Cell reprogramming therapy for Parkinson’s disease
Wenjing Dong ,Shuyi Liu ,Shangang Li,Zhengbo Wang
Abstract Parkinson’s disease is typically characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta.Many studies have been performed based on the supplementation of lost dopaminergic neurons to treat Parkinson’s disease.The initial strategy for cell replacement therapy used human fetal ventral midbrain and human embryonic stem cells to treat Parkinson’s disease,which could substantially alleviate the symptoms of Parkinson’s disease in clinical practice.However,ethical issues and tumor formation were limitations of its clinical application.Induced pluripotent stem cells can be acquired without sacrificing human embryos,which eliminates the huge ethical barriers of human stem cell therapy.Another widely considered neuronal regeneration strategy is to directly reprogram fibroblasts and astrocytes into neurons,without the need for intermediate proliferation states,thus avoiding issues of immune rejection and tumor formation.Both induced pluripotent stem cells and direct reprogramming of lineage cells have shown promising results in the treatment of Parkinson’s disease.However,there are also ethical concerns and the risk of tumor formation that need to be addressed.This review highlights the current application status of cell reprogramming in the treatment of Parkinson’s disease,focusing on the use of induced pluripotent stem cells in cell replacement therapy,including preclinical animal models and progress in clinical research.The review also discusses the advancements in direct reprogramming of lineage cells in the treatment of Parkinson’s disease,as well as the controversy surrounding in vivo reprogramming.These findings suggest that cell reprogramming may hold great promise as a potential strategy for treating Parkinson’s disease.
Key Words: animal models;astrocytes;autologous;cell reprogramming;cell therapy;direct lineage reprogramming;dopaminergic neurons;induced pluripotent stem cells;non-human primates;Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the second-most common neurodegenerative disease in the world and is characterized by the death of dopaminergic (DΑ) neurons in the substantia nigra pars compacta (SNpc),causing a range of motor symptoms such as resting tremor,muscle tonus,bradykinesia,and gait disturbances (Bj?rklund and Dunnett,2007;Lees et al.,2009;Dickson,2012;Kalia and Lang,2015;Mourtzi and Kazanis,2022).Current clinical treatments mainly include levodopa infusions (Cenci,2014) and deep brain stimulation therapies (Okun,2012;Fukaya and Yamamoto,2015;Ding et al.,2022;Yuan et al.,2023).In addition,researchers are trying to develop advanced therapy medicinal products such astissue engineered products and gene treatments (Kaplitt et al.,2007;Christine et al.,2009;Marks et al.,2010;Redmond et al.,2013;Ghosh et al.,2019;Gantner et al.,2020;Moriarty et al.,2022),but there is yet no effective preventive or curative treatment for PD (Redmond et al.,2013;Ghosh et al.,2019;Gantner et al.,2020;Moriarty et al.,2022).Because the characteristic pathological changes in PD are the degeneration of DΑ neurons in the SNpc with the progression of the disease,a number of studies are based on replenishing the lost DA neurons to treat PD.Accordingly,strategies aimed at cell replacement therapy have been investigated.Initially,human fetal ventral midbrain (fVM) and human embryonic stem cells (ESCs) were used for the treatment of PD(Kefalopoulou et al.,2014),which could significantly alleviate the symptoms of PD patients.However,ethical concerns and tumor formation are valid limitations for their clinical application.
Since Takahashi and Yamanaka’s team found in 2006 (Takahashi and Yamanaka,2006) that a few transcription factors (TFs)can reprogram terminally differentiated cells into stem cells(induced pluripotent stem cells [iPSCs])in vitro,these cells have been widely used in cell therapy research (Hallett et al.,2015;Kikuchi et al.,2017a,b;Schweitzer et al.,2020).The first advantage is that iPSC lines could be established without sacrificing human embryos,thereby eliminating a significant ethical obstacle to human stem cell treatments.iPSCs also permit human leukocyte antigen (HLΑ) matches in patient-specific treatments,effectively reducing the severity of postoperative immunosuppressants.However,reports of their tumorigenesis and damage to their own DNA after in vivotransplantation have also raised some safety concerns(Takahashi and Yamanaka,2016;Rouhani et al.,2022).
Αnother widely considered strategy for neuronal regeneration is the direct reprogramming of fibroblasts and astrocytes into neuronsin situ(Wang et al.,2021a;Talifu et al.,2023).In this strategy,neurons can be directly reprogrammed from somatic cells without going through the intermediate state of proliferation,thus avoiding the problem of immune rejection and tumor formation.Direct reprogramming provides broad prospects for regenerative medicine.However,in vivoreprogramming is still controversial regarding the conversion of glial cells to neurons in response to polypyrimidine tractbinding protein 1 (PTBP1) knockdown (Wang and Zhang,2023).
Here,we briefly review cell replacement therapies in the treatment of PD,focusing on iPSC replacement therapyin vivoand lineage reprogrammingin situ.
Search Strategy
An electronic search of the PubMed database for literature describing cell reprogramming of PD from 1979 to 2023 was performed using the following key terms: cell reprogramming(MeSH Terms) AND (PD Models,Animal (MeSH Terms)OR Behavior,Animal/Physiology (MeSH Terms) OR Animal Experimentation (MeSH Terms) OR clinical practice (MeSH Terms).The results were further screened by title and abstract to classify rats,mice,non-human primates,and clinical practices.
Research and Clinical Progress in the Use of Induced Pluripotent Stem Cells for Parkinson’s Disease
Cell replacement therapy has been a promising strategy for the treatment of PD.More than 30 years ago,mesencephalic DΑ neuronal precursors from allogeneic fetal tissue were first transplanted into the striatum of PD patients in open-label studies (Lindvall et al.,1989,1990,1992;Freed et al.,1990;Spencer et al.,1992;Molina et al.,1993;Peschanski et al.,1994;Wenning et al.,1997;Hagell et al.,1999;Brundin et al.,2000;Mendez et al.,2002).The first clinical trial demonstrated the feasibility of cell replacement therapy (Lindvall et al.,1989),with significant improvement in positron emission tomography results and symptoms of PD patients who received fVM transplants in a subsequent open-label study(Hallett et al.,2014;Li et al.,2016).However,patients who received transplants in two subsequent double-blind clinical trials showed variable recovery,and graft-induced dyskinesia was reported for the first time in 15–50% of transplanted patients (Freed et al.,2001;Olanow et al.,2003).In addition,in one of the double-blind studies,patients’ symptoms began to worsen after the immunosuppressive drugs were stopped (Olanow et al.,2003).In summary,although there are differences in clinical outcomes,the fVM tissue showed cell survival and long-term benefit.Since 2006,Bj?rklund et al.(2013) research has been dedicated to studying the factors that influence outcomes,with several important parameters including the cells,mode of implantation,immunosuppression,and patient selection.
In 2006,Takahashi and Yamanaka’s team (Takahashi and Yamanaka,2006) developed the iPSC technology.They successfully reprogrammed mouse fibroblasts back to a pluripotent state using four TFs,namely octamer-binding transcription factor 4,SRY-box transcription factor 2 (SOX2),Kruppel-like factor 4,and cellular-myelocytomatosis viral oncogene.This technique has since been used widely as a classic method,and other TF combinations have been developed (Yu et al.,2007;Park et al.,2008;Stadtfeld and Hochedlinger,2010;Dey et al.,2022).In addition,other groups have used microRNΑs (e.g.,miR-302,miR-367) and small-molecule compounds (e.g.,valproic acid,5-azacytidin)for iPSC reprogrammingin vitro(Huangfu et al.,2008;Anokye-Danso et al.,2011;Miyoshi et al.,2011;Borgohain et al.,2019;Dey et al.,2021).iPSCs also ushered in a new era for the treatment of PD.
iPSC-derived cells have several advantages over fetal cellbased therapies,including virtually unlimited availability,standardized manufacturing,and the ability to be cryopreserved.iPSC-derived DA neurons may be effective for future PD treatment (Parmar et al.,2020).This review summarizes the studies of iPSCs in rodents,non-human primates (NHPs),as well as clinical PD patients (Figure 1).PD mice and rats are modeled primarily by 6-hydroxydopamine(6-OHDA) and the NHPs PD model,modeled by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine,are the gold standard models (Cenci and Bj?rklund,2020).The details of the experiments with respect to animal model,sex,age,and transplantation location are shown inTable 1.

Table 1|A summary of research and clinical progress in the use of iPSCs for Parkinson’s disease
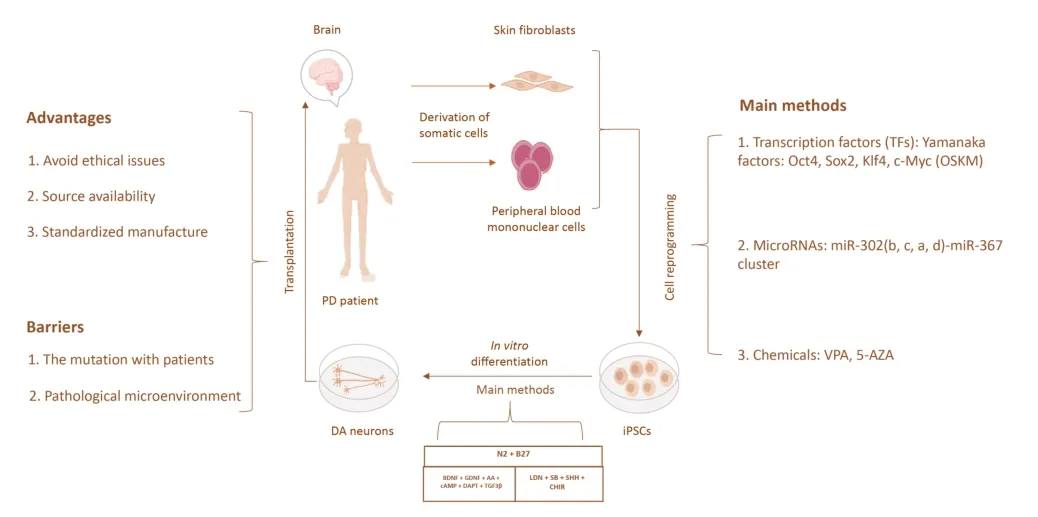
Figure 1|Strategy overview of hiPSC-based cell replacement therapy for PD.
Application of iPSCs in rodent PD models
Induction of differentiation of DA neurons is an important process in stem cell replacement therapy.In a previous study,ESCs were mainly induced into neural progenitors(supplemented with SB431542 and LDN193189) and then into the midbrain floor plate progenitors (supplemented with Sonic hedgehog,CHIR99021,surface antigen 1,and fibroblast growth factor-8b) until differentiation into mature DΑ neurons(supplemented with brain-derived neurotrophic factors,glial cell-derived neurotrophic factor,ascorbic acid,cyclic adenosine monophosphate,transforming growth factor β3,and compound E) (Xiong et al.,2021).By day 42,69% of the total cells were positive for tyrosine hydroxylase (TH) suggesting a midbrain DΑ (mDΑ) neuronal identity.Furthermore,most TH+neurons co-expressed G-protein-regulated inward-rectifier potassium channel 2 (>85%),a marker relatively enriched in Α9 mDΑ neurons.The cells normally used for transplantation are all DA neuronal progenitors (Xiong et al.,2021).The protocol of iPSCs to DA neurons is largely consistent with ESCs’ protocol (compound E was replaced with DAPT) (Gantner et al.,2020).Enrichment of DΑ was confirmed by TH+/FOXA2+co-expressionin vitro.Αfter the transplantation of human iPSC(hiPSC)-derived DΑ neurons into PD rats,significant survival of the transplanted cells and an improvement in apomorphineinduced rotational asymmetry were observed,but only a few neurons were observed to establish connections with the host striatum 16 weeks after transplantation (Hargus et al.,2010).Subsequent studies used cryopreserved iPSC-mDA neurons,and the results showed that transplanted DA neurons could extensively innervate the host brain and improve motor symptoms in PD mice (Wakeman et al.,2017).
However,iPSC applications are associated with low efficiency issues after transplantation (Li et al.,2015).Researchers established a feeder layer-free cell culture system and transgenic iPSCs (Bergstr?m et al.,2011;Lu et al.,2014),and Thompson and Clare’s team (de Luzy et al.,2021) showed that LIM homeobox transcription factor 1 (LMX1Α) could be used to isolate correctly specified cells for iPSC transplantation,which made cell grafts more predictable,with smaller grafts enriched in DA neurons,showing appropriate integration and accelerated functional recovery in PD rats (de Luzy et al.,2019).Furthermore,the activation of the suicide gene within weeks after transplantation could improve both the standardization and safety of iPSC-derived grafts in PD rats.These study findings indicate the feasibility of iPSCs in treating PD.Recently,Moriarty et al.(2022) showed that homotopic transplantation of iPSC-derived mDA neurons combined with the delivery of GDNF to the striatum could recapitulate brain-wide mDA target innervation.A study on rodents demonstrated the feasibility of iPSCs as a cell source for cell transplantation.Rodents are less expensive than NHPs,and there are fewer ethical constraints on the use of rodents than NHPs,so rodent models are often used in PD (Fox and Brotchie,2019).However,similarities in brain structure and behavioral phenotypes of NHPs facilitate experimental assessment of transplantation efficacy of iPSCs.Therefore,we further describe the use and application of iPSCs in NHP models.
Application of iPSCs in NHP PD models
Studies on the application of iPSCs in rodent PD models have provided invaluable information on neuronal survival,migration,and post-grafting integration.Nevertheless,PDrelevant behavioral outcomes (fine motor skills) can be more easily tested in NHP models (Camus et al.,2015).Furthermore,the neo-striatum of NHPs has a caudate nucleus and putamen,similar to humans (Howson et al.,2019).Therefore,more researchers choose NHP PD models for transplantation experiments (Camus et al.,2015;Howson et al.,2019).
In 2015,Ole Isacson’s team (Hallett et al.,2015) transplanted autologous iPSC-derived DA neurons into the striatum of a cynomolgus monkey PD model.The transplanted DA progenitor cells could survive for at least 2 years in the host brain,differentiate into DA neurons and grow widely in the putamen.This was the first report that proved the long-term innervation and functional efficacy of autologous iPSC-derived DA neurons in an NHP model without the requirement for immunosuppression.Αfter that,Takahashi’s team (Kikuchi et al.,2017b) transplanted progenitor DA neurons from iPSCs into NHP PD models and carried out a 2-year follow-up of the monkeys.The study found that PD symptoms improved significantly.Subsequent research used iPSCs derived from a major histocompatibility complex homozygous cynomolgus monkey (an NHP with a poor immune response) for transplantation,which significantly reduced immune rejection after the transplantation of cells and avoided the use of immunosuppressants (Morizane et al.,2017).
Furthermore,autologous transplantation of patient-specific iPSC-derived neurons is a better approach for the treatment of PD,and Emborg and Zhang’s team (Tao et al.,2021) compared the function of allogeneic and homologous autologous iPSC-derived progenitor DA neurons after transplantation in a monkey model.The results showed extensive axonal growth of DA neurons in transplanted cells from homozygous autologous grafts in the absence of immunosuppression and that motor and depressive symptoms recovered more markedly in monkeys that received autografts,which demonstrated that the use of autologous iPSCs obviates the need for immunosuppression.
In addition to autologous transplantation,HLA matching can also avoid the use of immunosuppressive drugs after transplantation.HLA matching refers to hiPSCs from super donors who have blood type O and are homozygous at the HLA loci,and whose HLA profiles make their cells more compatible for use in unrelated recipients (Taylor et al.,2011).Previous studies have used zinc finger nucleases (Torikai et al.,2013),adeno-associated virus (ΑΑV)-mediated gene editing to knock in HLA-E genes at the B2M locus in hiPSCs (Gornalusse et al.,2017),selective deletion ofHLAgenes using clustered regularly interspaced short palindromic repeats (CRISPRCas9) (Xu et al.,2019),and other gene editing methods to generate suitable HLA cell lines (Han et al.,2019).Based on these results,banks are currently being established in Japan,Europe,China,and USA,which make it possible to complete the supply of “off-the-shelf”cells (Wilmut et al.,2015).However,there are many challenges in the process of establishing the bank,such as the selection of suitable donors and related genotypes and the issues with international regulatory bodies.In addition,suitable donors are extremely rare;in a UK study,it was found that iPSCs from the top 50 ranked donors could only be HLA-matched to only 79% of recipients (Taylor et al.,2011).Wilmut et al.(2015) have described these issues in detail.Despite HLA matching based on the major HLΑ proteins,immunosuppression may still be beneficial (Morizane et al.,2017).Hence,research on NHP may provide a more valuable clinical reference.
Clinical treatment of PD with iPSC transplantation
Based on the above preclinical studies,Takahashi’s team launched the first clinical trial of iPSC transplantation for the treatment of PD in 2018 (JMΑ-IIΑ00384,UMIN000033564),and 2.4 million DA neurons were transplanted into the left brain of the first enrolled patient (Cyranoski,2018).The patient is presently in good health and has had no major adverse reactions (Doi et al.,2020).
In 2020,an investigation was conducted on a PD patient receiving treatment with DA neurons from autologous iPSCs(Schweitzer et al.,2020).This study was approved by US Food and Drug Administration.The patient had PD for more than 10 years,and the symptoms could not be controlled by drug treatment.Αutologous-derived iPSC-differentiated DΑ neurons were transplanted into the left and right putamen in 2017 and 2018,respectively.Two years after transplantation,the results of imaging examination showed that the transplanted DΑ neurons were alive in the patient’s brain and functioning in the expected way,without the need for immunosuppression.Clinical measures of PD symptoms after surgery stabilized or improved at 18–24 months after the implantation (Schweitzer et al.,2020).
Potential problems in iPSC transplantation for PD
The above studies showed that iPSCs have a self-renewable ability similar to ESCs and can differentiate into most somatic cell types,including neural precursor cells (Zhang et al.,2020).iPSC technology overcomes ethical and cell source problems and provides individualized cell therapy strategies that are considered to have great prospects in PD treatment.
However,there are some potential problems in the application of iPSCs.First,most iPSCs are generated by retrovirus or lentivirus vector gene transfer,and the genomes may be integrated into the host cell genome,leading to the potential risk of insertion mutations and teratoma formation after transplantation (Takahashi and Yamanaka,2016).Therefore,various studies have attempted to address the potential effects of the viral vectors through reprogramming by microRNAs,small molecules,or biocompatible materials(Yoo et al.,2011,2015;Ladewig et al.,2012;Xue et al.,2013;Ifkovits et al.,2014;Victor et al.,2014).
In addition,a research team from Cambridge University used whole-genome sequencing and whole-exome sequencing to conduct a comprehensive study on the mutation of hiPSCs in a large sample,and they explored the impact of mutations on the function of hiPSCs (Rouhani et al.,2022).They found that approximately 72% of skin fibroblast-derived iPSCs contained mutations associated with ultraviolet damage,and 26.9% of blood-derived iPSCs contained BCL6 corepressor mutations that affect pluripotency.This study demonstrated that mutations are common,but post-mitotic neurons are not usually considered to have cancer-related genetic mutations(Martínez-Jiménez et al.,2020).Furthermore,neither tumors nor the appearance of abnormal proliferation was reported in the transplantation of DΑ neurons derived from iPSCs (Kirkeby et al.,2017;Studer,2017;Takahashi,2017).These results suggest that the mutations generated by iPSC reprogramming have no safety concerns after transplantation.However,these approaches still require caution to be applied in the clinic.It is crucial to characterize the source cells,especially in large cell banks,where the presence of mutations in growth-related genes such as TP53 mutations has been shown (Garitaonandia et al.,2015;Αmir et al.,2017;Merkle et al.,2017).
Previous studies have also shown that iPSC-derived cells from PD patients are more vulnerable in the pathological environment (Devine et al.,2011;Beevers et al.,2013;Sanders et al.,2014;Rakovic et al.,2015;Chung et al.,2016;Bieri et al.,2019;Zambon et al.,2019).For example,mitochondrial autophagy and epigenomic and transcriptomic alterations can also be detected in DΑ neurons of sporadic PD patients (Fernández-Santiago et al.,2015;Hsieh et al.,2016).Therefore,genetic testing of autologous iPSCs is required prior to autologous transplantation.CRISPR-Cas9 technology might be able to overcome this challenge,because mutations can be introduced or corrected by Cas9-mediated genome editing(Tian et al.,2019;Chen et al.,2020a;Hernández et al.,2021).CRISPR-Cas9 can be used to express or inhibit the production of specific gene products,for example,some particular pathological point mutations in the SNCA,LRRK2,and GBA genes of PD (Soldner et al.,2016;di Domenico et al.,2019).These technologies offer exciting prospects for both modeling and cell transplantation therapy (Sen and Thummer,2022).
The selection of the transplantation site is also an important issue to be considered in cell replacement therapy.Many studies have focused on transplanting cells into the striatum to achieve the effect of supplementing dopamine,but mDA pathways were not reinstated,which may explain the incomplete restoration of motor function in patients(Hagell et al.,1999;Kikuchi et al.,2017a;Tao et al.,2021).Recent studies have attempted to transplant ESC-derived DA neurons into the SNpc of the midbrain,and at the same time,neurotrophic factors were added to promote transplanted neuron targeting to the striatum to achieve the effect of treating PD (Adler et al.,2019;Moriarty et al.,2022).Some progress in these studies has been made in rodents.Specifically,the grafts provided reinstatement of striatal dopamine levels and correction of motor function and also connectivity with additional mDA target nuclei,which suggested the feasibility of reconstruction of the long-distance circuitry with homotopic transplantation.However,further evidence is needed to determine whether these studies can be advanced to clinical research.
Finally,some results in clinical patients (Li et al.,2016) and animal PD models (Hoban et al.,2020) showed the spread of pathology from the host brain to the transplanted subject.However,to date,the long-term consequences of the presence of pathology in grafted neurons remain unknown.
Direct Lineage Reprogramming as a New Treatment Strategy for Parkinson’s Disease
Direct cell lineage conversion (also referred to as transdifferentiation) is a process that skips the pluripotent state to obtain target cells directly.Transdifferentiation can effectively reduce the generation of teratomas,providing another idea for regenerative medicine.For the treatment of central nervous system diseases,the key to application is to generate neurons through transdifferentiation.Through a combination of neural lineage-specific TFs (Ascl1,Brn2,and Myt1l),fibroblasts could be successfully transformed into induced neuronsin vitro(Vierbuchen et al.,2010;Qin et al.,2020).In addition,Corti et al.(2012) directly reprogrammed human cortical astrocytes into neural progenitor cells by expressing a single TF (octamer-binding transcription factor 4,SOX2,or NANOG)in vitro,which skipped the iPSC stage and reduced the risk of teratoma formation.This study provides a basis for the transdifferentiation of astrocytes into neuronsin vivo.Direct lineage conversion approaches could be used from patients’ fibroblasts (Capano et al.,2022;Drouin-Ouellet et al.,2022;Pircs et al.,2022;Table 2).

Table 2| A summary of direct lineage reprogramming as a new treatment strategy for Parkinson’s disease
Direct neural lineage reprogramming in vivo
Based on the above research,Niu et al.(2013) transferred Sox-2 into the striatum through lentivirus in 2013;successfully transformed astrocytes residing in the brain into neural stem cells (NSCs)in vivo,which were differentiated into mature neurons;and proved that these neurons could be locally integrated into the brain of rats.In addition,Liu et al.(2015) used ΑΑV encoding the TF achaete-scute family bHLH transcription factor 1 (Αscl1) driven by the glial fibrillary acidic protein promoter to reprogram dorsal midbrain astrocytes into mature neuronsin vivo.A study in 2019 used a single reprogramming factor,zinc finger protein 521,to generate NSCs from astrocytes,and these NSCs differentiated into neuronsin vivo(Zarei-Kheirabadi et al.,2019).The generation of these reprogrammed neuronsin vivosuggests the feasibility of transdifferentiation therapy for PD.A diagram of direct lineage reprogrammingin vivoandin vitrois shown inFigure 2.However,direct reprogramming of neural lineages is an emerging field of science,with mixed results to date.For example,there was some degree of leaky expression of ΑΑV2/5 in endogenous neurons (Mattugini et al.,2019;Qian et al.,2020),so that expression of neurogenic factors could also activate the GFAP promoter in endogenous neurons(Wang et al.,2020).In addition,ΑΑV could exert toxic effects on double cortex-positive neural progenitor cells (Johnston et al.,2021),which could prevent the conversion of virusinfected astrocytes into neurons.Previous studies have also shown that it is difficult to detect double cortex-positive intermediate cells using AAV genetic manipulation (Chen et al.,2020b;Lai et al.,2020;Leib et al.,2022).Therefore,experimental results targeting ΑΑV2/5 need to be considered comprehensively or by labeling endogenous neurons or glial cells to demonstrate that reprogrammed neurons are not preexisting neurons.
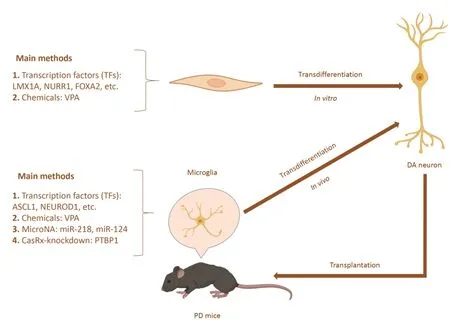
Figure 2|Schematic representation of direct lineage reprogramming for PD.
Direct neural lineage reprogramming in animal PD models
In a 2011 study,a combination of three TFs (Ascl1,NR4A2[Nurr1],and LMX1A) was used for the first time to directly transform mouse and human fibroblasts into functionally induced DA neuronsin vitro(Caiazzo et al.,2011).Then,the induced DA neurons were transplanted into PD model mice,where they could functionally integrate into the host neural circuit and alleviate the symptoms of mice (Kim et al.,2011).Subsequently,induced DA neurons were developedin situ(Jiang et al.,2015),and the first successful induction of DΑ neurons in PD mice was achieved in 2017 (Rivetti di Val Cervo et al.,2017),thus providing a new therapeutic perspective for PD treatment.
MicroRNAs can also be used to reprogram astrocytes into neurons.Several studies have shown that miR-9 and miR-124 target Brg/Brm-related factor 53a (a subunit of the BΑF complex) (Ghasemi-Kasman et al.,2015) can promote neuronal differentiation by targeting the multipyrimidine binding (PTB) protein and its homologous neuron PTB(Makeyev et al.,2007).Therefore,mouse midbrain and cortex astrocytes were reprogrammed into functional DA neuronsin vivoby manipulating PTB/neuron PTB circuits (Xue et al.,2013),and in PD model mice,astrocyte-derived DΑ neurons alleviated several symptoms of PD.Next,Zhou et al.(2020)used the CRISPR-CasRx system to knock down PTBP1 in the PD mice animal model,which could directly reprogram astrocytes into DA neurons in the striatum of PD micein vivo,and alleviate the motor defects in the PD mouse model.Qian et al.(2020) reduced the expression of PTBP1 gene through RNA interference technology,transformed astrocytes in the SNpc of PD mice into DA neurons,and further projected axons to the striatum,reconstructed the substantia nigra striatum circuit,and effectively restored the motor function of mice.These two studies suggested that the PTBP1 gene might be a potential target for treating PD.However,there are some controversies related to this approach.
Wang et al.(2021b) later demonstrated that astrocytes in the cortex or striatum could not be directly reprogrammed as neurons by overexpressing NEUROD1 or knocking down PTBP1.In these two studies,although the models were different,i.e.,in Fu’s team (Qian et al.,2020),the PD model was established by 6-OHDA injection in SN and in Zhang’s team (Wang et al.,2021b),it was established by cortical injury,the injection dose of ΑΑVs (1–4 μL per position) was similar,so the deviation in operation was minimal.Zhang’s team suggested that the so-called “newborn neurons”shown in previously published research from other laboratories were most likely the endogenous neurons in the brain that were mislabeled.Subsequent studies have shown that the loss of PTBP1 in both the retina and the brain of mice could not induce the conversion of glial cells into neurons (Hoang et al.,2022).Chen et al.(2022) also demonstrated that knockdown of PTBP1 from astrocytes in the SN or striatum of the 6-OHDA mouse model did not generate astrocyte-derived dopamine neurons using the Aldh1l1-CreERT2-mediated specific astrocyte lineage tracing method.Subsequently,the latest study by Yang et al.(2023) contradicts these previous results in cells that Cas13X-mediated PTBP1 knockdown does not induce glial cell to neuronal transformationin vivo.They believed that the previous results were attributed to GFAP-AAV leakage.Recently,Hoang et al.(2023) published a new study in Nature that showed that PTBP1 function loss could not induce astrocyte differentiation into neurons,and even could not cause gene expression changes in astrocytes.However,Hao et al.(2023) came to the opposite conclusion after analyzing the single-cell RNA sequencing data released by Hoang et al.(2023) Based on the above research results,it is necessary to further investigate whether astrocytes can be reprogrammed into iDNs by knocking down PTBP1 or overexpressing TFs(Weinberg et al.,2017;Maimon et al.,2021).Wang and Zhang (2023) reviewed the controversy surrounding PTBP1-based glial cells to neuronal transformation and summarized recommendations for future studies of glial cells to neuron transformationin vivoand PTBP1 in a recently published review.The above studies highlight the importance of using genetic manipulation,lineage tracing,and gene expression analysis in the study of cell type transformation.
At the same time,a recent study showed that elongated porous gold nanorods (AuNpRs) could be an enhancer of direct neural lineage reprogrammingin vivo(Lee et al.,2022).In this study,researchers transduced lentiviral particles together with AuNpRs into the striatum of 6-OHDA-induced PD mice.The results showed that AuNpRs promoted the transformation of astrocytes into DA neuronal lineage in 6-OHDA PD mouse models,by promoting the expression of antioxidant-related molecules.DA neurons reprogrammed with ΑuNpRs formed functional synapses with other neurons(Lee et al.,2022).Moreover,some other recent studies have shown that various biocompatible materials can be used to improve the efficiency,specificity,and safety of gene delivery to target cells,such as liposomes,polymeric nanoparticles,and inorganic nanoparticles (Tournebize et al.,2012;Amendola et al.,2017;Kang et al.,2020;Shin et al.,2022),indicating that the neuronal reprogramming approach may open up a new era in the treatment of PD.
Potential problems in direct neural lineage reprogramming for PD
Notably,direct neural lineage reprogramming has illustrated a potentially clinically feasible approach for the treatment of patients with PD.However,many obstacles need to be overcome for the eventual application of this approach in humans,including the age-related limits of reprogramming and the potential adverse effects caused by local astrocyte depletion.
First,it is unclear whether midbrain astrocytes from middleand advanced-aged animals are suitable for reprogramming,as the vast majority of PD patients are between 65 and 79 years of age,and astrocytes from patients with chronic PD and older people exhibit different reaction states and different transcriptome profiles (Xue et al.,2016;Boisvert et al.,2018;Clarke et al.,2018).
Next,reactive astrocytes in the SNpc release excessive GΑBΑ,leading to neurotransmitter imbalance in a PD model (Escartin et al.,2021),which may affect reprogramming efficiency as well as neuronal survival after astrocyte reprogramming.At the same time,astrocytes play an important role in regulating neuronal circuits through Ca2+signaling (Bush et al.,1999;Khakh and Deneen,2019),and Ca2+signal transduction in astrocytes may also affect a variety of signal transduction pathways related to maintaining neuronal circuits.Therefore,the consumption of astrocytes might destroy the local interaction between astrocytes and neurons,thus adversely affecting neuronal function.
In addition,the remaining DA neurons in the SNpc show signs of accelerated aging in PD,probably because of the continuous demand for oxidative phosphorylation leading to mitochondrial DNA damage,resulting in the generation of reactive oxygen species and free radicals in DA neurons(Barone,2010;Chan et al.,2010;Ma et al.,2016).Therefore,it remains to be investigated whether the transformation of astrocytes into DA neurons in older PD patients will further impair the redox balance in the SNpc,thereby inducing rapid death of the remaining DΑ neurons in the patient’s brain.
Each of these issues should be addressed experimentally for the development of this promising therapeutic strategy.
Summary and Outlook
Current PD treatment approaches primarily address motor dysfunction but are associated with significant drawbacks or decline in effectiveness over time.Over the past decades,attention has also been focused on gene therapy strategies.Cell replacement therapy provides a more promising treatment strategy,with human fVM and ESC-derived DA progenitor cells or NSCs producing satisfactory results in preclinical studies,but ethical issues and the risk of immune rejection limit their clinical applications.Autologous hiPSCs could overcome such ethical and immunological issues and therefore had advantages over other cell sources in regenerative medicine (Yasuhara et al.,2020).
The direct reprogramming of brain-resident astrocytes into functional DA neurons is another ideal strategy for PD treatment,and some progress has been made in mouse models.However,several problems need to be resolved before the reprogramming of astrocytes can be used in human experiments,and further preclinical and clinical studies are still needed (Tao et al.,2021).In addition,direct reprogramming can be used to treat other neurological diseases,for example,direct reprogramming of astrocytesin vivohas been used to treat brain injury,Alzheimer’s disease,spinal cord injury,and stroke mouse models.These studies have demonstrated the potential and promise of direct reprogramming in the treatment of neurological disorders beyond PD (Chang et al.,2023).
With the progress in these revolutionary technologies,it is not difficult to imagine that in the near future,iPSCs and direct lineage reprogramming therapies might become potential strategies to cure PD.However,this review did not provide a detailed explanation of the application of cell reprogramming in other neurodegenerative diseases,and extensive discussions might contribute to more comprehensive understanding of the applications of cell reprogramming.
Author contributions:WD and SLiu prepared the manuscript.ZW and SLi contributed to the definition of intellectual content and manuscript review.All authors approved the final version of the manuscript.
Conflicts of interest:The authors report no conflicts of interest in this work.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

