Mechanism of Cu entry into the brain: many unanswered questions
Shubhrajit Roy ,Svetlana Lutsenko
Abstract Brain tissue requires high amounts of copper (Cu) for its key physiological processes,such as energy production,neurotransmitter synthesis,maturation of neuropeptides,myelination,synaptic plasticity,and radical scavenging.The requirements for Cu in the brain vary depending on specific brain regions,cell types,organism age,and nutritional status.Cu imbalances cause or contribute to several life-threatening neurologic disorders including Menkes disease,Wilson disease,Alzheimer’s disease,Parkinson’s disease,and others.Despite the well-established role of Cu homeostasis in brain development and function,the mechanisms that govern Cu delivery to the brain are not well defined.This review summarizes available information on Cu transfer through the brain barriers and discusses issues that require further research.
Key Words: ATOX1;ATP7A;ATP7B;blood-brain barrier;brain;choroid plexus;copper;SLC31Α1
Introduction
Brain is a copper-enriched tissue that uses copper for many essential functions.The weight of the adult human brain is approximately 2% of the total body weight,yet the brain contains almost 9% of body copper (Cu) content (Zatta et al.,2008).The average Cu concentration in the brain (3–5 μg/gtissue) is the second highest among tissues after the liver (6–9μg/g tissue;Kardos et al.,2018),reflecting the importance of Cu for brain functions.Memory and learning,responses to stress and injury,emotional well-being,circadian rhythms,lipid metabolism,and other important physiological processes rely on Cu homeostasis.They are negatively affected,when Cu balance is perturbed (Beltramini et al.,2004;Yamada and Prosser,2020;Squitti et al.,2021;Xu et al.,2021;Zhao et al.,2021;Posadas et al.,2022;Liu et al.,2023b;Washington-Hughes et al.,2023).Under physiologic conditions,Cu can cycle between the two oxidation states: the oxidized cupric(Cu2+) and reduced cuprous (Cu+) forms.In biological fluids,the exchangeable Cu is present in the oxidized form,whereas intracellular Cu is mostly reduced.The brain,like all other tissues,utilizes the unique ability of Cu to easily donate and accept electrons in biochemical reactions that require electron transfer (Lech and Sadlik,2007;Rihel,2018).Cu cofactor enables activities of enzymes that participate in energy production (cytochrome c oxidase in mitochondria),iron metabolism (ceruloplasmin and hephaestin,enriched in basal ganglia),radical defense (superoxide dismutase 1,SOD1),vasculature formation (SOD3,LOX),synthesis of neurotransmitters (dopamine-β-hydroxylase and peptidylα-monooxygenase),synaptic plasticity (prion protein,PrPc),and others (Gupta and Lutsenko,2009).Another metal,iron,can accept and donate electrons and cycle between different oxidation states.However,the electronic configuration,redox potential,and coordination chemistry differ for Fe and Cu,allowing them to participate in different chemical reactions and have non-overlapping roles in biological systems.
In addition to its role in enzyme catalysis,Cu can modulate neuronal signaling and neuron-glia coupling by binding to NMDA,AMPA,and GABA receptors (Vlachova et al.,1996;Doreulee et al.,1997;Sharonova et al.,2000;Horning and Trombley,2001;Szabo et al.,2021) and regulate the metabolic status of cells (Xiao et al.,2018;Colombo et al.,2021;Raschke et al.,2023).In other words,Cu-dependent enzymes,Cu transporters,and their regulators are integral components of brain homeostatic and signaling networks.However,when present in excess Cu can be toxic,and the amount of Cu within the tissue is tightly controlled.In recent years,information on the regulation of Cu homeostasis in neuronal cells and glia has been rapidly accumulating (Niciu et al.,2006;El Meskini et al.,2007;Hatori et al.,2016;Comstra et al.,2017;Schmidt et al.,2018;Xiao et al.,2018;Hartwig et al.,2021;Chakraborty et al.,2022;McCann et al.,2022).However,specific mechanisms of Cu distribution within and between the various brain cells are still greatly under-explored.A better understanding of how Cu is distributed and utilized in various cells in the brain,both in norm and disease,may facilitate understanding of human neurological disorders,in which Cu dis-homeostasis is observed (Scheiber et al.,2013).In this review,we summarize the available information on how Cu enters the mammalian brain in order to highlight gaps in our knowledge and formulate questions that need to be addressed by further studies.
Search Strategy
The articles cited in this narrative review were identified through electronic searches of PubMed and Google Scholar databases for literature published in English prior to November 2023.The search was performed using the following terms:Copper transporters,blood-brain barrier,blood-CSF barrier,choroid plexus,Ctr1,Atp7b,Atp7a,brain,copper,Wilson disease,and Menkes disease.The results were further screened by reading the abstracts;and the content of relevant articles was used for this review.Single cell sequencing data were obtained through the single cell portal maintained by Broad Institute;the corresponding links to the dataset are included in the text of this review.
Copper Distribution throughout the Brain
Early atomic absorption studies and more recent quantitative metal-imaging studies using X-ray fluorescence and Laser Ablation Inductively Coupled Plasma Mass Spectrometry determined that Cu levels vary throughout the brain regions(Uitti et al.,1989;Portbury et al.,2016;Ashraf et al.,2019;Neely et al.,2019;Kim et al.,2020;Scholefield et al.,2021).Currently,there is a significant disagreement between the reported Cu concentrations in the brain.While most studies estimate average Cu to be within 2.5–3.5 μg/g (Αshraf et al.,2019),others show much higher values,such as 10–25 μg/g(Uitti et al.,1989;Liu et al.,2023a).This discrepancy is likely due to different imaging and/or normalization methodologies and needs to be eventually reconciled.Nevertheless,different methods agree on a relative enrichment of Cu in specific brain regions.Dentate gyrus molecular layer of the hippocampus,locus coeruleus,and subventricular zone (SVZ) in ventricles have higher Cu content than other brain regions (Palm et al.,1990;Madsen and Gitlin,2007).
High Cu concentration in specific brain regions can be linked,in part,to high expression of Cu requiring enzymes,such as peptidylglycine-α-amidating-monoxygenase in olfactory bulb and dopamine β hydroxylase in the locus coeruleus,respectively.Αt the same time,careful comparison of Cu levels and cuproenzyme levels (that was done in olfactory neurons)found that in secretory granules of these neurons Cu exceeds peptidylglycine-α-amidating-monoxygenase concentration by 10-fold (Bonnemaison et al.,2016).This finding points to a yet-to-be identified mechanism of Cu storage in secretory granules,and raises questions about physiologic significance of Cu accumulation in granules.In other cases,high Cu content appears to reflect the role of specific brain region/cells in Cu buffering.The highest Cu concentration is found in subventricular zone,in the intracellular structures called CSVs– “copper storage vesicles”– where Cu can reach astonishingly high 100 mM concentration (Sullivan et al.,2017b;Leary and Ralle,2020);the concentration and the size of the zone further increase with age (Pushkar et al.,2013;Leary and Ralle,2020).The Cu abundance in the subventricular zone inversely correlates with the expression of Ctr2 (Slc31a2),which is thought to facilitate Cu release from vesicles (Ohrvik et al.,2013),and was also linked to the expression of metallothioneins,the high capacity Cu storage proteins (Sullivan et al.,2017b).Experiments with different dozes of Cu chelator infused into ventricles suggested that Cu in SVZ is needed for stable adult neurogenesis (Liu et al.,2022a).Which specific cells types have a capacity to maintain such high Cu levels (possibly a subset of astrocytes (Sullivan et al.,2017a)),how exactly Cu is stored/buffered,and what physiological signals regulate Cu sequestration and release are fascinating questions for further studies.
Ceruloplasmin (Cp) is a Cu-dependent ferroxidase and a major Cu-containing protein in a blood,where it facilitates iron release from tissues.Peripheral Cp is secreted predominantly by the liver;Cp receives its Cu cofactor in hepatocytes from the Cu-transporter ATP7B (Hellman and Gitlin,2002).In the brain,astrocytes and microglia are major sources of Cp;in these cells Cp is either secreted or attached to the membrane surface by glycosylphosphatidylinositol anchor.Cp is also expressed in choroid plexus.In all these locations,Cp is likely involved in regulation of brain iron homeostasis,and the inherited Cp deficiency (aceruloplasminemia) is associated with significant deposition of iron in the brain.The data on the status of copper in aceruloplasminemia patients or animals are very limited and inconsistent.One study found copper rich particles in putamen,basal ganglia and cerebral cortex of Cp deficient patients (Yoshida et al.,2017),whereas mice with a conditional knock-out of Cp in astrocytes have no Cu deposition in cerebral cortex and hippocampus (Li et al.,2022).
In neurons,further redistribution of Cu occurs at the subcellular level.Available data suggest that Cu is more concentrated in grey matter (neuronal bodies,dendrites,and synapses),where signaling information is processed,compared to the brain’s white matter (myelinated axons),where information is transmitted (Bonilla et al.,1984).Cu enrichment is observed in dendritic protrusions in association with cytoskeletal elements,such as F-actin (Domart et al.,2020).High levels of the Cu-chelating proteins,metallothioneins Mt1 and Mt3,are found in epithelial cells of choroid plexus and ependymal cells and strongly support the role of these cells in regulating Cu entry into the brain (see below).Very little is known about Cu pools,and the relationship between the various modes of copper sequestration and storage remains to be elucidated.In general,neuronal and non-neuronal cells have different roles in brain Cu physiology.Cu in neurons is essential for brain functional activities: production of neurotransmitters,signaling,and synaptic plasticity (D’Αmbrosi and Rossi,2015).In contrast,astrocytes and ependymal cells are brain Cu homeostatic cells,which readily absorb and release Cu,and may be involved in Cu buffering and storage (Dringen et al.,2013;Chakraborty et al.,2022).Oligodendrocytes are unique in their high sensitivity to Cu depletion (Benardais et al.,2013;Colombo et al.,2021).Treatment of animals with Cuchelating agent cuprizone induces demyelination owning to a diminished ability of oligodendrocytes to produce myelin(Jhelum et al.,2020;Zirngibl et al.,2022).Extensive loss of myelin is also seen in patients with Menkes disease (MD)(Hodgkinson et al.,2015),who have significant brain Cu deficiency caused by an impaired Cu entry into the brain.The appreciation of many roles that Cu has in the brain has been growing exponentially.We are now entering an exciting phase of studies of brain Cu homeostasis,when important mechanistic questions are being posed and answered.One of these questions is how is the entry of Cu in the brain regulated during brain development and aging?
Cu Entry into the Brain Parenchyma Varies during Brain Development and Aging
Major physiological processes like myelination,establishing synaptic connectivity,cell proliferation,and neurotransmitter synthesis take place during early development in human and animals (Semple et al.,2013;Jiang and Nardelli,2016).These processes directly or indirectly rely on Cu availability,and the development of the brain can be compromised significantly under suboptimal Cu levels.The importance of Cu for embryonic brain development was noticed in early studies of enzootic ataxia,spastic paralysis of hindlimbs in lambs.The brains of the affected animals had reduced cerebral hemispheres with notable hypomyelination (Howell and Davison,1959).Further studies revealed that Cu supplementation to mothers of ataxic lambs decreased the severity of symptoms.In rats,the E9-10 embryos obtained from dams maintained on a low Cu diet showed abnormal neuronal development (Hawk et al.,2003).Importance of Cu entry during early postnatal development is also apparent from a phenotype of mottled-brindle (mo-br)mice,an animal model for MD,characterized by global inactivation of Cu transporterAtp7aand systemic Cu deficiency.These animals show neuronal loss and axonal degeneration,enlarged dendrites of Purkinje neurons(Niciu et al.,2006).Severely Cu-deficientAtp7a–/–mo-brmice do not survive beyond 14 days unless rescued by injection of Cu in a form of Cu histidine or Cu elescomol or byAtp7agene delivery into the brain ventricles (Donsante et al.,2011).Adding small Cu-containing molecules along with viral delivery enhances the beneficial effect of gene therapy(Haddad et al.,2018;Guthrie et al.,2020).
Human patients with MD (or corresponding animal models),who suffer from severe developmental delays caused by systemic Cu deficiency (Hodgkinson et al.,2015) can also show some improvement in their symptoms if they received early Cu supplementation (Kaler,2014;Kim et al.,2015;Guthrie et al.,2020;Gohil,2021).However,the success of Cu supplementation in MD is limited,demonstrating that Cu not only has to enter the brain but also be distributed to the correct intracellular destinations.Studies comparing mo-br mice with a systemic Cu deficiency and mice,which lackedAtp7agene within neural and glial cell precursors provided compelling evidence that “the severe neuropathology in themo-brmice is not due to intrinsic loss of Atp7a within neurons and glia but,rather,to a loss of Atp7a-dependent copper transport into the CNS”(Hodgkinson et al.,2015).
Postnatally,the rates of Cu entry in brain parenchyma change with age,as demonstrated by the radiotracer-based imaging experiments (Peng et al.,2018).Studies in animals confirmed that there is an age-dependent increase in the concentration of Cu in the brain (Haywood and Vaillant,2014;Peng et al.,2018;Liu et al.,2022b).In rodents,the most significant increase in Cu levels takes place between the postnatal days 7 and 14,due to Cu influx through brain barriers leading to its accumulation in the striatum,thalamus,and superior colliculus (Tarohda et al.,2005).Subsequent changes in Cu uptake are less dramatic: in mice,64Cu uptake followed by PET scan found a gradual increase from 5 months to 13 months of age and then decline at 26 months (Peng et al.,2018).These data suggests that Cu accumulation in ageing brain is due to its less clearance through the barriers and not because of increased influx.Analysis of Cu content from different brain regions isolated from 3 weeks,10 weeks,and 9 months old rats found that most of the entering Cu is deposited around the SVZ and choroid plexus (Fu et al.,2015).The Cu deposits are especially high in the GFΑP-positive cells of SVZ (Pushkar et al.,2013).
Experiments with different forms of Cu (free Cu64,Cu64bound to albumin or ceruloplasmin) established that a free rather than a protein-bound Cu preferentially enters the brain(Choi and Zheng,2009),in agreement with the major role of specialized Cu transporters in the brain Cu entry (below).Cu-Histidine complex penetrates brain in the neonatal period but is not effective as a Cu delivery agent in adults.GTSM (sp-4-2)-((2,2′-(1,2-ethanediylidene)bis(n-methylhydrazine-carbothio-amidato-κn2,κs))(2-)) is a small Cu-binding molecule that was shown to be more effective in transferring Cu to the brain than Cu-acetate (Αndreozzi et al.,2017).Peritoneal injection of64Cu-GTSM complex results in significant Cu uptake by the brain in contrast to low uptake of64Cu administered via oral gavage: all regions of the brain show Cu absorption within 30 minutes with the thalamus,midbrain,cerebellum and pons area having 15–20% higher Cu content compared to average(Baguna Torres et al.,2019).The variable amount of Cu uptake within the brain regions can be attributed to the capillary density,the rate of blood flow,and the abundance of Cu transporters (Gromadzka et al.,2020).
The Sites of Cu Uptake into the Brain
Cu enters the brain from the peripheral circulation through the blood-brain barrier (BBB) and blood-cerebrospinal fluid(CSF) barrier (BCB;Zheng and Monnot,2012).BBB separates blood and brain parenchyma,whereas BCB separates blood and CSF.The BBB is comprised of endothelial cells connected by tight junctions followed by a layer of pericytes and astroglial cells (Figure 1).Astrocytes may regulate brain Cu metabolism,as they are strategically placed between the neuronal cells and blood capillaries of BBB.These cells extend their cellular processes that cover the blood-capillaries and are first to encounter metal ions that cross BBB.The BCB is composed of choroid plexus (ChPl) epithelial cells surrounding the endothelial cells of capillaries.The ChPl endothelial cells are fenestrated and,unlike endothelial cells of BBB,are significantly more permeable (Solar et al.,2020).The BCB barrier is formed by polarized cuboidal epithelial cells of ChPl that are connected by junctional protein complexes and directly face CSF (Figure 2).The anatomical location of the ChPl in ventricles,in the vicinity of Cu-enriched SVZ,suggests the importance of ChPl in regulating Cu homeostasis of the brain.The concentration of Cu in CSF is extremely low compared to blood plasma (Stuerenburg,2000).The difference in Cu concentration between the blood and CSF further illustrates the critical role of barriers in regulating the brain’s Cu homeostasis.
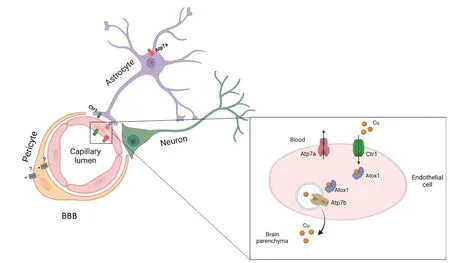
Figure 1|Cu transport in BBB.

Figure 2|Cu transporters in BCB.
The absorptive surface of either BBB or BCB,which is involved in nutrient transport,is vast.The estimated surface of the BBB– 12–18 m2(Kadry et al.,2020)– is approximately 2-fold larger than that of BCB (Johanson et al.,2011).The pioneering studies using64Cu perfusion in the adult rats and subsequent metal analysis in wet tissue,found that the initial rate of Cu uptake was highest by ChPl (1034.5μL/sec/g),followed by blood capillaries (319 μL/sec/g),brain parenchyma (115 μL/sec/g) and CSF (0.8 μL/sec/g) (Choi and Zheng,2009).Based on these results,Cu was suggested to enter the brain parenchyma through BBB,whereas BCB was thought to export Cu out of the brain (Choi and Zheng,2009).However,this model could be oversimplified as the measurement time of Cu entry was limited to 120 seconds.The fate of large amounts of Cu entering ChPl during this time remains unknown.This Cu may potentially be stored or used for the biosynthesis of Cu-dependent enzymes and Cu-binding proteins in the ChPl epithelium.BCB is a very active secretorytissue responsible for the production of CSF.Αlthough protein content of CSF is low compared to serum,such proteins as albumin,cystatin C,serotransferrin,and transthyretin are among the most abundant proteins in the CSF (Lardinois et al.,2014),with albumin and transthyretin capable of binding Cu2+(Ciccone et al.,2018).Thus,current data do not exclude that at a lengthier time-point BCB contributes to both Cu entry into the brain and removal of excess Cu from the CSF.Experiments with rodents also demonstrated that Cu export through the ChPl decreases with age and Cu eventually gets deposited in the ChPl epithelial cells and around the SVZ (Liu et al.,2022b).
Cu Transporting Proteins Are Abundant in Neural Barriers
Although key Cu transporters essential for Cu entry into the brain (CTR1 and ATP7A,see below) have been identified,understanding of their function in the context of brain development and aging (in either norm or disease) is still in infancy.In most cells,including brain tissue,cellular Cu homeostasis is maintained through (i) Cu uptake,which is mediated primarily by the high affinity Cu transporter CTR1(SLC31Α1),(ii) Cu utilization by Cu-dependent enzymes,and(iii) Cu efflux mediated by one or two Cu-transporting P-type ATPases,ATP7A and ATP7B (Lutsenko,2021).Metallothioneins(MT1,MT2,MT3) serve to bind excess Cu,when cells Cu efflux capacity is exceeded.Since ATP7A,ATP7B,and CTR1 are specific for Cu+,it is thought that the extracellular Cu2+is reduced prior to entering cells.The precise mechanism of coupling between the Cu2+>Cu+conversion and Cu+entry into the CTR1 metal translocation pathways is currently being investigated.How and when Cu+that is exported out of the cells by ATP7A and ATP7B becomes Cu2+is not clear.
Dmt1 (Slc11a2) is a divalent-metal transporter expressed in brain barriers (Zheng et al.,2012),which predominantly transports iron and manganese (Mackenzie et al.,2007).The role of Dmt1 as a Cu importer has been suggested by studies of Caco-2 cells and intestinal tissue (Αrredondo et al.,2003).Thein vitrostudies using ChPl-derived Z310 cells,further demonstrated that down-regulation of Ctr1 or Dmt1 resulted in a significantly lower Cu uptake,whereas down-regulation of Αtox1 or Αtp7a increases of cellular retention of Cu (Monnot et al.,2012).Recent study demonstrated the ability an aminoacid transporter LAT1 (L-type amino-acid transporter type 1),which is highly expressed in the brain endothelial cells and choroid plexus,to transport copper-histidine Cu(His)2in vitro(Scanga et al.,2023).Whether this activity LΑT1 contributes to Cu distribution in the brain is an important question for further investigations.
Αlthough all major Cu transporters (Αtp7a,Slc31a1,Αtp7b,Dmt1) and a cytosolic Cu-chaperone Atox1 are expressed in cells forming brain barriers (Fu et al.,2015;He et al.,2022),the expression levels ofSlc31a1(Ctr1),Atp7aandAtox1are especially high (Figure 3).Single cell RNA expression analysis of fetal brain shows abundant signal forCtr1transcript in the endothelial cells,neurons and glial cells (Batzios et al.,2022) and even higher expression in ChPl (Figure 3).Changing Cu levels and other signals regulate the abundance and localization of Cu transporters in ChPl (Zheng et al.,2012;Washington-Hughes et al.,2023).Further studies revealed that ChPl epithelial cells express significantly higher levels ofCtr1andAtp7acompared to that of the cerebral vasculatures(Fu et al.,2015;Hodgkinson et al.,2015) and ChPl maintains a higher expression ofCtr1in all age groups (Fu et al.,2015).In fact,the expression levels ofCtr1in ChPl are on par withGapdh,an abundant metabolic enzyme.In contrast,Atp7bis not significantly expressed in the endothelial cells of capillaries,but shows moderate expression in the ChPl epithelial cells (Choi and Zheng,2009).These findings are in good agreement with the single cell sequencing data in mouse brain available in public databases (Figure 3).High expression of Cu transporters in ChPl further suggests the importance of ChPl epithelia in Cu fluxes that maintain Cu levels in the brain.The challenge arises when one attempts to rationalize the directionality of Cu fluxes in the endothelial cells of BBB or epithelial cells of ChPl based on known localization of Ctr1 and Atp7a in peripheral tissues (such as liver or kidney),and in neuronal cells,or astrocytes.In these cells,Ctr1 typically transfers Cu from blood circulation or from extracellular milleu into cells,whereas Atp7a exports Cu from cells into the bloodstream/extracellular milleu.These functional characteristics place Ctr1 and Atp7a at the same membrane,but working in opposite directions.However,inactivation of either Ctr1 or Αtp7a results in brain Cu deficiency (see below);these findings argue against the Ctr1 and Atp7a targeting to the same membrane in the endothelial cells of BBB.Thus,Cu transport mechanisms in barriers are distinct from Cu transport in peripheral tissue or in the CNS.This notion is supported by the distinct polarity of cells forming barriers,in which a well-known marker of basolateral membrane,Na+,K+-ATPase,is located at the apical membrane facing CSF in ChPl epithelium (Ernst et al.,1986)and in the abluminal (facing brain parenchyma) membrane in BBB (Worzfeld and Schwaninger,2016).
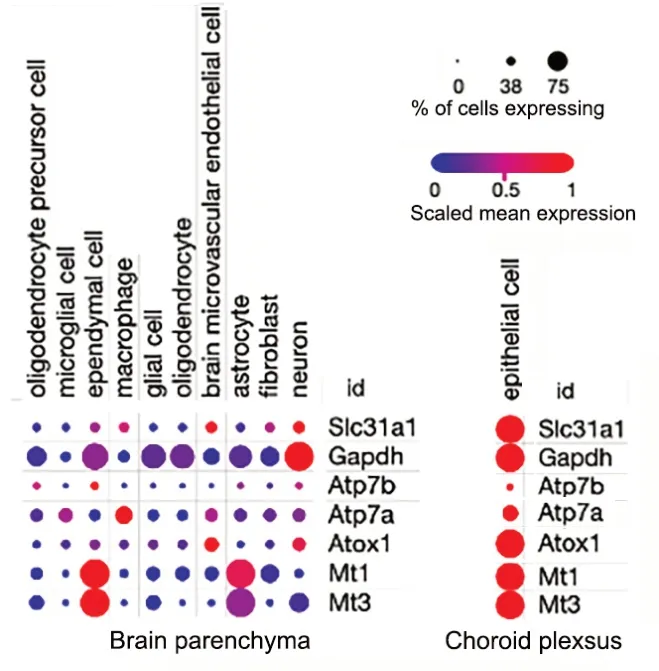
Figure 3|Relative expression of Cu transporters in different cell types of adult mouse brain (left) and choroid plexus epithelium (right).
Ctr1 Is Essential for Cu Transfer into the Brain and Is Abundant in Both Barriers
Recently,new neurological disorders were discovered,that were caused by a functional deficit of a high affinity Cu transporter CTR1 (Dame et al.,2023).Α missense mutation inCTR1was associated with a respiratory distress in a newborn infant,the development of multifocal brain hemorrhages at 2 weeks after birth,tortuosity of cerebral arteries,uncontrolled seizures,coma and death at 1 month.In another case,twins born with a homozygous mutation inCTR1gene showed severe cerebral atrophy and ventriculomegaly.The Cu-His treatment partially improved the symptoms in these patients (Batzios et al.,2022).The marked Cu deficiency in patients with aCTR1mutation suggests that CTR1 mediates Cu uptake either into the endothelial cells of brain capillaries during early tissue development or/and into the epithelial cells of ChPl for further utilization by ATP7A (Figure 2).This predicted localization remains to be experimentally verified.In the mouse ChPl epithelia,localization of Ctr1 was examined;Ctr1 was found to be located at the apical membrane facing CSF (Washington-Hughes et al.,2023).This localization is consistent with a known epithelial cell polarity of ChPl and the role of Ctr1 in regulating Cu levels in CSF.However,these observations are inconsistent with a high rate of Cu uptake from the blood into the CSF epithelial cells (above),unless another Cu transporter performs this function.Further studies are needed to determine whether the basolateral Cu entry into ChPl is mediated by DMT1,by CTR1 that is targeted to both membranes of ChPl epithelial cells,LAT1,or by yet to be characterized Cu transporter.
ATP7A Facilitates Cu Transport into the Brain
A critical role of ATP7A in Cu entry in the brain is firmly established.Inactivating mutations inATP7Acause a fatal neurodegenerative disorder,MD (La Fontaine et al.,2010),which is characterized by defective export of dietary Cu from the gut and is further exacerbated by an impaired Cu entry into the brain (Hodgkinson et al.,2015).Cu deficiency in MD can be diminished (but only partially) by Cu supplementation,which is beneficial only if initiated soon after birth,in both humans and in animal models of MD.The narrow window of therapeutic opportunity highlights significant developmental changes,which occur in the barriers with regard to their permeability to Cu.Since the brain barriers are established prenatally (Ek et al.,2012);changes in Cu permeation during brain development and aging likely involve changes in the expression,intracellular targeting and/or regulation of Cu transporters.The precise molecular nature of these changes awaits further investigation.
While the role of ATP7A in Cu transfer is clear,the relative contribution of ATP7A in BBB and BCB to the entry of Cu in the brain is less certain and may change during brain development.ATP7A is expressed in blood vessels and is important for neovascularization (Αsh et al.,2021).Tortuosity of intracranial blood vessels is characteristic clinical findings in MD patients (Guo et al.,2022),which illustrates functional significance of ΑTP7Α in the vasculature.Αs discussed above,in order to facilitate Cu entry into the brain parenchyma,the localization of ΑTP7Α in endothelial cells should be opposite to periphery (i.e.ATP7A does not export Cu from the endothelial cells into the blood).Αlternatively,ΑTP7Α in ChPl epithelium may be the major route of Cu entry into the brain.Consistent with this latter idea,ATP7A levels were found to be highest in the choroid plexus/ependymal cells of the lateral and third ventricles (Niciu et al.,2006).
An unusual MD patient with a somatic mosaicism for an inactivating ATP7A P1001L mutation provided further evidence for ATP7A-mediated Cu entry into the brain via ChPl.In this patient,cells of ectodermal origin (ChPl epithelia) had ATP7A mutation more frequently than cells of mesodermal origin (endothelial cells of BBB) (Donsante et al.,2010).These results in MD patient with brain Cu deficiency suggest a relatively larger contribution of ChPl to Cu entry compared to BBB.Furthermore,Atp7alevels in the choroid plexus are about 3.4-fold higher than in the cerebral capillaries (Choi and Zheng,2009).Targeted virus-mediated delivery ofAtp7agene to ChPl of an animal model of MD restored Cu delivery to the brain,decreased symptoms of Cu deficiency and prevented mice lethality (Donsante et al.,2011).InAtp7aNesmice,which have Atp7a inactivated specifically within neurons and glial cells,expression of Αtp7a in ChPl is increased,illustrating the ability of ChPl to sense the Cu status of brain parenchyma and regulate Cu flux into the brain (Hodgkinson et al.,2015).
Immunostaining of ChPl epithelia revealed the mostly intracellular localization of Atp7a (Washington-Hughes et al.,2023) and the ability of Αtp7a to traffic to the apical membrane (facing the CSF) upon Cu elevation (Choi and Zheng,2009).Taken together,all these data strongly suggest that Atp7a in BCB contributes significantly to Cu entry into the brain.Furtherin vivo(orin situ) studies of localization and regulation of Αtp7a in endothelial cells in the context of fully formed BBB are needed to better understand how brain barriers work together to maintain Cu influx into the brain.
ATP7B Buffers Cu and Regulates Cu Availability
ATP7B is biochemically similar to ATP7A.Like ATP7A,ATP7B uses the energy of ATP hydrolysis to transfer Cu from the cytosol into the trans-Golgi network for activation of Cudependent enzymes or to endocytic vesicles -for further export and/or storage (Lutsenko,2021).The expression of ATP7B in either BBB or BCB is lower than expression of ATP7A(Figure 3) and ΑTP7B’ role in Cu homeostasis is less defined.Cu elevation in CSF in adult mice triggers Αtp7b trafficking to the basolateral membrane of ChPl epithelia,suggesting the role of Αtp7b in Cu export from the ChPl back into circulation(Figure 2).Αnalysis of Cu concentration in young adult Wilson disease patients,which have mutations in ATP7B,found elevated Cu in CSF in agreement with this model.
Patients with Wilson disease are treated with Cu chelators,such as D-penicillamine or trientine,to remove excess Cu fromtissues (Hedera,2019).Chelation therapy with D-penicillamine was shown to reduce the concentration of Cu in CSF and the extent of this decrease showed negative correlation with the duration of treatment.Nonetheless,during the early phase of chelation,patients can develop secondary neurological deficits including dysarthria,dysphagia,extrapyramidal symptoms and cognitive dysfunctions (Stuerenburg,2000).The basis of this neurologic worsening is unclear and can be ascribed either to excessive Cu sequestration,transient mobilization of the excess Cu by the chelator or more complex mechanisms involving changes in the expression and/or localization of Cu transporters.
Recent study found a transient decrease in Cu levels in the postnatally developing 4-week-oldAtp7b–/–mice brains compared to the controls,which was reversed in adult 20-week-old mice (Washington-Hughes et al.,2023).The reduced levels of Cu observed in the brain parenchyma ofAtp7b–/–mice could be due to Cu being entrapped either in ChPl or in endothelial cells of capillaries (Washington-Hughes et al.,2023).Further studies ofAtp7b–/–mice found that the loss of Αtp7b was associated with significant upregulation of Atp7a and the loss of Ctr1 from the apical membrane of ChPl epithelial cells.These changes in both the expression and localization of Cu transporters,other than Atp7b,illustrate co-dependence of Cu-transporting proteins and highlight the need for careful analysis of consequences of gene knock-outs,as they could be compensatory and very complex.The agedependent changes in the expression and localization of the Cu transporters regulating either influx or efflux of Cu also require better understanding.
Conclusions
Both Cu deficiency and Cu excess are detrimental to brain development and function.Copper deficiency is associated with a decreased activity of essential Cu-dependent enzymes and a wide range of metabolic changes.Excess copper,in addition to causing oxidative stress,also causes protein aggregation,abnormal signaling,and unwanted inhibition of enzymatic activities,all of which contribute to copper toxicity.Studies of Cu entry into the brain have demonstrated the major role of the Cu-transporting P-type ATPase ATP7A and CTR1 (SLC31Α1) in facilitating Cu delivery into the brain parenchyma.Both BBB and BCB are likely to be involved in Cu entry.The Cu flux into the brain changes depending on the developmental stages,nutritional status and under disease conditions.Α better understanding of the mechanism of Cu entry into the brain will be aided by studies of spatial distribution and regulation of the key Cu transporters.The capillaries of the BBB are surrounded by different types of cells,which have different levels of Cu transporter expression and varying Cu storage capacities.Hence,this small population of cells may be important regulators of Cu flux into the brain.The recent advent of high throughput single-cell sequencing and spatial transcriptomics/ proteomics may help to understand the spatiotemporal changes in Cu transporters under different physiologic conditions.In addition,high resolution XRFM images of brain tissues at different ages and/or disease conditions could be informative for studies focusing on Cu storage and compartmentalization in cells.
Acknowledgments:SL thanks Dr.Peter Ott for helpful discussions.The illustrations were created with BioRender.com(agreement ## MR262GOIDX,WA262GOA7H,and AB262GNYB1).
Author contributions:SR wrote parts of the review and created illustrations;SL wrote parts of the review and edited the final text.Both authors read and approved the final version of the manuscript for publication.
Conflicts of interest:The authors declare that there is no conflict of interest regarding the publication of this paper.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Nikolay Solovyev,Helmholtz Center Munich,Germany;David Benn Lovejoy,Faculty of Medicine,Health and Human Sciences,Macquarie University,Australia
Additional file:Open peer review reports 1,2.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

