhesus macaques,pain was evaluated by mechanical stimulus,and no development of hyperalgesia was reported (Zheng et al.,2023).
In summary,a large number of behavioral assessments of motor function have been developed and adapted from tests used in other species.NHPs due to their proximity to human,allow the investigation of unique,clinically relevant motor function alterations and recovery of hand functions,as well as,for some species,bipedal walking.Few studies have reported the investigation of sensory impairments.This area warrants further investigation,particularly because SCI is often associated with the development of neuropathic pain in human.
Electrical Activity Assessments
Behavioral assessments can be implemented by electromyography (EMG) and evoked potential testing to establish objective evidence of sensory-motor impairments and evaluate potential motor recovery after SCI.
Electromyography:To assess functional muscle activity,Rosenzweig et al.(2010) initially generated probability density distributions of normalized EMG amplitudes of specific pairs of muscles during continuous treadmill stepping sequences.This approach allowed them to assess coactivation between muscles in rhesus monkeys with a C7 hemisection.EMG electrodes were implanted in hindlimb and/or forelimb muscles,revealing reduced EMG activity after SCI.Similarly,Nout et al.(2012b) conducted EMG recordings during pulling motor tasks using bipolar intramuscular electrodes implanted in selected muscles following C7 hemisection in rhesus monkeys.Αlso,Slotkin et al.(2017) analyzed EMG activity in green monkeys after T9–T10 lateral hemisection.Krucoff et al.(2017) employed EMG recordings in a model of transient paraplegia in macaques.In this model,anesthetic was delivered through an epidural catheter,inducing a temporary loss of voluntary leg movement.EMG electrodes allowed for the recording of quadriceps and gastrocnemius activities;with comparison made before and after anesthetic injection,revealing a reduction of EMG amplitude.
Brain electrical recordings:To assess motor and sensory SCIinduced alterations in electrical conduction within the spinal cord,evoked potential tests have also been utilized in NHPs.
To evaluate neuronal function ofcynomolgusmonkeys following SCI,Ye et al.(2016) employed transcranial electrical stimulation-motor evoked potentials and somatosensory evoked potentials (SSEPs).They compared recordings before and after a T6–T7 contusive injury.For transcranial electrical stimulation-motor evoked potentials,stimulating electrodes were placed over the motor cortex,and a short train of transcranial electrical stimulations was initiated.Myogenic MEPs were recorded in several muscles of the upper and lower limbs.For SSEPs,stimulating electrodes were placed on nerves in the upper limbs,intercostals nerves and nerves in the lower limbs.This study showed that,caudal to the lesion,evoked potentials were modified after injury and correlated with motor function.Similarly,Ma et al.(2016)recorded MEP induced by transcranial magnetic stimulation and SSEP in rhesus monkeys after T9 contusion.They found a significant decrease or abolition of MEP depending on the severity of the lesion.Moreover,hindlimb SSEP revealed a bilaterally decrease in amplitude and an increase in latency(Ma et al.,2016).During a reach-and-grasp task following SCI at the cervical level in macaques,electrocorticography in the sensorimotor cortex and local field potentials in the nucleusaccumbenshighlighted the role of nucleusaccumbensin the recovery of motor function (Sawada et al.,2015).Αs a result,assessments of electrical activities complement behavioral motor tests.Importantly,due to the challenges encountered in investigating sensory outcomes in animals using behavioral tests,electrical activity assessments may provide a reliable measure of SCI-induced sensory function.In particular,cortical recordings represent a unique methodology to investigate supraspinal reorganization induced by SCI.
Magnetic Resonance Imaging
MRI of NHPs after SCI is invaluable for highlighting signal abnormalities in the spinal cord,not only at the lesion site and its vicinity but also at more distant locations.Modifications in signal intensity allow for the evaluation of the severity of the lesion,and longitudinal MRI follow-up can provide insights into the lesion evolution over time.Additionally,functional MRI (fMRI) can be used to investigate activated brain areas and assess the supraspinal consequences of a SCI.
In vivo conventional T1 and T2 sequences
Le Corre et al.(2018) utilized a 9.4 Tesla apparatus to acquire longitudinal MRI scans inMicrocebus murinussubjected to L1 spinal cord hemisection.A Multiple Echo Multi Slices protocol was used to obtain axial images.MRI quantification of lesion percentage and lesion extension did not show the evolution over three months after injury,although a peak in lesion volume was observed 1 week post-injury.Three months after injury,the lesion quantification by MRI was consistent with that obtained through histology.In common marmosets with contusive SCI,sagittal T1-weighted (T1W),and sagittal and axial T2W fast spin echo MRI were acquired using a 1.5 Tesla apparatus (Iwanami et al.,2005;Okano et al.,2012).The MRI follow-up revealed significant differences among groups displaying three severities of contusion.Αfter C4/C5 dorsal column lesion in squirrel monkeys,T1-and T2W MRI demonstrated the formation of fluid-type cysts (Wang et al.,2015).T1 and/or T2W sequences were also employed to quantify lesion volume and extension in macaques.T1-and T2W fast spin-echo sequences in macaques identified a high signal lesion at the acute stage after T9 spinal cord contusion(Ma et al.,2016) and at 1,12,and 24 weeks after T10 hemisection (Zheng et al.,2023).MRI outcomes of injuries at cervical levels were also investigated following C5 hemicontusion (Liu et al.,2020) and C6/C7 unilateral contusion(Salegio et al.,2016).Finally,following C4/C6 dorsal column section on squirrel monkeys a T2*-weighted acquisition was performed in the cortex to evaluate injury-induced supraspinal reorganization (Chen et al.,2012).
In vivo quantitative MRI
In hemisected marmosets,diffusion tensor imaging (DTI)demonstrated the disruption of the CST 2 weeks after injury (Fujiyoshi et al.,2007).Wang et al.(2015,2016)employed several quantitative 9.4T MRI modalities,including DTI,magnetization transfer (MT),and chemical exchange saturation transfer (CEST),in squirrel monkeys after C4–C5 dorsal column lesion.The apparent diffusion coefficient and fractional anisotropy obtained through DTI reflected microstructural changes,as well as cell and white matter fiber densities.MT allowed for the assessment of macromolecular content,enhancing the visualization of the lesion,while CEST quantified the micromolecular composition of the cyst.The combination of these MRI modalities highlighted dynamic changes in tissue properties over time after the injury.Quantitative MT specifically detected demyelination processes after SCI (Wang et al.,2016).A combination of CEST and nuclear Overhauser enhancement further reduced imaging times to approximately 13 minutes (Wang et al.,2021).In rhesus macaque monkeys,DTI enabled the visualization of interrupted neural tracts at both 4 hours and 6 months after T9 contusive injury (Ma et al.,2016).Αlso in macaques,Zhao et al.(2017) used DTI to correlate changes and gait parameters after T9 hemisection with the ratio of residual fibers.
Functional MRI of the brain
Functional MRI of the brain allows for the assessment of supraspinal consequences of SCI and cortical reorganization.SCI-induced reorganizations in the brain are observed because SCI leads to the loss of sensory information reaching the corresponding cortex area as well as the alteration of signal transmission from the motor cortex.
In squirrel monkeys with a dorsal column lesion,blood oxygen level dependent (BOLD) fMRI of the brain highlighted the dynamic reorganization of digit representations in the somatosensory cortex (Chen et al.,2012;Yang et al.,2014).Similarly,BOLD fMRI after T7–T9 unilateral removal of the spinal cord in rhesus monkeys revealed changes in cerebral function states in the sensory and motor systems and atypical spontaneous activation in the cerebellum,thalamus,lateral geniculate nucleus,the superior parietal lobule,and the posterior cingulate gyrus (Rao et al.,2014,2015).
Ex vivo MRI
To characterize tissue alterations after spinal cord hemisection inMicrocebus murinus,ex vivoT2W-MRI was used to discriminate between injured and spared tissues.The lesion size was similar when quantified using bothin vivoandex vivoT2W MRI (Le Corre et al.,2018).In the same model,ex vivodiffusion-weighted MRI found no difference between lesions in lemurs treated with an inhibitor of microglia proliferation and untreated animals (Poulen et al.,2021).Post-mortem diffusion tensor tractography on common marmosets after a cervical hemisection revealed a disruption of white matter tracts,particularly the CST,at the lesion site (Fujiyoshi et al.,2007).Ex vivoT2W MRI analysis highlighted that elezanumab injection following SCI in African green monkeys improved the structural integrity of extralesional tissue (Jacobson et al.,2021).
The use of several modalities of functional MRI following SCI in NHPs could certainly be beneficial for preclinical studies,especially considering the availability of fMRI in clinical routine.In particular,CEST imaging permits the detection of modifications in the concentration of molecules such as glycogen (van Zijl et al.,2007),amide (Jones et al.,2006),and specific brain metabolites associated with neurotransmitter degradation.Further studies using fMRI will certainly help investigate supraspinal reorganization induced by SCI.
Histology,immunohistochemistry,and tracing
To characterize damaged tissues and functional consequences of SCI,several staining/immunohistochemistry,and tracing studies have been conducted.
The degree and extent of spinal cord lesions have been quantified using various dyes including toluidine blue inMicrocebus murinus(Le Corre et al.,2018),hematoxylin and eosin as well as an amino cupric silver stain in green monkeys(Slotkin et al.,2017),and Nissl staining in rhesus macaques(Nout et al.,2012b).To investigate myelin alterations after SCI,fluoromyelin staining has been utilized in lemurs (Poulen et al.,2021),luxol fast blue staining in marmosets (Iwanami et al.,2005),eriochrome cyanine staining inMacaca mulatta(Salegio et al.,2016),while others have employed solochrome cyanine staining and myelin binding protein immunolabeling in green monkeys (Slotkin et al.,2017).
Immunostainings have also been used to quantify various cell populations such as neurons and glial cells.An SCI-induced increase in gliosis,involving both microglia and astrocytes,has been reported inMicrocebus murinususing IBA1 and glial fibrillary acidic protein (GFΑP),respectively (Le Corre et al.,2018).Astrogliosis (assessed using GFAP),a decrease in the number of ChΑT-positive motor neurons,and the loss of CST fibers (detected through calmodulin-dependent protein kinase IIα) were identified by immunostaining after injury in marmosets (Iwanami et al.,2005).To assess the therapeutic potential of implanting biodegradable scaffolds in green monkeys after SCI,non-phosphorylated neurofilament H(SMI32) was used to assess the presence of axons,and GΑP43 staining was performed to evaluate neurite sprouting,in addition to makers for glial cells (GFΑP,IBΑ1) and myelin (SMI-99) markers (Slotkin et al.,2017).
CST tracing following injection of biotinylated dextran amine(BDA) has been conducted in macaques following incomplete cervical sections (Levi et al.,2002;Courtine et al.,2005b;Freund et al.,2006,2009;Brock et al.,2010;Darian-Smith et al.,2014;Salegio et al.,2016;Nakagawa et al.,2018;Fisher et al.,2020;Wang et al.,2020).Similarly,on macaques displaying various types of SCI,the analysis of corticospinal projections using BDA and lucifer yellow dextran tracing highlighted that corticospinal sprouting depends on the type of injury (Darian-Smith et al.,2013,2014).
Gene Expression
To understand molecular mechanisms underlying the pathophysiology of SCI,it is crucial to investigate the modification of gene expression patterns in the injured spinal cord is critical.Gene expression analysis conducted at 1,2,4,and 6 weeks after spinal cord contusion in marmosets,utilizing microarray and RNAseq techniques,has revealed that the inflammatory response is prolonged,and glial scar formation is delayed in NHPs as compared to rodents(Nishimura et al.,2014).Additionally,a post-injury decrease in myelin-associated proteins (MBP,proteolipid protein 1) has been reported (Nishimura et al.,2014).Interestingly,similar results were obtained inMicrocebus murinus1 week after hemisection (personal data).
Therapeutic Strategies
Therapeutic strategies had been developed and tested in characterized NHP models of SCI.These strategies aim to promote axonal regeneration and/or sprouting and to modulate the expression of detrimental molecules induced by an SCI.Cell transplantations,which are the focus of numerous studies,have been excluded from our review.
In one approach,the implantation of a biodegradable scaffold onChlorocebus sabaeus(green monkey) promoted tissue remodeling and functional recovery after a lateral hemisection of the spinal cord (Slotkin et al.,2017).Han et al.(2019)demonstrated significant electrophysiological and locomotor recovery after complete transection in rhesus monkeys that were implanted with a linear-order collagen scaffold combined with collagen-binding neurotrohpin-3.Similarly,chitosan served as a matrix scaffold loaded with neurotrophin3 (NT3)and was transplanted into rhesus macaques that underwent spinal cord hemisection at thoracic level T8 (Rao et al.,2018),resulting in vigorous axonal regeneration associated with sensory-motor recovery (Rao et al.,2018).
Pharmacological strategies have also been tested in NHPs following SCI.Inhibition of microglia proliferation using an oral delivery of GW2580,a CSF-1R inhibitor,after lateral hemisection,improved functional motor recovery and promoted tissue protection in lemur (Poulen et al.,2021).Another study used intrathecal administration of recombinant human hepatocyte growth factor,a potent neurotrophic factor in the CNS.Treated marmosets with contusion injuries displayed improved motor recovery,preservation of corticospinal fibers,and myelinated areas(Kitamura et al.,2011).Similarly,intrathecal administration of papaverine,a vasodilator,in macaques with contusion injuries,reduced secondary injury (Yong et al.,2008).Ye et al.(2018) demonstrated that methylprednisolone inhibits the proliferation of endogenous neural stem cells incynomolgusmonkeys with SCI.
In the frame of antibody-based therapies,the administration of an antibody neutralizing the neurotrophic factor plateletderived growth factor-B to hemisected rhesus monkeys resulted in decreased motor function recovery,suggesting the crucial role of platelet-derived growth factor-B in SCI(Xiyang et al.,2009).Spontaneous axonal regrowth in the adult CNS is rather limited after SCI,partly due to inhibitory factors released or upregulated following the injury.Repulsive guidance molecule-a (RGMa) has been reported to be expressed by glial cells at the spinal cord lesion site(Siebold et al.,2017).Nakagawa et al.(2018) hypothesized that inhibition of RGMa may promote axonal sprouting and regrowth.They delivered anti-RGMa antibody through a catheter connected to an osmotic pump directly to the lesion site,in macaque monkeys after a lateral CST lesion at the cervical level.This led to an increase in axonal sprouting associated with improved manual dexterity recovery.Recently,systemic intravenous administration of elezanumab,a human anti-RGMa monoclonal antibody,within 24 hours following hemicompression of the T9/10 thoracic SCI in African green monkeys efficiently reduced SCI-induced RGMa in cerebrospinal fluid,improved microstructural integrity of extralesional tissue,increased the density of CST fibers emerging from the ipsilesional and the sprouting of serotonergic fibers rostral to the lesion (Jacobson et al.,2021).Another recognized inhibitory factor to axonal growth is the myelin-associated growth inhibitory protein Nogo-A.Freund et al.(2006,2009) tested the effect of anti-Nogo Α antibody as a treatment after a unilateral cervical cord lesion on 13 rhesus andcynomolgusmonkeys.Animals were implanted with an osmotic pump immediately after the lesion.Six monkeys benefited from an immediate infusion of anti-Nogo-A antibody,one received the infusion of anti-Nogo-A starting 7 days post-lesion,and other animals received the control antibody.Αnti-Nogo-Α treated animals presented a faster and better recovery of manual dexterity,along with enhanced regenerative axonal sprouting and elongation in the damaged spinal cord.The combination of anti-Nogo-A and delivery of BDNF was also tested and demonstrated a better recovery with the anti-Nogo-A treatment alone (Beaud et al.,2020).These results in NHPs have led to clinical trials showing that intrathecal injection of anti-Nogo-Α was well tolerated (Kucher et al.,2018).Another study in NHPs modulating the activity of the Nogo-A receptor promoted recovery (Wang et al.,2020).It was followed by a clinical trial where the molecule was delivered at the chronic stage after SCI,and similarly,the molecule was well tolerated (Maynard et al.,2023).Finally,the results of the SCI NISCI study (Nogo Inhibition in Spinal Cord Injury: NCT03935321) will be available soon.
Non-Human Primates Are Adapted Models for Studying Supraspinal Consequences of Spinal Cord Injury
After SCI,the brain undergoes complex changes that affect various neural networks.The motor cortex experiences significant alterations and losses of inputs after SCI,resulting from the disruption of the communication between the brain and muscles.For instance,Rao et al.(2015) reported modifications in the regional synchrony of brain activity,including the sensorimotor cortex,in rhesus monkeys following a thoracic SCI using a resting-state functional MRI.Studies in marmosets with thoracic contusions undergoing treadmill exercise have demonstrated improved locomotor recovery (Kondo et al.,2022).In addition,when intracortical microstimulation triggered hindlimb muscle movements,it further promoted brain network reorganization (Kondo et al.,2022).fMRI studies in primates have also described reorganizations in somatosensory areas 3b,1 et S2 after SCI (Yang et al.,2014;Wu et al.,2017).Chao et al.(2019) suggested that cortical compensation involving interdependent motor networks from the contralateral hemisphere may promote functional recovery after SCI in monkeys.
Beyond the sensory and motor cortex,Suzuki et al.(2020)demonstrated that the ventral striatum plays a key role in finger dexterity functional recovery in monkeys after SCI.Αdditionally,Sawada et al.(2015) reported the involvement of thenucleus accumbensin motor recovery in monkeys,making it a potential target for therapeutic strategies.
Furthermore,cognitive consequences of SCI are noteworthy,as patients often report difficulties in attention,memory,and executive functions.NHPs provide a unique model to investigate cognitive consequences.Understanding the supraspinal consequences of SCI is pivotal for the development of effective rehabilitation strategies and therapeutic interventions to enhance the quality of life.
Issues Associated with Non-Human Primate Research for Spinal Cord Injury
The use of animal models in scientific research,particularly NHPs,has been a subject of debate and ethical concern.Critics argue that it raises questions about animal welfare and the morality of experimenting on high-profile species.However,it is important to acknowledge the crucial role of NHP models in advancing medical knowledge.In the pursuit of new therapeutic strategies for a wide range of diseases,such as SCIs,NHP models allow researchers to investigate the safety and efficacy of potential treatments before progressing to human clinical trials.Therefore,while the use of NHP models necessitates strict ethical guidelines and efforts to minimize suffering,they remain an indispensable step on the path toward medical breakthroughs.
Research into SCI often faces a significant drawback due to difficulties associated with caring for critically injured animals.Incomplete spinal cord sections in NHPs are a common choice,with variations in surgical techniques based on study goals.These sections generally provide less experimental variability than contusion-based approaches.The use of complete SCI in NHPs has garnered criticism due to the significant deficits and morbidity they induce,as well as the associated animal suffering.While these models offer advantages in terms of controlled injury levels,they often result in severe and irreversible damage,leading to profound and enduring motor and sensory impairments in animals.Moreover,NHPs exhibit better spontaneous recovery from SCI compared to humans,which may partially limit their ability to accurately replicate the complex pathophysiological changes observed in human SCI.Therefore,the use of complete SCI in NHPs not only raises ethical concerns regarding animal welfare but also limits the translational relevance of the findings and should be strictly limited.
Exploring novel treatment interventions necessitates comprehensive sensory assessments in addition to motor evaluations.Efforts are being made to expand sensory assessment techniques,including mechanical allodynia and cold hyperalgesia testing,recognizing the importance of accounting for changes in sensory function in the context of SCI research.One of the remaining challenges lies in assessing“pain”in animals,including NHPs,as it involves reporting the affective state associated with altered sensation,which differs from human pain perception.
Author contributions:GP wrote the first draft of the manuscript.FEP participated in the writing of the final version of the manuscript.Both authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long asappropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1:Studies using NHP models of SCI.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

